1. Introduction
In March 2024, the United Nations Environment Programme (UNEP) released the 2024 Food Waste Index Report ("The Report"). The Report indicates that in 2022, a total of 1.05 billion tons of food waste was generated globally, equivalent to an average of 132 kg of food wasted per person, accounting for approximately 20% of the per capita available food. Greenhouse gas emissions from food loss and waste contribute 8% to 10% of the global total greenhouse gas emissions, nearly five times that of the aviation industry. Additionally, food waste occupies one-third of the world's agricultural land and consumes 60.5% of global water resources. Every year, food loss and waste lead to an economic loss of 1 trillion USD globally [1]. Food waste, added to the environmental costs of food production, such as extensive land and water use for crop cultivation and livestock farming, increases greenhouse gas emissions, accelerating global climate change further. The major causes of food waste include overproduction, an imperfect transportation chain, and spoilage of food items. Among these, spoilage caused by microorganisms mainly resulted in global food waste.
Different types of food are susceptible to different spoilage microorganisms. For instance, protein-rich foods (such as meat and dairy products) are prone to contamination by Lactobacillus and Staphylococcus species. In contrast, Penicillium and Aspergillus species often spoil fiber-rich foods (such as vegetables and fruits). Prevention of microbial contamination plays a crucial role in minimizing the economic loss of food due to spoilage. The primary concern with fungal spoilage is the production of mycotoxins, secondary metabolites produced by fungi, which can be fatal and pose severe health threats to both humans and livestock. Therefore, the safe and efficient prevention of fungal growth has become one of the critical areas of research in the food industry. Currently, most food industry uses chemical preservatives to inhibit mold growth. This paper will systematically review two essential preservatives: Natamycin and potassium sorbate. These two preservatives are widely used in the food industry, particularly in preserving dairy products and fruits/vegetables. We will introduce their chemical structures, mechanisms of action, and current research to improve their production or preservation efficiency.
2. Natamycin
Natamycin is a polyene macrolide antimycotic agent primarily produced by submerged fermentation of Streptomyces natalensis and Streptomyces chattanogenesis [2]. Its main structure consists of a macrolide ring connected to multiple hydroxyl groups, as shown in Figure 1. As a result, Natamycin is categorized as a macrolide antibiotic, consisting of a mycosamine moiety (3-amino-3,6-dideoxy-D-mannose) linked by an ether bond [3]. Due to four conjugated double bonds, Natamycin can also be classified as a tetraene antibiotic. A carboxyl group on one end of Natamycin's macrolide ring ensures the molecule's acidic properties. Additionally, due to the mycosamine group in its structure, Natamycin is considered amphoteric [4].
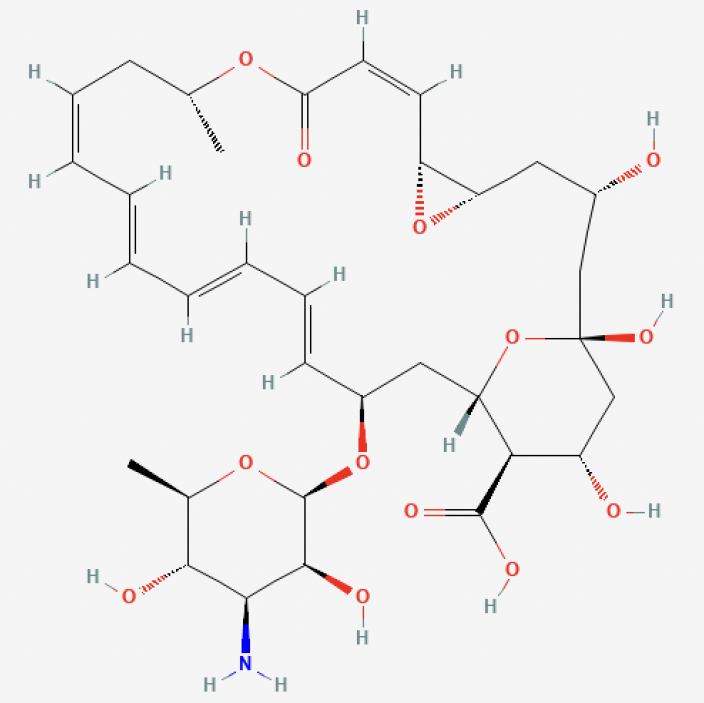
Figure 1: The chemical structure of Natamycin
Natamycin has a molecular weight of 665.75 g/mol and appears as a white powder. The empirical formula of Natamycin is C33H47NO13 and its isoelectric pH is 6.5[5]. Natamycin is insoluble in water and soluble in organic solvents. Because it has low water solubility, Natamycin is usually applied to the surfaces of food for the best preservative effects instead of being embedded in the food itself. Natamycin will show its maximum solubility at 30°C, 1 atm pressure in a 75% aqueous methanol solution with a pH of 2.0[6]. The parameters affecting the stability of Natamycin are the pH, solvent type, temperature, and the exposure to the UV and infrared light. Natamycin was first discovered by Struyk in 1955 at the Gistbrocades research laboratories and later officially published in Antibiotics Annual [7]. It was isolated from S. natalensis, an organism encountered in South African soil and was named Pimaricin [8]. Later the World Health Organization (WHO) renamed it Natamycin based on the name of the organism from which it was isolated. Natamycin is employed in the food industry over 30 years as a preservative and is one of the effective inhibitors of fungal growth [2, 9].
