1. Introduction
With the intensification of global environmental issues and resource crises, the chemical industry is confronted with huge challenges regarding the pollutants and resource waste generated during the chemical synthesis process. Therefore, achieving greening and sustainable chemical reactions has become an important challenge in the field of chemistry. Against this backdrop, research on green catalysts has emerged as a key technology promoting the development of green chemistry.
Green catalysts are defined as those that can enhance reaction efficiency, reduce by-products, lower energy consumption and minimize environmental pollution. They typically possess biodegradability, low toxicity and recyclability. The classification of green catalysts includes metal catalysts, organic small molecule catalysts, enzyme catalysts and solid acid-base catalysts, etc. These catalysts exhibit high catalytic activity and selectivity, enhancing atomic utilization and reducing energy consumption while being environmentally friendly.
This article explores the application of green catalysts in sustainable chemical reactions, highlighting their ability to optimize reaction pathways, enhance atomic utilization, reduce pollution from the source, and meet global demands for low-carbon transformation. By promoting material design and process innovation, the industry can reduce energy consumption and enhance competitiveness, providing green technological support in fields like medicine and energy. This approach contributes to achieving the "dual carbon" goals, balancing economic development and ecological protection, and serves as a core driving force for a sustainable chemical industry.
2. The application of green catalysts in organic synthesis reactions
Enzyme catalysis, a green biocatalyzer, features high selectivity, efficiency and environmental friendliness. This approach is widely applied in asymmetric synthesis, preparation of chiral compounds, construction of complex molecules and other fields. Enzyme catalysis, with its excellent properties, has become an important means in modern organic synthesis.
The Mannich reaction is widely used and economical methods for forming C-C bonds and introducing nitrogen-containing compounds in organic synthesis, with important applications in the synthesis of various drugs and natural products [1-2]. Ideally, this reaction is performed by the three-component one-pot method. However, most reaction methods are carried out in two components step by step, where electrophilic imines or nucleophilic enols and enamines are generated sequentially [3]. The traditional Mannich reaction route has a low atomic utilization rate due to multiple separation and purification steps, making it less suitable for green synthesis. In contrast, the one-pot Mannich reaction achieves step integration by in-situ generation of active intermediates, significantly enhancing atomic economy (up to over 92%) and simplifying the process (reducing reaction time by 50%). Therefore, it is regarded as an environmentally friendly and efficient synthetic strategy [4]. In 2009, Yu Xiaoqi and Wang Na et al. reported the direct Mannich reaction catalyzed by lipase. The lipase catalytic activities derived from Mucor oryzae and Candida were relatively high, achieving yields of 72% and 48% respectively. The authors also found that the water content in the reaction system had a significant impact on the enzyme activity (in Figure 1) [5]. In 2010, they reported a direct Mannich reaction catalyzed by lipase from Candida albicans, expanding the enzyme's versatility and substrate range [6]. In the same year, Zhang Pengfei's research group also reported the direct Mannich reaction catalyzed by porcine trypsin and achieved excellent results as well (in Figure 2) [7].
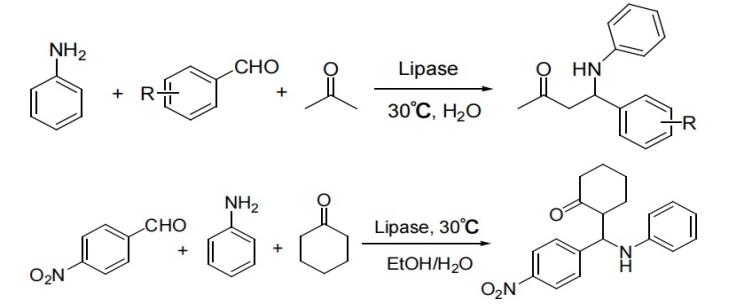
Figure 1: Direct Mannich reaction catalyzed by lipase [5]
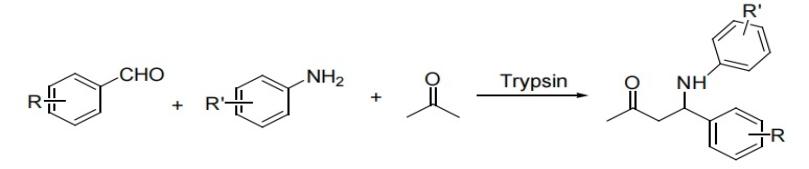
Figure 2: Direct Mannich reaction catalyzed by porcine trypsin [7]
