1. Introduction
Melanoma arises from the malignant transformation of melanocytes. This insidious disease not only threatens the integrity of the skin but also has the potential to metastasize, and ultimately lead to death, making early detection and intervention of paramount importance. According to data from the majority of countries, once a rare malignancy a century ago, melanoma has now evolved into a significant health concern around the world, with the average lifetime risk soaring to an alarming 1 in 50 among many Western populations [1].
One of the treatments for primary melanoma is wide excision, a surgical procedure meticulously designed to ensure the complete removal of the tumor. The extent of the surgical margins, which are crucial for minimizing the risk of recurrence, is precisely determined based on the thickness of the tumor [2]. For advanced melanoma, the treatment is relatively more complex and varied, mainly including surgery, immunotherapy, targeted therapy, radiotherapy and so on [2]. For patients with resectable locoregional metastases, systemic drug therapies are employed as a complementary measure to surgical intervention, enhancing the overall therapeutic efficacy [2]. However, traditional cancer treatments, including chemotherapy and surgery, show constrained applicability which is limited by significant side effects and the complex nature of advanced malignancies [3]. This reality underscores the pressing need to explore and develop more effective, targeted, and less invasive therapeutic strategies.
Due to the complex interactions between melanoma and the immune system, immunotherapy is emerging as a crucial therapeutic tool in the treatment of melanoma. About 30 to 40 percent of all subtypes of melanoma have a significant tumor mutational burden (TMB), which are often more responsive to immunotherapy.
With the continuous development of immunotherapy, the main immunotherapies that can be applied today include immune checkpoint inhibitors (PD-1/L1 and CTLA-4 inhibitors), adoptive cell transfer herapy, cytokine therapies and tumour vaccines, etc. Immunocheckpoint inhibitors, such anti-programmed cell death protein-1 monoclonal antibodies, are the most widely used immunotherapies for melanoma. Despite the promising efficacy of anti-PD-1 therapy, a significant proportion of patients ultimately develop resistance to these immunotherapeutic agents. Notably, the predominant melanoma subtypes in Asian populations—acral lentiginous and mucosal melanomas—exhibit distinct biological characteristics associated with heightened aggressiveness and demonstrate substantially reduced responsiveness to anti-PD-1 treatment regimens [4].
One of the emerging immunotherapies, oncolytic virotherapy, is gradually developing and making new advances, which may solve the problem. Therapeutically beneficial anticancer viruses known as oncolytic viruses (OV) will specifically infect and kill malignant tissues while sparing healthy tissues. In addition to evading immune recognition or elimination, the majority of tumors have developed the ability to withstand apoptosis and translational repression, two important defense mechanisms that healthy cells employ to prevent viral infection. OV can take advantage of this to selectively target and lyse tumors. This certainly provides new ideas for cancer treatment. Simultaneously, OVs remodel the tumor microenvironment (TME) through the release of associated substances following tumor lysis. This remodeling activates antigen-presenting cells, enhances the infiltration of cytotoxic T lymphocytes, recruits other immune-related molecules, and amplifies tumor-specific immune responses. Consequently, this cascade of events leads to the effective clearance of both distant and uninfected tumor cells (Fig. 1). This transformation converts immunologically 'cold' tumors, which are non-responsive to immune checkpoint inhibitors (ICIs), into 'hot' tumors that are highly immunogenic. Consequently, this enhances the tumor's sensitivity to ICI therapy and offers a promising avenue to overcome resistance to PD-1 inhibitors.
There are numerous oncolytic virotherapy treatments that can be used to treat melanoma. like OH2, VSV-NDV, etc. However, oncolytic virotherapy still faces considerable challenges, such as variability in clinical response and individualised differences in patients.
In this review, we summarise the latest research advances in oncolytic virotherapy for the treatment of melanoma, with a focus on the effects of this therapy in combination with a variety of other therapies. Additionally, we go over the difficulties and evaluate the future of this new field critically. Our goals are to benefit melanoma patients' prognosis and shed light on the function of oncolytic virotherapy in melanoma treatment.