Nowadays, yeast and molds are the two major food spoilage microorganisms. Weak organic acids, azole derivative, fluorocytosine, allylamines and polyenes [10,11] are some of the agents applied in the food industry to inhibit the growth of fungi. However, their use has not yet been widespread in the food industry because they are synthetic and can serve as a source of resistance. The mechanism of Natamycin on fungi killing is mainly related to ergosterol which is found in cell membranes of yeast and molds (Figure 2). Ergosterol performs the function of structural support in cell membranes, which is equivalent to that of sterols in eukaryotic cell membranes It also acts as Natamycin binding sites. Natamycin complexes with ergosterol to form a high-affinity, irreversible complex that is a polyene-sterol complex in the cell membrane. This complex changes the fluidity of the membrane, leading to the cell losing essential ions and peptides thus killing the yeast and molds.
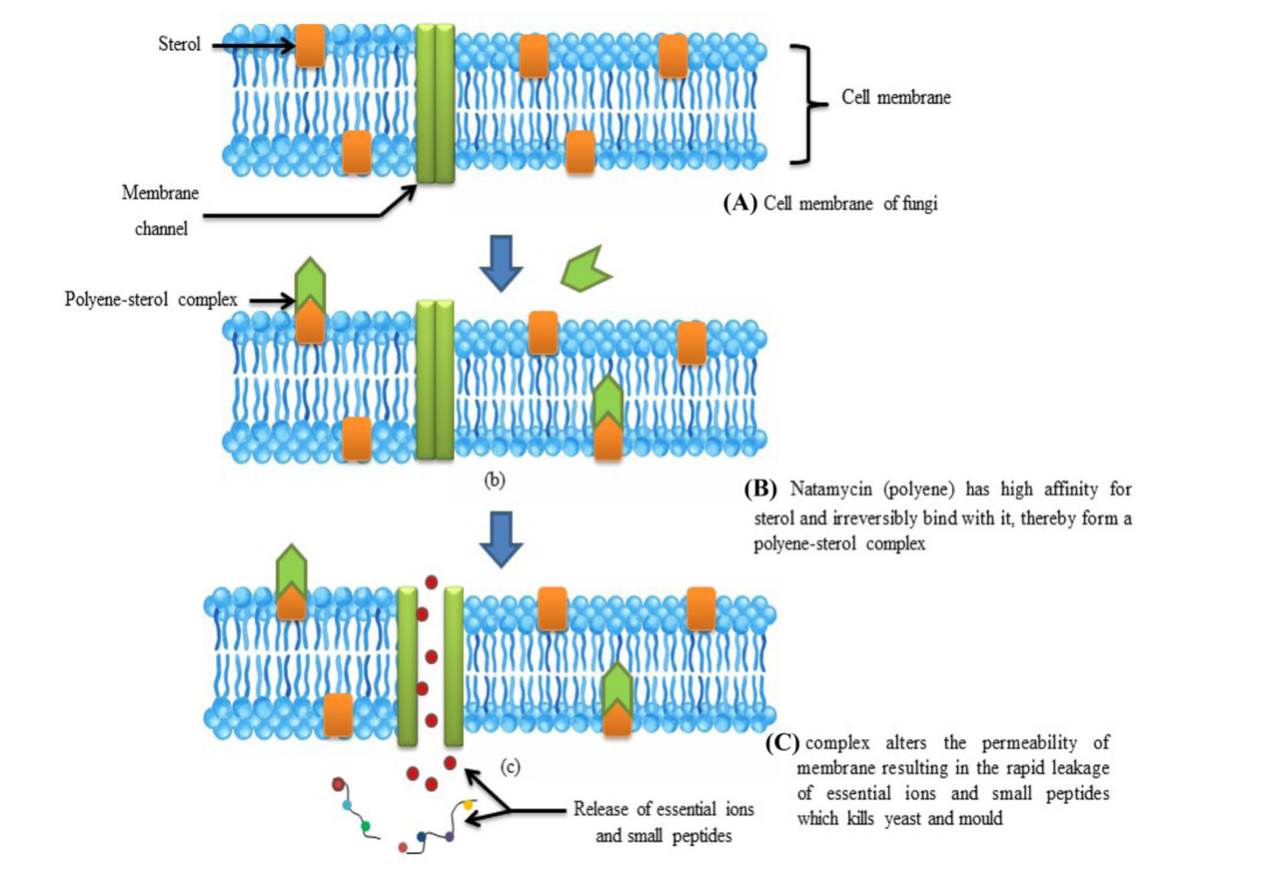
Figure 2: Mode of action of Natamycin on fungal cells [12]: (A). Shows the fungal cell membrane structure (B). Natamycin has a strong affinity for sterol in the fungal membrane, forming a polyene-sterol complex. (C). The formation of the polyene-sterol complex increases the membrane's permeability, leading to the rapid leakage of essential ions and small peptides from the cell
The intracellular redox state can regulate natamycin production. Regulating hydrogen peroxide (H₂O₂) synthesis could be a solution to improve natamycin production. The cholesterol oxidase SgnE catalyzes the oxidation of the 3β-hydroxyl group of sterols (cholesterol, ergosterol, and phytosterol, et al.) in the natamycin-producing strain Streptomyces gilvosporeus to yield Δ4-en-3-ketosteroids and H₂O₂. The H₂O₂ functions as a second messenger that activates a cascade of redox reactions. OxyR is a redox-sensing transcription factor that is activated through the accumulation of H₂O₂ inside the cell. When the level of H₂O₂ rises, OxyR will undergo a conformational change. More specifically, two essential cysteine residues (Cys212 and Cys221) of OxyR are oxidized to initiate a disulfide bridge, thereby switching