In studying the catalytic organic reactions catalyzed by REDOX enzymes in organic solvents, several typical examples illustrate these processes. A prominent case is the oxidation of polyphenols to o-quinones, catalyzed by mushroom polyphenol oxidase, and the polymerization of phenols via oxidase. [8]. In enzyme-catalyzed REDOX reactions, biocatalysts achieve the efficient construction of chiral centers through precise three-dimensional control. Dehydrogenase enzymes, such as alcohol dehydrogenase ADH, utilize NAD(P)H cofactor to drive the asymmetric reduction of ketone groups, resulting in chiral alcohols with high optical purity (e.g., key intermediate of β -receptor blockers, ee value >99%). Their reverse catalytic ability can also selectively oxidize primary alcohols to aldehyde/ketone compounds. Additionally, mono-oxygenases achieve selective hydroxylation of inert C-H bonds by activating molecular oxygen. This strategy is of milestone significance in the synthesis of natural products, such as converting artemisinic acid to the antimalarial drug artemisinin via P450 enzymes, and hydroxylating key paclitaxane precursors in the semi-synthesis of paclitaxel, effectively replacing traditional multi-step chemical methods. In industrial applications, transaminase catalyzes the efficient preparation of chiral amine intermediates, enabling enantioselectivity to exceed 99.9% while reducing process waste by 90%.
3. Application of green catalysts in environmental remediation
Green catalysts, especially photocatalysts like TiO₂, are essential for degrading organic pollutants, effectively transforming harmful substances into harmless ones. In photocatalytic reactions, the catalyst is excited under the action of light to generate electron-hole pairs. These electron-hole pairs respectively participate in reduction reactions and oxidation reactions, thereby achieving the purposes of energy conversion and pollutant degradation, etc. The reaction mechanism can be divided into light absorption, formation of electron-hole pairs, their separation and migration, generation of active species, REDOX reactions, and maintenance of the catalytic cycle.
3.1. TiO2 photocatalytic mchanism
The TiO2 crystal mainly has three structures: rutile type, anatase type and brookite type (in Figure 3)[9]. The rutile type belongs to the tetragonal crystal system. Each unit cell contains two TiO2 molecules. The oxygen ions are hexagonal and closely packed. The octahedral chains extend along the C-axis, and the chains share angular apex connections. The anatase type is also a tetragonal crystal system. The unit cell contains four TiO2 molecules. The octahedrons are connected through shared vertices, and the structure is relatively open. The brookite type belongs to the orthorhombic crystal system. The unit cell contains 6 TiO2 molecules. The octahedral arrangement is unique, and the common edge and common vertex situations are complex, with poor stability.
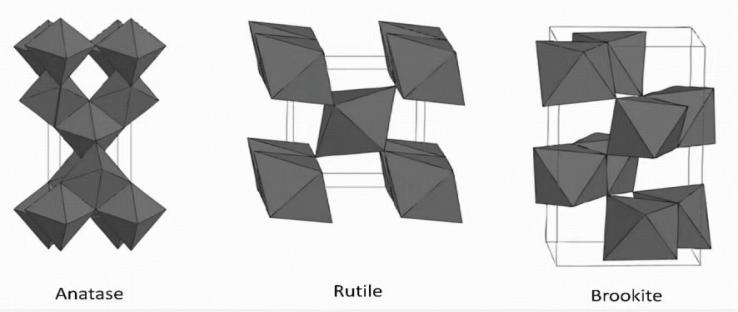
Figure 3: Schematic diagrams of three crystal forms of TiO2 [9]
In 1972, Fujishima and Honda first demonstrated that TiO2 electrodes in electrochemical cells could decompose water into hydrogen and oxygen under ultraviolet light [10]. This discovery sparked significant interest in TiO₂ photocatalysts for applications in environmental remediation and hydrogen production [11]. TiO2 is an N-type semiconductor material. The band gap width of the anatase phase is 3.2 eV. It can absorb ultraviolet rays with wavelengths lower than 380nm to excite itself and generate catalytic activity [12]. The catalytic process mechanism of TiO2 involves several stages (in Figure 4). First, when the light energy absorbed by TiO2 reaches or exceeds its bandgap energy, the valence band electrons will be excited to the conduction band, generating electron-hole pairs [11]. In the second stage, some electron-hole pairs recombine inside or on the surface of TiO2. In the third stage, others separate and transfer to the surface. In the fourth and fifth stages, the holes and electrons transferred to the surface of TiO2 can respectively act as strong oxidants and strong reductants, triggering oxidation and reduction reactions on the surface.
