1. Introduction
1.1. Background of peptic ulcer
Peptic ulcer disease is a wound or lesion in the digestive system's lining caused by the direct contact of cells and stomach acid or digestive enzymes like pepsin [1]. A common misconception is that the disease is primarily caused by psychological stress or dietary irritants, which explains why it is more common in regions of the world that have habits of consuming spices and heavy workloads. However, modern medicine has implied that H. pylori is the main culprit instead [2]. Despite being a common health concern that can be cured under relatively simple treatments, peptic ulcers are often neglected by the public because many carriers of H. pylori are asymptomatic. The bacteria affects the population globally; even in developed countries like the United States, about 36% of the population have H. pylori in their digestive system [3], and approximately 5-10% of the adult population have peptic ulcers, while H. pylori is responsible for 80-90% of these cases [4]. Moreover, in less developed areas like Asia, the prevalence of H. pylori is estimated at 54.7% [5].
H. pylori is a gram-negative and spiral-shaped bacterium that can survive in acidic environments [6]. This explains why it can colonize the mucosa and spread via fecal-oral and oral-oral transmission. Its unique property increases the risk of exacerbating the infection chain in third-world or developing countries where improper hygiene practices and contaminated water sources are prevalent [7].
1.2. Infecting mechanism and impacts of H. pylori
Common routes that spread the bacteria are through eating from the same utensils or consuming water from unfiltered water sources; once H. pylori enters the human digestive system, the bacterium will inhabit within gastric epithelium cells, specifically the mucus-secreting foveolar cells that line the stomach. As stated by Dr. Doohan in his paper Helicobacter pylori BabA–SabA Key Roles in the Adherence Phase: The Synergic Mechanism for Successful Colonization and Disease Development [8]: “BabA is believed to be the most important protein in the early infection phase due to its ability to interact with various Lewis antigens, whereas SabA interaction with sialylated Lewis antigens may prove important for the adherence process in the inflamed gastric mucosal tissue in the ongoing-infection phase.” The bacterium attaches to the foveolar cells by utilizing outer membrane proteins such as blood-group antigen-binding adhesion (BabA) and sialic acid-binding adhesion (SabA). The linkage between the bacterium and the cell allows H. pylori to inject virulence factors directly into the host cell. One of the main virulence factors is the cytotoxin-associated gene A (AagA) protein transported by type IV secretion systems (T4SSs), large protein complexes that traverse the cell envelope of many bacteria to convey effectors to the host cell; the AagA cause cell malfunction by interfering with the cell's signal transduction pathways. The AagA can modify G proteins, which are the proteins that transmit signals from cell surface receptors to the inside of the cell. Receiving inappropriate signals from modified G protein can alter cell morphology, increase cell proliferation, and invoke an inflammatory response. Another virulence factor, the vacuolating cytotoxin A (VacA) protein, also from H. pylori, has a different effect on the host cell compared with AagA; it can cause the formation of large vacuoles in healthy cells, break the cellular homeostasis by creating an imbalance in ion concentrations.
The virulence proteins, AagA and VacA from H. pylori infection can trigger a prolonged immune system response by initiating the production of pro-inflammatory cytokines from the cell, such as interleukin-8 (IL-8), that command neutrophils and other body defense mechanisms to the site of infection [9]. However, by residing within gastric epithelial cells, H. pylori can avoid complete eradication from the human immune system. The lasting bacteria will keep replicating, therefore leading to persistent inflammation, which increases the chances of more severe health concerns, including gastric cancer and gastric atrophy [3].
The stomach lining is typically protected by a layer of mucus, known as gastric mucosa, that shields the gastric epithelial cells from gastric acid and digestive enzymes. However, continuous inflammation from H. pylori dissolves that thin layer of protection; the gastric acid and enzymes come into complete contact with the epithelial cells, killing the cells on contact and thus causing ulcers in the digestive tract [10].
1.3. Treatments of peptic ulcer induced by H. pylori
Though there are many treatments for peptic ulcers induced by H. pylori, almost all include antibiotic therapy. Amoxicillin and clarithromycin are the most popular and broadly utilized antibiotics for treating H. pylori-induced peptic ulcers. The two drugs are practical, relatively cheap, and accessible anywhere [11].