OxyR from its reduced form to its oxidized form. After oxidation, OxyR binds to the PsgnM promoter and increases expression of the sgnM gene. SgnM is a cluster-situated regulator that regulates the expression of up to 12 genes in the natamycin biosynthetic gene cluster. Among these genes are polyketide synthase and transporter protein genes involved in natamycin biosynthesis. As a result, when OxyR is activated by the accumulation of H₂O₂, it turns on the transcription of a number of genes in the natamycin biosynthesis pathway, thus promoting the production of natamycin [13].
However, this method faces challenges during the implementation phase. First, precise control of H₂O₂ concentration is required. H₂O₂ is a strong oxidant, and high levels of this reactant can contribute to oxidative stress in cells, which may induce cellular damage or even death. Thus, realizing high-precision control over H₂O₂ concentration is essential. Setting a feedback regulatory mechanism can control the range of H₂O₂ concentration when OxyR is activated. This feedback loop will ensure no toxic effect on the cell caused by excessive H2O2. The second challenge is the effective screening of SgnE expression. Since SgnE is involved in producing H₂O₂ throughout the pathway, H₂O₂ will be produced less effectively if SgnE expression is too low or its activity is too weak to facilitate the reaction. This can be achieved with enzyme engineering of SgnE that maximizes SgnE expression, such as promoter selection or fermentation conditions to maximize SgnE activity. The third challenge is the maintenance of redox homeostasis of the cell. Redox molecules in the cells are not limited to H₂O₂ and include superoxide and nitric oxide, which keep the cellular redox balance in equilibrium. It remains to be determined if the redox state of OxyR can stably control sgnM and any other related genes without disrupting other redox pathways, and this will be an essential area of research going forward. One practical option is to supplement with additional antioxidants or alter components of the culture from media, keeping the redox balance during growth to limit the amount of ROS that could interfere with cell function.