2. Research on melanoma in the last five years
On February 24, 2025, a systematic literature review was carried out by searching the PubMed database using the keywords "Oncolytic Virotherapy" and "Melanoma," filtering the results for clinical trials and randomized controlled trials from 2021 to 2025. 26 manuscripts were found using these search criteria and chosen for preliminary screening. It was discovered that 15 of these included first-hand accounts of clinical trial data employing an OV[4-18]. Reports of other diseases were the primary reason for the rejection of articles for further review, including four of breast neoplasm and one of soft-tissue sarcomas. Seven reports were studies of solid tumours, three of which provided comprehensive treatment and patient data specifically for melanoma cases, making them eligible for inclusion in the study. However, four reports was excluded as it only mentioned solid tumors without specifying the exact disease type, rendering them insufficient for detailed analysis. In addition, two reports were excluded due to the lack of complete full-text information.There are two papers that analyze the results of the same trial from different perspectives[15,16], so the data is only counted once during inclusion.
The 15 papers that reported the findings of clinical trials were chosen for additional examination, and each study was assessed for a number of factors. Clinical trial phase, number of patients treated, virus type, viral backbone, transgene expression, and use of single agent or combination regimens were among the parameters evaluated.
3. Clinical studying phase
We identified 15 independent clinical trials spanning various years, all reporting on oncolytic virus (OV) research for melanoma, including treatments for multiple melanoma patients. Among these trials, 9 studies (60%, N=164) were Phase I trials. This includes 3 Phase Ib studies (20%, N=67), such as one evaluating the efficacy of V937 combined with ipilimumab in uveal melanoma patients; 1 Phase Ia/Ib study (6.7%, N=42); and 5 full Phase I studies (33.3%, N=55). Additionally, there were 2 Phase I/II trials (13.3%, N=123), such as one examining the safety, tolerability, and efficacy of T-VEC combined with ipilimumab (T-VEC-ipilimumab) in treatment-naïve, unresectable Stage IIIB-IVM1c melanoma patients. There were also 3 Phase II trials (20%, N=547). Only 1 study (6.7%, N=346) was a Phase III trial. This distribution emphasizes the need for more research to maximize therapeutic approaches and enhance patient outcomes because a sizable amount of the material currently in publication still concentrates on early-phase clinical trials in the treatment of melanoma.
4. Types of lysosomal viruses
Among the 15 analyzed studies, a variety of DNA and RNA viruses were investigated as oncolytic viruses (OVs). The majority of the studies (10 studies, 66.7%) utilized DNA viruses, while a smaller portion (5 studies, 33.3%) employed RNA viruses (Figure 1). The most frequently used virus was herpes simplex virus type 1 (HSV-1, 9 studies, 60%), followed by coxsackievirus A21 (V937, 2 studies, 13.3%) and the rhinovirus-poliovirus chimera (PVSRIPO, 2 studies, 13.3%). Additionally, one study utilized herpes simplex virus type 2 (HSV-2, 6.7%), and another study employed vesicular stomatitis virus (VSV, 6.7%). Despite the potential of using multiple oncolytic viruses, none of the 18 clinical trials investigated the use of more than one type of oncolytic virus.
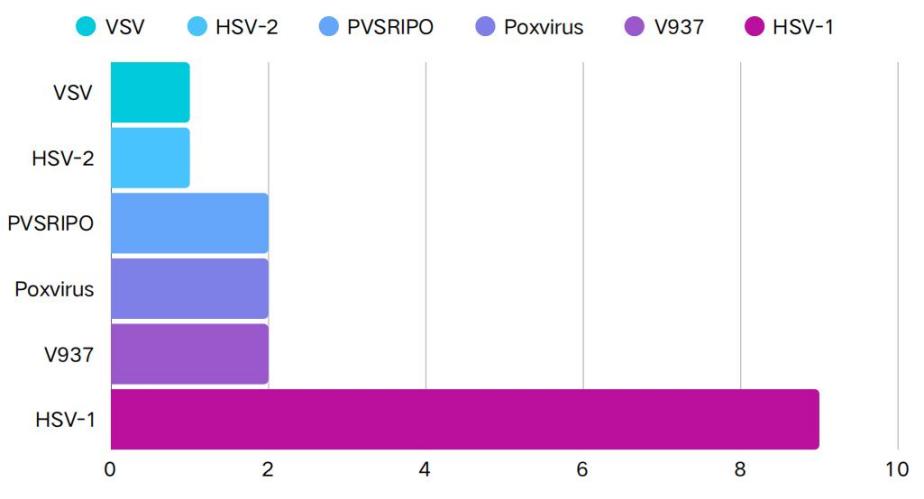
Figure 1: Types of lysosomal viruses
5. Native viruses and genetically modified viruses
Based on the analysis of these 15 studies, the oncolytic virus (OV) clinical trials associated with these investigations. Among the 15 clinical trials, 3 (20%) utilized naturally occurring viruses, while 12 studies (80%) employed genetically modified viruses. In order to allow the virus to replicate selectively in tumor cells and lessen its pathogenicity, these changes mostly involved the deletion of non-essential viral genes. In 4 of these clinical trials, genetic engineering was further extended to include the expression of multiple transgenes, with a total of 7 distinct recombinant genes being utilized.