1.4. Amoxicillin treatment mechanisms
The first antibiotic to mention can be obtained easily in most countries is amoxicillin. This beta-lactam antibiotic suppresses bacterial cell wall synthesis by binding to penicillin proteins (PBPs) that dwell on the surface of H. pylori [12]. By inhibiting the cross-linking of peptidoglycan chains, the cell wall, an essential organelle of prokaryotes providing many functions such as keeping the cell in shape, giving protection, and maintaining integrity, loses its rigidity, which causes osmotic imbalances, leading to cellular swelling and eventual rupture, effectively killing the bacteria.
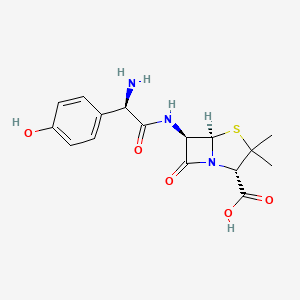
Figure 1: Amoxicillin chemical structure [13]
1.5. Clarithromycin treatment mechanisms
On the contrary, clarithromycin is an antibiotic that eradicates both extracellular and intracellular bacteria by binding to the 50S ribosomal subunit. Alternating the ribosomal subunit will pause the elongation of peptide chains, inhibiting bacteria from producing essential proteins for growth and survival [14]. Compared with amoxicillin, clarithromycin is more effective against H. pylori because of its 6-methoxy group modification, which provides stability in acidic environments, in this case, stomach acids, and its ability to accumulate within gastric epithelial cells. These properties ensure that the drug particles can reach the bacteria within the gastric epithelial cells.
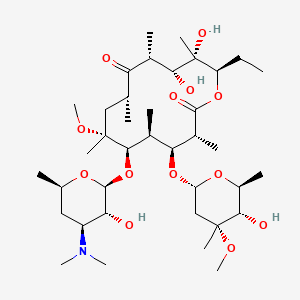
Figure 2: Clarithromycin chemical structure [15]
1.6. Triple therapy
As mentioned in the abstract, both antibiotics are effective in treating peptic ulcers, but the most productive treatment is still triple therapy, a combination therapy. Triple therapy consists of a proton pump inhibitor (PPI), such as omeprazole, and two additional antibiotics, in this case, amoxicillin and clarithromycin [16]. The function of PPI is to reduce gastric acid secretions and, therefore, increase the environment's pH level, allowing more stable antibiotics to eradicate bacteria while providing a more temperate environment for mucosa to heal [17].
Eliminating H. pylori using appropriate antibiotic therapy will return the inflammatory and digestive systems to homeostasis. Nevertheless, monitoring an individual’s antibiotic resistance, allergy, and adherence to the treatment regimen is crucial for complete recovery.
2. Discussion
2.1. Difference between amoxicillin & clarithromycin
Two antibiotics eradicate bacteria in different ways: amoxicillin binds to the surface of the bacteria's cell wall and causes cell lysis; it is particularly effective for actively dividing bacteria in the digestive system. In contrast, clarithromycin inhibits the 50S ribosomal units, which pause the production of peptide chains, preventing the bacteria from carrying out essential functions and ultimately leading to cell death. Clarithromycin, however, is always adequate despite the stage the bacteria is in; it can still eliminate the bacteria even if it is dormant.
2.2. Benefits of amoxicillin
Amoxicillin is preferable to clarithromycin in certain circumstances. For instance, H. pylori has a low resistance rate towards amoxicillin despite any mutational kind around the globe, and this property helps maintain a treatment regimen; the drug can be used multiple times if the initial treatment is unsuccessful or the patient has a recurring infection. Additionally, amoxicillin is more tolerated by most individuals with few side effects [18]; unlike clarithromycin, amoxicillin does not wholly inhibit cytochrome P450 enzymes, resulting in minimal drug interactions with other medications. In contrast, amoxicillin is more affordable than clarithromycin, making it more accessible and produced to supply developing countries.
2.3. Limitations of amoxicillin
Amoxicillin is preferable to clarithromycin in certain circumstances. For instance, H. pylori has a low resistance rate towards amoxicillin despite any mutational kind around the globe, and this property helps maintain a treatment regimen; the drug can be used multiple times if the initial treatment is unsuccessful or the patient has a recurring infection. Additionally, amoxicillin is more tolerated by most individuals with few side effects [18]; unlike clarithromycin, amoxicillin does not wholly inhibit cytochrome P450 enzymes, resulting in minimal drug interactions with other medications. In contrast, amoxicillin is more affordable than clarithromycin, making it more accessible and produced to supply developing countries.