Natamycin is essential for dairy food preservation. Dairy products are especially prone to spoilage by fungi abundantly present in nature, such as Debaryomyces hansenii, Kluyveromyces lactis, and Yarrowia lipolytica, due to their high protein content, high water activity, and moderate pH [14]. Improper food packaging and machine contamination during portioning also leave dairy products vulnerable to mold and yeast contamination. Natamycin has served as a vital preservative for many years in dairy products like cheese. In a study of mold growth in Egyptian fresh soft cheese by Ombarak and Shelaby [15], 20 ppm of natamycin achieved the best inhibition and extended the cheese's shelf life by four weeks. Similarly, Sara et al. established that the shelf life of plain yogurt with 10 ppm natamycin had an approximate 40-day shelf-life increase [16].
3. Potassium sorbate
Potassium sorbate (E 202), the potassium salt of sorbic acid, commonly comprises unsaturated fatty acids [17]. The chemical structure is shown in Figure 3. Moreover, it is an antimicrobial chemical compound of sorbic acid and potassium chloride [17], which protects food from spoilage and prolongs the food shelf life. To be specific, inhibiting the reproduction of molds and yeats by changing the cell membrane morphology [18], integrity and binding with sulfhydryl groups of microbial enzyme systems and then destroying the enzyme systems and disrupting the transportation and metabolic activity, which is different with the Natamycin that we mentioned before.
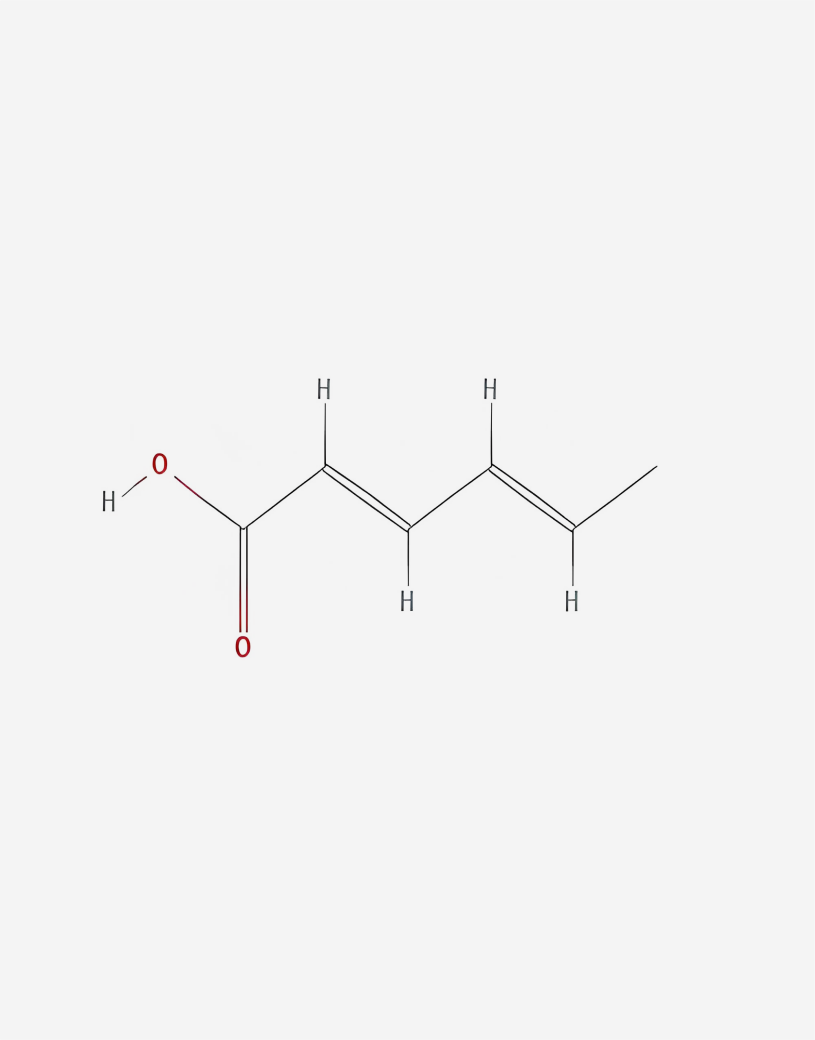
Figure 3: The chemical structure of potassium sorbate
Potassium sorbate appears as a white powder or granule; its molar mass is 150.22 g/mol. Besides, its empirical formula is C6H7KO2. It has high water solubility, especially showing significant preservative effects in acidic conditions. Furthermore, potassium sorbate acts more efficiently in certain circumstances, such as in an acidic environment with a pH ideally between 4.0 and 6.0 [19]. The undissociated form of potassium sorbate can cross the cell membranes of microorganisms more easily in these conditions, thereby preventing their growth. Concentration is also necessary for potassium sorbate to be effective. It is typically used in food products at concentrations of 0.025% to 0.1% (by weight). In addition, lower temperatures also improve the efficacy of potassium sorbate, with some microorganisms having a slower growth at lower temperatures making them more amenable to preservatives. In addition, reduced temperatures can increase the efficacy of potassium sorbate: certain microorganisms grow less rapidly (and thus slower) at colder temperatures, increasing their susceptibility to preservatives. Moreover, certain food components, such as high fat or protein levels, may interfere with potassium sorbate solubility or preservation capacity to increase its efficiency.