6. Gene use in genetically modified viruses
The article presents the genes utilized in the 15 relevant studies. The most commonly utilized transgene among these was granulocyte-macrophage colony-stimulating factor (GM-CSF), which was intended to encourage the development of local dendritic cells in order to boost the host's immunological response (9 studies, 60%). The second most commonly used transgenes were ICP34.5 and ICP47 (2 studies, 13.3%). Deleting ICP34.5 and ICP47 genes reduces virulence and enhances oncolytic activity: the deletion of ICP34.5 allows the oncolytic virus to enhance viral replication in cancer cells, while the deletion of ICP47 enhances antigen presentation and T-cell priming, making tumor cells more recognizable to the immune system, thereby improving the efficacy of OrienX010. The ICP6 gene (1 study, 6.7%) is often used in conjunction with these. Inactivating ICP6 reduces the growth of oncolytic HSV-1 virus in neurons, thereby lowering the risk of neurotoxicity post-injection. Additionally, one study employed transgene technologies such as IFN-β, TYRP1, and IRES replacement, which has immunomodulatory effects in addition to suppressing angiogenesis, apoptosis, and cell proliferation(Figure 2).
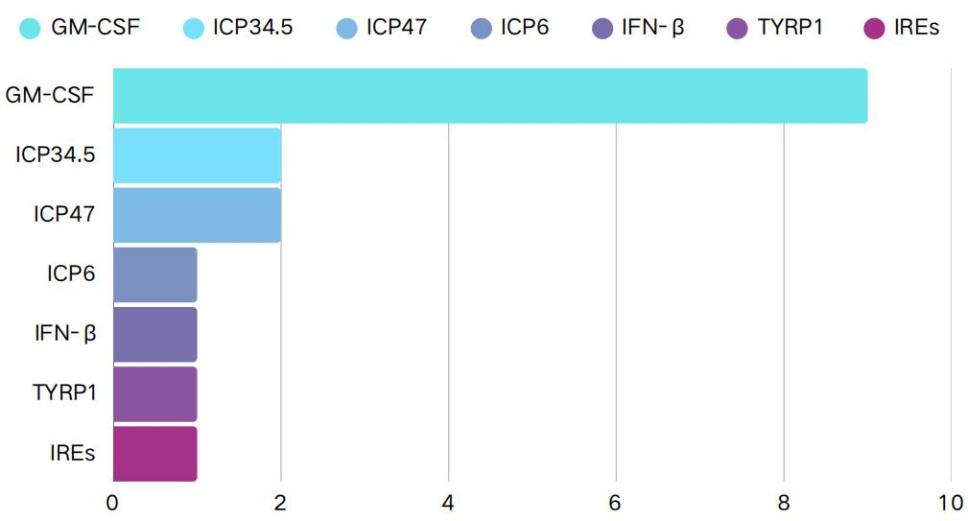
Figure 2: Type of genetic modification involved
7. Delivery route in oncolytic virus clinical trials
The route of administration for oncolytic viruses (OVs) has been a contentious topic, prompting us to identify the methods employed in reported clinical trials. The most common route is intratumoral (IT) injection, utilized in 12 clinical trials (80%). Other routes include intravenous injection, used in 1 study (6.7%). Additionally, 1 study did not report the method of OV administration. Although preclinical studies have described the use of stem cells or nanovesicles for delivering oncolytic viruses, no clinical trials have yet reported using these methods. When evaluating the routes based on patient numbers (Table 1), intratumoral injection remains the most prevalent (1,132 patients, 96.0%). In one study, 12 patients received OVs through multiple routes, most commonly a combination of intravenous and intratumoral injections (12 patients, 1.0%).