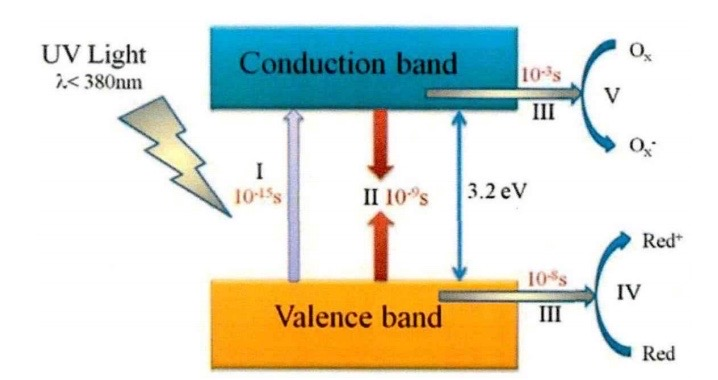
Figure 4: Schematic diagram of the photocatalytic mechanism of TiO2 [11]
3.2. Influencing factors of TiO2 photocatalytic activity
Firstly, different crystal forms of TiO2 have a significant impact on photocatalytic activity. Crystal forms with a narrower band gap will hinder the occurrence of reduction reactions, while those with more defects and dislocations have more vacancies to capture electrons, reducing the likelihood of electron-hole recombination and allowing for sustained high catalytic activity. However, special treatment during the synthesis may also decrease catalytic activity [13]. In addition, the differences in electron receptors can also have an impact on photocatalytic activity.
Secondly, the influence of temperature on the photocatalytic process shows a bidirectional regulation characteristic. Heating can increase the proportion of reactive molecules and promote the chemical surface reactions but increased thermal motion will reduce the adsorption efficiency of reactants. By precisely controlling the heat treatment, the particle size, crystal structure and surface characteristics of nano-TiO2 can be regulated, thereby optimizing its catalytic performance. In practical applications, the rate of photocatalytic reactions can be precisely controlled by adjusting temperature parameters, thus facilitating optimization of the photocatalytic process.
In addition, lattice defects can reduce atomic spacing, thereby enhancing the adsorption effect of reactants and promoting the photocatalytic reaction. However, these defect sites may also become the recombination centers of photogenerated electron-hole pairs, reducing the catalytic efficiency. This highlights the need for balanced regulation of defect concentration.
Finally, introducing oxidants into the reaction system can effectively capture photogenerated electrons, thereby significantly inhibiting the recombination process of electron-hole pairs and further enhancing the efficiency of the photocatalytic reaction. The addition of this electron acceptor optimizes the carrier separation efficiency of the photocatalytic system by altering the charge transfer path, ultimately manifested as a significant increase in the reaction rate.
4. Application of green catalysts in biofuel production
Green catalysts have shown broad application prospects in the field of biofuel production. Their environmentally friendly characteristics and highly efficient catalytic performance provide innovative solutions for the sustainable development. In the process of biodiesel preparation, solid base catalysts (such as CaO/ZrO2) can replace traditional homogeneous catalysts, achieving a conversion rate of over 98% for fatty acid methyl esters while avoiding wastewater pollution.
4.1. Reaction mechanism of traditional homogeneous catalysts
Take the NaOH alkaline homogeneous catalyst as an example. The first step of the reaction is that sodium hydroxide will dissociate sodium hydroxide ions in methanol. Due to their strong nucleophilicity, hydroxide ion attacks the hydrogen atom on the hydroxyl group in the methanol molecule, causing the methanol to deprotonate and form a methoxy anion. In the second step, the generated CH3O⁻ acts as a nucleophile to attack the carbonyl carbon atom (C=O) in the ester group of the triglyceride molecule. The carbonyl carbon atom, due to the high electronegativity of the oxygen atom, has a certain positive charge and is vulnerable to attack by nucleophiles. After the nucleophilic reaction, a tetrahedral intermediate is formed. Next, in the third step, the tetrahedral intermediate undergoes rearrangement. The redistribution of the electron cloud causes the bond with the alkoxy group (-O-R, where R is the alkyl group from the fatty acids in vegetable oil) to break, while the methoxy group (-OCH3) forms a new ester bond with the carbonyl carbon. Meanwhile, a fatty acid methyl ester molecule (one of the main components of biodiesel) and a diglycerol molecule were released. Then the reaction is repeated. The generated diglycerol molecules will undergo a similar nucleophilic attack, intermediate formation and rearrangement process with the methoxy anion. This reaction will repeat, resulting in the formation of fatty acid methyl ester molecules and glycerol monoester molecules. Then the glycerol monoester molecule undergoes the final reaction with the methoxy anion to generate the methyl ester molecule of the third fatty acid and the glycerol molecule (as shown in Figure 5).