In 1859, Potassium sorbate was first extracted from the fruit of rowan. Even though the preservative property of sorbic acid was discovered very early, it was not used in the food preservation industry until the mid-20th century. In the 1940s, with the continuing advances in microbiology and chemistry, the positive role potassium sorbate can have in food preservation became apparent. Due to its antimicrobial properties, sorbic acid is viewed as a successful natural preservative against molds, yeasts, and some bacteria. But sorbic acid has a low solubility, which restricts its application in certain food products. To solve this problem, scientists have modified potassium sorbate to retain the effectiveness of sorbic acid while also having greater solubility than sorbic acid, making it easier to distribute during food processing.
Mold and yeast are the main culprits that cause food spoilage. In addition, preservatives such as benzoates and nitrates are very effective in inhibiting the growth of yeast and mold. There are also agents that target cell membranes, increasing their permeability and causing microbial death. Others work by inhibiting essential enzymes involved in cell metabolism, effectively halting their growth and reproduction. Sorbic acid is a widely used preservative in various food products due to its ability to control yeast and mold spoilage. It is considered safe, with low toxicity, and research shows it is metabolized like other fatty acids into carbon dioxide and water. Potassium sorbate, a derivative, dissolves easily in water at room temperature and breaks down into sorbic acid, its active antimicrobial form. Sorbic acid acidifies food substrates, creating an environment that inhibits the growth and reproduction of harmful bacteria such as E. coli and Salmonella.
Sorbic acid is also known as an unsaturated six-carbon fatty acid, 2,4-hexadienoic acid, as shown in Figure 4. This compound is similar to those found in some edible fats and oils. Both sorbic acid and its salt form, potassium sorbate, have a broad spectrum of activity, mostly against yeast and mold. It is primarily used when bottling to prevent refermentation by fermentative strains of Saccharomyces cerevisiae in sweetened wine. Wine's pH generally falls in the 3.0 – 4.0 range, an ideal range for sorbic acid action. Additionally, at 20 °C the solubility of sorbic acid in water is a maximum of 0.15% by weight and potassium sorbate has a much greater solubility of 58.2%. This salt is a white crystalline powder which needs to be stored away from moisture, heat, and light at maximum temperatures of 38 °C.
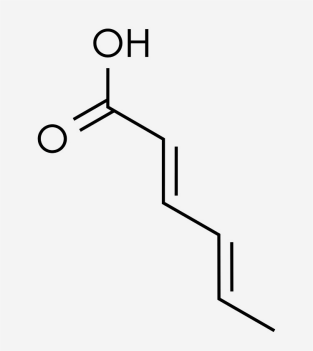
Figure 4: The chemical structure of sorbic acid [20]
The serious aspect that people need to be concerned about is toxicity; according to the FDA, it is safe for people to eat and consume (U.S. Food & Drug Administration). Owing to it not accumulating in the body, it breaks down into carbon dioxide and then water in the body. (Encyclopedia of Food Sciences and Nutrition). Due to its low toxicity and high- safety, potassium sorbate is often used to treat wines because sorbic acid has lower water- solubility. Potassium sorbate is functional in any water-based food item or baked good and is added directly to any "wet" ingredient (eggs, dairy, water) to integrate within a recipe. It is stable and retains its preservative action at high temperatures (above 390˚F), making it suitable for stovetop and oven use. The proper concentration of potassium sorbate depends on different factors, including the initial level of the contamination before preservation and the hygiene of your working environment. To determine if the dilution is suitable, the best practice is to begin at the low end with approximately 0.1% total weight and conduct a shelf stability test at room temperature [21].