Table 1: Delivery route in oncolytic virus clinical trials
Characteristics | N |
Intratumoral | 1132 |
Intravenous | 11 |
Multiple | 12 |
Not mention | 25 |
Total | 1180 |
8. Whether or not to combine treatments
Overall, among the 15 clinical trials analyzed from the literature, 7 trials (46.7%) employed oncolytic virus (OV) monotherapy, while 8 trials (53.3%) reported the use of oncolytic viruses in conjunction with at least one anticancer medication or other therapeutic approach. Among these combination therapies, the most frequently co-administered agents were monoclonal antibodies (6 studies, 75%). Additionally, one study combined oncolytic viruses with autologous CD1c (BDCA-1)+ and/or CD141 (BDCA-3) myeloid dendritic cells. Other treatment modalities used in combination with oncolytic viruses included surgical intervention (2 studies, 25%), radiotherapy (1 study, 12.5%), and chemotherapy (1 study, 12.5%). Different combination therapies could also be used in conjunction with each other.
9. Analysis of results
Although the majority of clinical trials are in the early stages and not specifically designed to assess treatment responses, most studies did report clinical outcomes. Notably, 10 trials (66.7%) reported clinical responses using some version of the Response Evaluation Criteria in Solid Tumors (RECIST). This includes 4 trials (26.7%) using the standard RECIST, 1 study (6.7%) using modified RECIST, and 7 studies (46.7%) employing the immune-related RECIST (irRECIST). One clinical trial (6.7%) applied modified World Health Organization (WHO) criteria, while the remaining 4 studies (26.7%) did not report the use of specific evaluation standards. Since some trials used more than one evaluation criterion, the total count exceeds the actual number of trials.
288 patients (24.4%) had an overall objective response rate out of the 1,180 patients treated in the reviewed studies. This includes 101 patients (8.6%) who achieved a complete response and 187 patients (15.8%) who achieved a partial response.
Table 2 lists the clinical reactions linked to particular OV treatments. The most notable responses were observed in patients treated with HSV-1, with 253 patients achieving an objective response. 4 patients experienced objective responses in response to the PVSRIPO virus, whereas 8 patients experienced objective responses in response to V937 therapy.
Table 2: Clinical reactions associated with specific oncolytic virus (OV) therapy
Characteristics | N |
HSV-1 | 253 |
- OrienX010(ori) | 16 |
- T-VEC | 237 |
PVSRIPO | 4 |
V937 | 8 |
Lastly, we looked into whether the method of administration affected how well patients responded to treatment. We discovered that patients who received intratumoral (IT) injections had better clinical responses, with 280 of them obtaining an objective response. On the other hand, patients receiving intravenous administration did not exhibit any objective responses. Furthermore, 8 patients exhibited objective responses. However, the precise administration route was not disclosed.
10. Conclusion
In the treatment of cancer, especially melanoma, oncolytic viruses (OVs) have shown great promise. This review systematically summarizes the clinical applications of OVs in melanoma therapy based on literature from 2021 to 2025. The findings reveal that herpes simplex virus type 1 (HSV-1) and V937 are the most commonly used OVs, with GM-CSF serving as the predominant transgene to enhance local immune responses by promoting dendritic cell maturation and cross-presentation of tumor antigens, thereby boosting host antitumor immunity. In melanoma treatment, OVs have shown considerable therapeutic success, particularly when paired with immune checkpoint inhibitors (ICIs) like PD-1/PD-L1 inhibitors. According to studies, OVs can cause tumor cells to undergo immunogenic cell death, while release antigens linked to the tumor and increase the effectiveness of ICIs. For instance, patients with advanced melanoma have demonstrated impressive therapeutic results when T-VEC is combined with either pembrolizumab (a PD-1 inhibitor) or ipilimumab (a CTLA-4 inhibitor), significantly improving objective response rates.