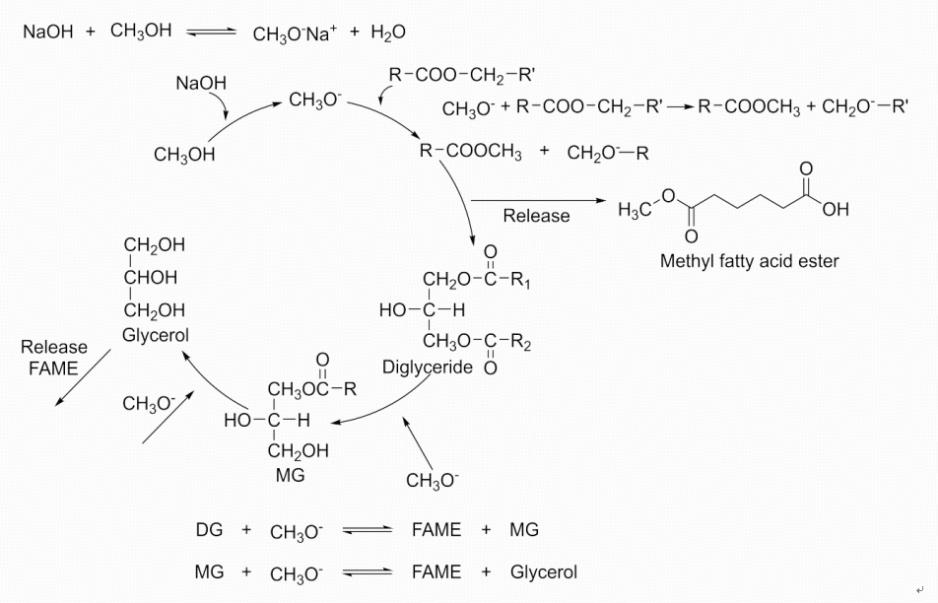
Figure 5: Homogeneous catalytic process with NaOH catalyst
4.2. Esterification reaction of ethanol and oleic acid catalyzed by Zro2
During the esterification reaction process, oleic acid molecules first undergo chemical adsorption with the Lewis acid sites on the surface of the ZrO2 catalyst through their carbonyl oxygen atoms. Subsequently, under catalytic action, the adsorbed oleic acid molecules undergo protonation to form carbocation intermediates. This intermediate undergoes nucleophilic addition with the hydroxyl oxygen atom of the ethanol molecule to form a tetramer transition state complex. In this transition state, the esterification reaction proceeds rapidly, leading to the formation of ethyl oleate through intramolecular dehydration. Ultimately, the generated ethyl oleate molecules are desorbed from the surface of the catalyst, completing the entire catalytic cycle.
According to the Eley-Rideal mechanism, the heterogeneous catalytic reaction of ethanol and oleic acid on ZrO2 proceeds in the following three-step sequence. First, the carboxyl oxygen of the oleic acid molecule forms a coordination bond with the Lewis acid sites on the surface of ZrO2, completing the chemical adsorption. Second, in the nucleophilic substitution stage, the body-phase ethanol molecules attack the carbonyl carbon of the adsorbed oleic acid, generating ethyl oleate through a nucleophilic substitution reaction and releasing water molecules. The final desorption and regeneration stage is that the product ethyl oleate dissociates from the active site, restoring the catalyst to its initial state and completing the catalytic cycle [14].
4.3. Influencing factors for the preparation of biodiesel
There are several influencing factors in the transesterification of vegetable oil to prepare biodiesel.
Firstly, the molar ratio of reactants has a significant regulatory effect on the equilibrium of esterification reactions. According to Le Chatelier's principle, by increasing the amount of methanol (the molar ratio of alcohol to acid is usually controlled between 6:1 and 12:1), the chemical equilibrium can be effectively shifted towards the formation of fatty acid methyl esters (FAME).