It can be seen that potassium sorbate plays a very important role in food preservation. We've come up with a lot of ways to increase the yield and quickly produce potassium sorbate. We believe that one of the simplest and most efficient methods, suitable for large-scale production, is the neutralization method using sorbic acid as a raw material. First, raw materials such as sorbic acid, potassium carbonate or potassium hydroxide, and industrial ethanol are prepared. A certain amount of sorbic acid is first added to a jacketed reactor, then ionized water is added and stirred at a specific temperature. However, in order to produce potassium sorbate, a neutralization reaction needs to be initiated by adding potassium carbonate or potassium hydroxide. Upon completion of the reaction, the final product is obtained through steps such as filtration, washing and drying. The entire production process consists of several stages, including raw material preparation, the reaction itself and post-treatment procedures. Raw material preparation is a critical stage that requires strict control to maintain the quality and purity of the material. Prior to production, however, experimenters must carefully manage parameters such as temperature, pressure, and other experimental conditions to ensure that the reaction runs smoothly. Post-production processing steps such as filtration, washing and drying are equally important to ensure that the finished product meets the required quality standards.
4. Conclusion
This paper summarizes the chemical and physical properties of two chemicals, natamycin and potassium sorbate, and the important role they play in food preservation. We analyze the mechanisms and principles of how they prevent the growth of yeasts or molds. The main principle of both preventing the growth of microorganisms is to change the structure of the cell membrane of microorganisms so that the target dies due to the lack of essential ions and peptides. Also, based on these principles, we analyzed feasible ways to increase yield. For natamycin, we believe that a feasible way to increase the yield is to increase the expression of OxyR to obtain more H2O2 , which activates the natamycin biosynthetic pathway more efficiently. For potassium sorbate, we believe that a neutralization method using sorbic acid as a feedstock could increase its productivity. We also analyzed potential problems that may be encountered with both methods, which need more attention in future applications. Also, with the development of food science, we believe it is important to understand the chemicals that can prevent food spoilage. Both natamycin and potassium sorbate, as we examined above, will play important roles in the world's food industrial system.
References
[1]. Emissions Gap Report 2024. (n.d.). UNEP - UN Environment Programme. https://www.unep.org/resources/emissions-gap-report-2024
[2]. Davidson PM, Doan C. Natamycin. In Antimicrobials in Food (pp 339–356). CRC Press (2020)
[3]. Natamycin (Pimaricin). Its properties and possibilities in medicine. Von Wolfgang P. Raab (Wien). (2009). Mycoses, 17(1), 21. https://doi.org/10.1111/j.1439-0507.1974.tb04240.x
[4]. Bolard, J. (1986). How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochimica Et Biophysica Acta (BBA) - Reviews on Biomembranes, 864(3–4), 257–304. https://doi.org/10.1016/0304-4157(86)90002-x
[5]. Brik H. Natamycin. In Analytical profile of drug resistance. Academic Press (1981)