However, despite the promising potential of OVs in melanoma treatment, several challenges remain in their clinical development. First, the selection of viral species and genetic modification strategies requires further optimization. Despite the positive safety and effectiveness profiles that HSV-1 has shown in clinical trials, the adaptability of different viruses to various tumor types warrants deeper investigation. Second, the choice of administration routes is a critical consideration. While intratumoral injection allows for direct targeting of tumor tissues, its applicability to deep-seated or metastatic lesions is limited. Conversely, intravenous administration can address multiple lesions but faces obstacles such as rapid viral clearance in the bloodstream. Thus, the creation of novel delivery systems, like cellular carriers or nanocarriers, offers a promising direction for further study.
Moreover, oncolytic virotherapy not only exerts direct tumor cell lysis but also remodels the tumor microenvironment, producing "hot" tumors from immunologically "cold" ones, thereby enhancing the efficacy of immune checkpoint inhibitors and other immunotherapies. Recent clinical trials, including those involving OH2, have demonstrated durable antitumor responses, especially in patients who are receiving anti-PD-1 therapy and have made progress. The potential of OVs as a second-line treatment is highlighted by their capacity to overcome resistance to immune checkpoint inhibitors, particularly in difficult subtypes like acral melanoma. Furthermore, oncolytic virotherapy may have abscopal effects, focusing on both local and distant metastases, based on the systemic immune activation seen in preclinical and clinical studies.
Nevertheless, challenges remain, including variability in clinical responses, the potential for preexisting immunity to limit efficacy, and the need for optimized delivery methods. Combining OVs with other immunotherapies, like adoptive cell therapy and checkpoint inhibitors, presents a viable way to improve treatment results. Furthermore, the identification of biomarkers, such as neutrophil activation states, may help tailor treatments to patients most likely to benefit.
In conclusion, oncolytic virotherapy represents a multifaceted approach to melanoma treatment, combining direct oncolysis with immune stimulation. As research progresses, the development of next-generation OVs and their combination with other therapies will likely expand their role in the treatment of melanoma, offering hope for improved survival and quality of life for patients.
References
[1]. Huang J, Chan S C, Ko S, et al. Global Incidence, Mortality, Risk Factors and Trends of Melanoma: A Systematic Analysis of Registries. American Journal of Clinical Dermatology, 2023, 24(6): 965-975.
[2]. Long G V, Swetter S M, Menzies A M, et al. Cutaneous melanoma. Lancet, 2023, 402(10400): 485-502.
[3]. Mirzayans R, Murray D. What Are the Reasons for Continuing Failures in Cancer Therapy? Are Misleading/Inappropriate Preclinical Assays to Be Blamed? Might Some Modern Therapies Cause More Harm than Benefit? International Journal of Molecular Sciences, 2022, 23(21): 13217.
[4]. Wang X, Tian H, Chi Z, et al. Oncolytic virus OH2 extends survival in patients with PD-1 pretreated melanoma: phase Ia/Ib trial results and biomarker insights. Journal for ImmunoTherapy of Cancer, 2025, 13(2): e010662.
[5]. Smith K E R, Peng K W, Pulido J S, et al. A phase I oncolytic virus trial with vesicular stomatitis virus expressing human interferon beta and tyrosinase related protein 1 administered intratumorally and intravenously in uveal melanoma: safety, efficacy, and T cell responses. Frontiers in Immunology, 2023, 14: 1279387.
[6]. Liu J, Wang X, Li Z, et al. Neoadjuvant oncolytic virus orienx010 and toripalimab in resectable acral melanoma: a phase Ib trial. Signal Transduction and Targeted Therapy, 2024, 9(1): 318.
[7]. Chesney J A, Ribas A, Long G V, et al. Randomized, Double-Blind, Placebo-Controlled, Global Phase III Trial of Talimogene Laherparepvec Combined With Pembrolizumab for Advanced Melanoma. Journal of Clinical Oncology, 2023, 41(3): 528-540.
[8]. Cui C, Wang X, Lian B, et al. OrienX010, an oncolytic virus, in patients with unresectable stage IIIC-IV melanoma: a phase Ib study. Journal for ImmunoTherapy of Cancer, 2022, 10(4): e004307.