Secondly, the dosage of the catalyst affects the number of active sites and their dynamic equilibrium. If a low dose is used, the insufficient active sites lead to a slow reaction rate, causing the equilibrium to shift to the reactants. When the optimal dose is used, a high-yield balance is achieved rapidly. However, when an excessive dose is used, it will inhibit the shift of balance.
Finally, temperature affects the kinetics of the reaction system. According to the Arrhenius equation, raising the temperature can exponentially increase the reaction rate constant. Experimental data shows that within the optimized range of 50-60℃, for every 10℃ increase in temperature, the reaction rate can increase by 2-3 times. Furthermore, from the perspective of reaction equilibrium, this temperature zone can ensure a thermodynamic equilibrium conversion rate of over 90% while avoiding the increase of side reactions caused by high temperatures.
5. Conclusion
This article systematically explores the innovative applications of green catalysts in fields such as organic synthesis, environmental remediation, and biofuel production. Studies have shown that enzyme catalysis significantly enhances chiral synthesis efficiency through precise three-dimensional control. The photocatalyst TiO2 achieves a high mineralization rate in pollutant degradation, while the solid base catalyst further breaks through the conversion rate of biodiesel. All these highlight the core value of green catalysts in enhancing atomic economy, reducing energy consumption and minimizing waste.
However, this paper has limitations. For example, the literature coverage is not comprehensive, and the mechanism analysis is mostly based on theoretical models.
The application of green catalysts in sustainable chemical reactions shows great potential but also faces many challenges. In enzyme-catalyzed organic synthesis, substrate selectivity, activity, and stability are critical issues. Enhancing the performance and reusability through protein engineering and immobilization techniques can help reduce costs and improve efficiency. In the photocatalytic reaction of TiO2, its large band gap limits visible light utilization, and the photogenerated carriers are prone to recombine. By doping and compounding semiconductors, the light absorption range of TiO2 can be expanded to the visible light region, thereby improving the photocatalytic efficiency. In biodiesel production, the high cost and low efficiency of enzyme catalysis are the main challenges. Screening for efficient enzyme types and optimizing production process can effectively reduce costs and enhance conversion efficiency, making the process more sustainable. Through continuous technological innovation, green catalysts are expected to play a more significant role in the chemical industry, promoting a greener and more sustainable direction.
References
[1]. Muller, R., Goesmann, H., & Waldmann, H. (1999). N, N-Phthaloylamino acids as chiral auxiliaries in asymmetric Mannich-type reactions. Angewandte Chemie International Edition, 38(1), 184-187.
[2]. Bohme, H., & Haake, M. (1976). Advances in organic chemistry. New York: Interscience.
[3]. Trost, B. M., & Terrell, L. R. (2003). A direct catalytic asymmetric Mannich-type reaction to syn-amino alcohols. Journal of the American Chemical Society, 125(2), 338-339.
[4]. Hu, J., Fu, J., Han, X., Xu, G., Wang, H., & Xiong, W. (2016). Advances in enzyme-catalyzed multifunctionality. Biochemical Engineering, (1), 59-64.
[5]. Li, K., He, T., Li, C., et al. (2009). Lipase-catalysed direct Mannich reaction in water: Utilization of biocatalytic promiscuity for C-C bond formation in a "one-pot" synthesis. Green Chemistry, 11(6), 777-779.
[6]. He, T., Li, K., Wu, M. Y., et al. (2010). Utilization of biocatalytic promiscuity for direct Mannich reaction. Journal of Molecular Catalysis B: Enzymatic, 67(s3-4): 189-194.
[7]. Chai, S. J., Lai, Y. F., Zheng, H., et al. (2010). A novel trypsin-catalyzed three-component Mannich reaction. Helvetica Chimica Acta, 93(11), 2231-2236.
[8]. Liu, Z. P. (2014). Enzyme multifunctionality in catalyzing organic synthesis reactions in organic solvents. Chemical Engineering Management, (2), 138-138.
[9]. Chai, Y. D., Pang, Y. L., Lim, S., Chong, W. C., Lai, C. W., & Abdullah, A. Z. (2022). Recent progress on tailoring the biomass-derived cellulose hybrid composite photocatalysts. Polymers, 14(23), 5244.
[10]. Fujishima, A., & Honda, K. (1972). Electrochemical photolysis of water at a semiconductor electrode. Nature, 238(5358), 37-38.