[6]. Zhang X, Chi YL, Miao T, Jia DY, Yao K. Antibacterial effects of different preservatives on the major spoilage microbes in traditional fermented ham. China Condiment. 01 (2013)
[7]. Struyk AP, Drost G, Haisvisz JM, Van Eek T, Hoogerheide JC. Pimaricin, a new antifungal antibiotic. 878–885 (1958)
[8]. Stark J. Natamycin: an effective fungicide for food and beverages. Woodhead Publishing Ltd (2003)
[9]. He, C., Zhang, Z., Li, B., Xu, Y., & Tian, S. (2019). Effect of Natamycin on Botrytis cinerea and Penicillium expansum—Postharvest pathogens of grape berries and jujube fruit. Postharvest Biology and Technology, 151, 134–141. https://doi.org/10.1016/j.postharvbio.2019.02.009
[10]. Brul, S., & Coote, P. (1999). Preservative agents in foods Mode of action and microbial resistance mechanisms. International Journal of Food Microbiology, 50(1–2), 1–17. https://doi.org/10.1016/s0168-1605(99)00072-0
[11]. Ghannoum MA, Rice LB. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clinical Microbiology Reviews. 12: 501–517 (1999)
[12]. Meena, M., Prajapati, P., Ravichandran, C., & Sehrawat, R. (2021). Natamycin: a natural preservative for food applications—a review. Food Science and Biotechnology, 30(12), 1481–1496. https://doi.org/10.1007/s10068-021-00981-1
[13]. Zong, G., Cao, G., Fu, J., Zhang, P., Chen, X., Yan, W., Xin, L., Wang, Z., Xu, Y., & Zhang, R. (2023). Novel mechanism of hydrogen peroxide for promoting efficient natamycin synthesis in Streptomyces. Microbiology Spectrum, 11(5). https://doi.org/10.1128/spectrum.00879-23
[14]. Delavenne, E., Ismail, R., Pawtowski, A., Mounier, J., Barbier, G., & Blay, G. L. (2012). Assessment of lactobacilli strains as yogurt bioprotective cultures. Food Control, 30(1), 206–213. https://doi.org/10.1016/j.foodcont.2012.06.043
[15]. Ombarak, R., & Shelaby, H. (2017). The inhibitory effect of Natamycin and Potassium Sorbate on mold growth in Egyptian Fresh soft cheese (Tallaga Cheese). Alexandria Journal of Veterinary Sciences, 1. https://doi.org/10.5455/ajvs.264557
[16]. Sara AE, Ekbal MA, Adham MA, Hamdi AM. The role of natamycin fortification to extend shelf life of plain yoghurt. Benha Veterinary Medical Journal. 27: 140–149 (2014)
[17]. Han, J. (2021, January 13). What is Potassium Sorbate (E202) in Food & Why Add it in wine? FOODADDITIVES. https://foodadditives.net/preservatives/potassium-sorbate/#easy-footnote-bottom-8-972
[18]. Handbook of Cosmeceutical Excipients and their Safeties. (2014). In Elsevier eBooks. https://doi.org/10.1016/c2013-0-18182-2
[19]. LINGYUE-FOODCHEM. (2024, March 1). The complete guide to potassium sorbate: Everything you need to know - LINGYUE-FOODCHEM. LINGYUE-FOODCHEM. https://lingyue-foodchem.com/potassium-sorbate/
[20]. Younes, M., Aquilina, G., Castle, L., Engel, K., Fowler, P., Fernandez, M. J. F., Fürst, P., Gürtler, R., Gundert‐Remy, U., Husøy, T., Mennes, W., Moldeus, P., Oskarsson, A., Shah, R., Wölfle, D., Lambré, C., Christodoulidou, A., & Waalkens‐Berendsen, I. (2019). Opinion on the follow‐up of the re‐evaluation of sorbic acid (E200) and potassium sorbate (E202) as food additives. EFSA Journal, 17(3). https://doi.org/10.2903/j.efsa.2019.5625
[21]. Whitney, C. (n.d.). Understanding potassium sorbate – Kitchen Alchemy. Kitchen Alchemy. https://blog.modernistpantry.com/advice/understanding-potassium-sorbate/#:~:text=When%20potassium%20sorbate%20mixes%20with%20water%2C%20it%20dissolves,water%29%20into%20sorbic%20acid%2C%20an%20active%20antimicrobial%20agent.