[9]. Ahamadi M, Kast J, Chen P W, et al. Oncolytic viral kinetics mechanistic modeling of Talimogene Laherparepvec (T-VEC) a first-in-class oncolytic viral therapy in patients with advanced melanoma. CPT: Pharmacometrics & Systems Pharmacology, 2023, 12(2): 250-260.
[10]. Lutzky J, Sullivan R J, Cohen J V, et al. Phase 1b study of intravenous coxsackievirus A21 (V937) and ipilimumab for patients with metastatic uveal melanoma. Journal of Cancer Research and Clinical Oncology, 2023, 149(9): 6059-6066.
[11]. Chesney J A, Puzanov I, Collichio F A, et al. Talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone for advanced melanoma: 5-year final analysis of a multicenter, randomized, open-label, phase II trial. Journal for ImmunoTherapy of Cancer, 2023, 11(5): e006270.
[12]. Schwarze J K, Tijtgat J, Awada G, et al. Intratumoral administration of CD1c (BDCA-1)+ and CD141 (BDCA-3)+ myeloid dendritic cells in combination with talimogene laherparepvec in immune checkpoint blockade refractory advanced melanoma patients: a phase I clinical trial. Journal for ImmunoTherapy of Cancer, 2022, 10(9): e005141.
[13]. Yamazaki N, Isei T, Kiyohara Y, et al. A phase I study of the safety and efficacy of talimogene laherparepvec in Japanese patients with advanced melanoma. Cancer Science, 2022, 113(8): 2798-2806.
[14]. Dummer R, Gyorki D E, Hyngstrom J, et al. Neoadjuvant talimogene laherparepvec plus surgery versus surgery alone for resectable stage IIIB-IVM1a melanoma: a randomized, open-label, phase 2 trial. Nature Medicine, 2021, 27(10): 1789-1796.
[15]. Beasley G M, Nair S K, Farrow N E, et al. Phase I trial of intratumoral PVSRIPO in patients with unresectable, treatment-refractory melanoma. Journal for ImmunoTherapy of Cancer, 2021, 9(4): e002203.
[16]. Beasley G M, Brown M C, Farrow N E, et al. Multimodality analysis confers a prognostic benefit of a T-cell infiltrated tumor microenvironment and peripheral immune status in patients with melanoma. Journal for ImmunoTherapy of Cancer, 2022, 10(9): e005052.
[17]. Dummer R, Robert C, Scolyer R A, et al. Neoadjuvant anti-PD-1 alone or in combination with anti-TIGIT or an oncolytic virus in resectable stage IIIB-D melanoma: a phase 1/2 trial. Nature Medicine, 2025, 31(1): 144-151.
[18]. Malvehy J, Samoylenko I, Schadendorf D, et al. Talimogene laherparepvec upregulates immune-cell populations in non-injected lesions: findings from a phase II, multicenter, open-label study in patients with stage IIIB-IVM1c melanoma. Journal for ImmunoTherapy of Cancer, 2021, 9(3): e001621.
Cite this article
Liu,S. (2025). Oncolytic Virotherapy's Current and Prospective Applications in the Management of Melanoma. Theoretical and Natural Science,113,1-8.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: AI for Healthcare: Advanced Medical Data Analytics and Smart Rehabilitation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Huang J, Chan S C, Ko S, et al. Global Incidence, Mortality, Risk Factors and Trends of Melanoma: A Systematic Analysis of Registries. American Journal of Clinical Dermatology, 2023, 24(6): 965-975.
[2]. Long G V, Swetter S M, Menzies A M, et al. Cutaneous melanoma. Lancet, 2023, 402(10400): 485-502.
[3]. Mirzayans R, Murray D. What Are the Reasons for Continuing Failures in Cancer Therapy? Are Misleading/Inappropriate Preclinical Assays to Be Blamed? Might Some Modern Therapies Cause More Harm than Benefit? International Journal of Molecular Sciences, 2022, 23(21): 13217.
[4]. Wang X, Tian H, Chi Z, et al. Oncolytic virus OH2 extends survival in patients with PD-1 pretreated melanoma: phase Ia/Ib trial results and biomarker insights. Journal for ImmunoTherapy of Cancer, 2025, 13(2): e010662.