[11]. Wang, Y., Wang, Q., Zhan, X., et al. (2013). Visible light driven type II heterostructures and their enhanced photocatalysis properties: A review. Nanoscale, 5(18), 8326-8339.
[12]. Zhang, Y. G. (2021). Preparation and performance study of TiO2-based photonic crystal color materials (Doctoral dissertation, Dalian University of Technology). Retrieved from https://link.cnki.net/doi/10.26991/d.cnki.gdllu.2021.003886
[13]. Yang, J. Y. (2023). Principles and influencing factors of TiO2 photocatalysis. Contemporary Chemical Research, (22), 15-17. doi:10.20087/j.cnki.1672-8114.2023.22.005
[14]. Qu, J. Y. (2021). Experimental study and system optimization of subcritical/supercritical heterogeneous catalytic preparation of biodiesel (Master's thesis, Xi'an University of Technology). Retrieved from https://link.cnki.net/doi/10.27398/d.cnki.gxalu.2021.001422
Cite this article
Ge,X. (2025). Research on the Application of Green Catalysts in Sustainable Chemical Reactions. Theoretical and Natural Science,102,41-47.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICEGEE 2025 Symposium: Impact of Technological Innovation on Energy Efficiency in European Countries
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Muller, R., Goesmann, H., & Waldmann, H. (1999). N, N-Phthaloylamino acids as chiral auxiliaries in asymmetric Mannich-type reactions. Angewandte Chemie International Edition, 38(1), 184-187.
[2]. Bohme, H., & Haake, M. (1976). Advances in organic chemistry. New York: Interscience.
[3]. Trost, B. M., & Terrell, L. R. (2003). A direct catalytic asymmetric Mannich-type reaction to syn-amino alcohols. Journal of the American Chemical Society, 125(2), 338-339.
[4]. Hu, J., Fu, J., Han, X., Xu, G., Wang, H., & Xiong, W. (2016). Advances in enzyme-catalyzed multifunctionality. Biochemical Engineering, (1), 59-64.
[5]. Li, K., He, T., Li, C., et al. (2009). Lipase-catalysed direct Mannich reaction in water: Utilization of biocatalytic promiscuity for C-C bond formation in a "one-pot" synthesis. Green Chemistry, 11(6), 777-779.
[6]. He, T., Li, K., Wu, M. Y., et al. (2010). Utilization of biocatalytic promiscuity for direct Mannich reaction. Journal of Molecular Catalysis B: Enzymatic, 67(s3-4): 189-194.
[7]. Chai, S. J., Lai, Y. F., Zheng, H., et al. (2010). A novel trypsin-catalyzed three-component Mannich reaction. Helvetica Chimica Acta, 93(11), 2231-2236.
[8]. Liu, Z. P. (2014). Enzyme multifunctionality in catalyzing organic synthesis reactions in organic solvents. Chemical Engineering Management, (2), 138-138.
[9]. Chai, Y. D., Pang, Y. L., Lim, S., Chong, W. C., Lai, C. W., & Abdullah, A. Z. (2022). Recent progress on tailoring the biomass-derived cellulose hybrid composite photocatalysts. Polymers, 14(23), 5244.
[10]. Fujishima, A., & Honda, K. (1972). Electrochemical photolysis of water at a semiconductor electrode. Nature, 238(5358), 37-38.
[11]. Wang, Y., Wang, Q., Zhan, X., et al. (2013). Visible light driven type II heterostructures and their enhanced photocatalysis properties: A review. Nanoscale, 5(18), 8326-8339.
[12]. Zhang, Y. G. (2021). Preparation and performance study of TiO2-based photonic crystal color materials (Doctoral dissertation, Dalian University of Technology). Retrieved from https://link.cnki.net/doi/10.26991/d.cnki.gdllu.2021.003886
[13]. Yang, J. Y. (2023). Principles and influencing factors of TiO2 photocatalysis. Contemporary Chemical Research, (22), 15-17. doi:10.20087/j.cnki.1672-8114.2023.22.005
[14]. Qu, J. Y. (2021). Experimental study and system optimization of subcritical/supercritical heterogeneous catalytic preparation of biodiesel (Master's thesis, Xi'an University of Technology). Retrieved from https://link.cnki.net/doi/10.27398/d.cnki.gxalu.2021.001422