Cite this article
Li,Q.;Sun,Y. (2025). Addressing Global Food Waste with Antifungal Preservatives: Insights into Natamycin and Potassium Sorbate. Theoretical and Natural Science,116,88-95.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Emissions Gap Report 2024. (n.d.). UNEP - UN Environment Programme. https://www.unep.org/resources/emissions-gap-report-2024
[2]. Davidson PM, Doan C. Natamycin. In Antimicrobials in Food (pp 339–356). CRC Press (2020)
[3]. Natamycin (Pimaricin). Its properties and possibilities in medicine. Von Wolfgang P. Raab (Wien). (2009). Mycoses, 17(1), 21. https://doi.org/10.1111/j.1439-0507.1974.tb04240.x
[4]. Bolard, J. (1986). How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochimica Et Biophysica Acta (BBA) - Reviews on Biomembranes, 864(3–4), 257–304. https://doi.org/10.1016/0304-4157(86)90002-x
[5]. Brik H. Natamycin. In Analytical profile of drug resistance. Academic Press (1981)
[6]. Zhang X, Chi YL, Miao T, Jia DY, Yao K. Antibacterial effects of different preservatives on the major spoilage microbes in traditional fermented ham. China Condiment. 01 (2013)
[7]. Struyk AP, Drost G, Haisvisz JM, Van Eek T, Hoogerheide JC. Pimaricin, a new antifungal antibiotic. 878–885 (1958)
[8]. Stark J. Natamycin: an effective fungicide for food and beverages. Woodhead Publishing Ltd (2003)
[9]. He, C., Zhang, Z., Li, B., Xu, Y., & Tian, S. (2019). Effect of Natamycin on Botrytis cinerea and Penicillium expansum—Postharvest pathogens of grape berries and jujube fruit. Postharvest Biology and Technology, 151, 134–141. https://doi.org/10.1016/j.postharvbio.2019.02.009
[10]. Brul, S., & Coote, P. (1999). Preservative agents in foods Mode of action and microbial resistance mechanisms. International Journal of Food Microbiology, 50(1–2), 1–17. https://doi.org/10.1016/s0168-1605(99)00072-0
[11]. Ghannoum MA, Rice LB. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clinical Microbiology Reviews. 12: 501–517 (1999)
[12]. Meena, M., Prajapati, P., Ravichandran, C., & Sehrawat, R. (2021). Natamycin: a natural preservative for food applications—a review. Food Science and Biotechnology, 30(12), 1481–1496. https://doi.org/10.1007/s10068-021-00981-1
[13]. Zong, G., Cao, G., Fu, J., Zhang, P., Chen, X., Yan, W., Xin, L., Wang, Z., Xu, Y., & Zhang, R. (2023). Novel mechanism of hydrogen peroxide for promoting efficient natamycin synthesis in Streptomyces. Microbiology Spectrum, 11(5). https://doi.org/10.1128/spectrum.00879-23
[14]. Delavenne, E., Ismail, R., Pawtowski, A., Mounier, J., Barbier, G., & Blay, G. L. (2012). Assessment of lactobacilli strains as yogurt bioprotective cultures. Food Control, 30(1), 206–213. https://doi.org/10.1016/j.foodcont.2012.06.043
[15]. Ombarak, R., & Shelaby, H. (2017). The inhibitory effect of Natamycin and Potassium Sorbate on mold growth in Egyptian Fresh soft cheese (Tallaga Cheese). Alexandria Journal of Veterinary Sciences, 1. https://doi.org/10.5455/ajvs.264557
[16]. Sara AE, Ekbal MA, Adham MA, Hamdi AM. The role of natamycin fortification to extend shelf life of plain yoghurt. Benha Veterinary Medical Journal. 27: 140–149 (2014)
[17]. Han, J. (2021, January 13). What is Potassium Sorbate (E202) in Food & Why Add it in wine? FOODADDITIVES. https://foodadditives.net/preservatives/potassium-sorbate/#easy-footnote-bottom-8-972
[18]. Handbook of Cosmeceutical Excipients and their Safeties. (2014). In Elsevier eBooks. https://doi.org/10.1016/c2013-0-18182-2
[19]. LINGYUE-FOODCHEM. (2024, March 1). The complete guide to potassium sorbate: Everything you need to know - LINGYUE-FOODCHEM. LINGYUE-FOODCHEM. https://lingyue-foodchem.com/potassium-sorbate/
[20]. Younes, M., Aquilina, G., Castle, L., Engel, K., Fowler, P., Fernandez, M. J. F., Fürst, P., Gürtler, R., Gundert‐Remy, U., Husøy, T., Mennes, W., Moldeus, P., Oskarsson, A., Shah, R., Wölfle, D., Lambré, C., Christodoulidou, A., & Waalkens‐Berendsen, I. (2019). Opinion on the follow‐up of the re‐evaluation of sorbic acid (E200) and potassium sorbate (E202) as food additives. EFSA Journal, 17(3). https://doi.org/10.2903/j.efsa.2019.5625
[21]. Whitney, C. (n.d.). Understanding potassium sorbate – Kitchen Alchemy. Kitchen Alchemy. https://blog.modernistpantry.com/advice/understanding-potassium-sorbate/#:~:text=When%20potassium%20sorbate%20mixes%20with%20water%2C%20it%20dissolves,water%29%20into%20sorbic%20acid%2C%20an%20active%20antimicrobial%20agent.