[5]. Smith K E R, Peng K W, Pulido J S, et al. A phase I oncolytic virus trial with vesicular stomatitis virus expressing human interferon beta and tyrosinase related protein 1 administered intratumorally and intravenously in uveal melanoma: safety, efficacy, and T cell responses. Frontiers in Immunology, 2023, 14: 1279387.
[6]. Liu J, Wang X, Li Z, et al. Neoadjuvant oncolytic virus orienx010 and toripalimab in resectable acral melanoma: a phase Ib trial. Signal Transduction and Targeted Therapy, 2024, 9(1): 318.
[7]. Chesney J A, Ribas A, Long G V, et al. Randomized, Double-Blind, Placebo-Controlled, Global Phase III Trial of Talimogene Laherparepvec Combined With Pembrolizumab for Advanced Melanoma. Journal of Clinical Oncology, 2023, 41(3): 528-540.
[8]. Cui C, Wang X, Lian B, et al. OrienX010, an oncolytic virus, in patients with unresectable stage IIIC-IV melanoma: a phase Ib study. Journal for ImmunoTherapy of Cancer, 2022, 10(4): e004307.
[9]. Ahamadi M, Kast J, Chen P W, et al. Oncolytic viral kinetics mechanistic modeling of Talimogene Laherparepvec (T-VEC) a first-in-class oncolytic viral therapy in patients with advanced melanoma. CPT: Pharmacometrics & Systems Pharmacology, 2023, 12(2): 250-260.
[10]. Lutzky J, Sullivan R J, Cohen J V, et al. Phase 1b study of intravenous coxsackievirus A21 (V937) and ipilimumab for patients with metastatic uveal melanoma. Journal of Cancer Research and Clinical Oncology, 2023, 149(9): 6059-6066.
[11]. Chesney J A, Puzanov I, Collichio F A, et al. Talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone for advanced melanoma: 5-year final analysis of a multicenter, randomized, open-label, phase II trial. Journal for ImmunoTherapy of Cancer, 2023, 11(5): e006270.
[12]. Schwarze J K, Tijtgat J, Awada G, et al. Intratumoral administration of CD1c (BDCA-1)+ and CD141 (BDCA-3)+ myeloid dendritic cells in combination with talimogene laherparepvec in immune checkpoint blockade refractory advanced melanoma patients: a phase I clinical trial. Journal for ImmunoTherapy of Cancer, 2022, 10(9): e005141.
[13]. Yamazaki N, Isei T, Kiyohara Y, et al. A phase I study of the safety and efficacy of talimogene laherparepvec in Japanese patients with advanced melanoma. Cancer Science, 2022, 113(8): 2798-2806.
[14]. Dummer R, Gyorki D E, Hyngstrom J, et al. Neoadjuvant talimogene laherparepvec plus surgery versus surgery alone for resectable stage IIIB-IVM1a melanoma: a randomized, open-label, phase 2 trial. Nature Medicine, 2021, 27(10): 1789-1796.
[15]. Beasley G M, Nair S K, Farrow N E, et al. Phase I trial of intratumoral PVSRIPO in patients with unresectable, treatment-refractory melanoma. Journal for ImmunoTherapy of Cancer, 2021, 9(4): e002203.
[16]. Beasley G M, Brown M C, Farrow N E, et al. Multimodality analysis confers a prognostic benefit of a T-cell infiltrated tumor microenvironment and peripheral immune status in patients with melanoma. Journal for ImmunoTherapy of Cancer, 2022, 10(9): e005052.
[17]. Dummer R, Robert C, Scolyer R A, et al. Neoadjuvant anti-PD-1 alone or in combination with anti-TIGIT or an oncolytic virus in resectable stage IIIB-D melanoma: a phase 1/2 trial. Nature Medicine, 2025, 31(1): 144-151.
[18]. Malvehy J, Samoylenko I, Schadendorf D, et al. Talimogene laherparepvec upregulates immune-cell populations in non-injected lesions: findings from a phase II, multicenter, open-label study in patients with stage IIIB-IVM1c melanoma. Journal for ImmunoTherapy of Cancer, 2021, 9(3): e001621.