2.4. Benefits of clarithromycin
Conversely, the other antibiotic mentioned, clarithromycin, is more effective than amoxicillin since it binds to the 50S ribosomal subunit inside the bacterial cell, which blocks off peptide chain elongation that forms protein. The lack of these proteins can lead to a bacteriostatic effect; the antibiotic can effectively kill the bacteria as the concentration increases [19]. Compared with amoxicillin, clarithromycin offers more advantages; it can penetrate the intracellular space deeper than amoxicillin, reaching the H. pylori that resides inside the host cell. According to many data, clarithromycin has demonstrated superior capability in eradicating bacteria, especially in triple therapy regimens. When clarithromycin is included in triple therapy, combined with PPI, the treatment can eliminate bacterial infections and offer a post-antibiotic effect, suppressing bacterial growth and lowering the surrounding pH level to create a moderate environment for the gastric tissues to repair.
Furthermore, clarithromycin offers a longer half-life expectancy than amoxicillin, suggesting two doses daily, which is more manageable for patients to consume [20]. At the same time, the drug also provides more resistance against an acidic environment, making clarithromycin ideal for treating diseases in the digestive system. What differentiates clarithromycin and amoxicillin the most is that clarithromycin is even effective against dormant bacteria, which is critical for eradicating all H. pylori on site to avoid failure in treatment or recurrence of the infection.
2.5. Limitations of clarithromycin
Nonetheless, clarithromycin offers benefits like penetrating the intracellular spaces and inhibiting protein synthesis. The trade-off is that H. pylori can swiftly adapt resistance against the antibiotics. Therefore, the drug is not recommended if the initial treatment is unsuccessful or the patient has a relapsing infection. Using clarithromycin also has higher drug interactions with other medications. In terms of price, clarithromycin generally surpasses amoxicillin by a moderate amount despite the region of the world.
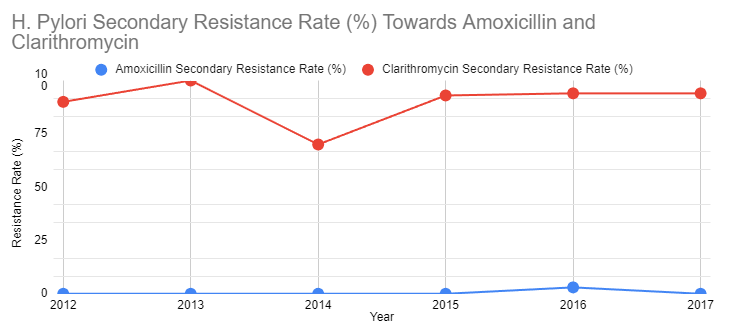
Figure 3: The graph demonstrates the table [21] of secondary resistance rate of 154 patients, with a median age of 45, mainly from Asia or Australia, to amoxicillin and clarithromycin after the initial treatment from 2012 to 2017
Similarly, according to “Alarming antibiotics resistance of Helicobacter pylori from children in Southeast China over 6 years” [22], the overall resistance to clarithromycin increased from 11.1% in 2012 to 43.1% in 2020 by 2.8% each year. It stayed above 15% from 2013 to 2020, a threshold of clarithromycin resistance rate for the standard triple therapy in the Maastricht IV/Florence consensus report.
2.6. Applicability for both antibiotics
After identifying the advantages and disadvantages of these two similar but distinct antibiotics, selecting the two drugs should be individualized depending on the patient’s allergy, local resistance patterns, and potential drug interactions. Amoxicillin is a reliable antibiotic with good safety but is less effective against intracellular H. pylori. Clarithromycin offers a higher eradication rate due to its ability to inhibit protein synthesis and reach intracellular bacteria, but it faces burdens like high resistance and side effects. It is always ideal to utilize triple therapy to maximize the eradication rate and target H. pylori through different mechanisms. Due to triple therapy's potential cardiac and hepatic risks, it is vital to monitor for side effects closely, and drug interactions are also essential, especially with clarithromycin.
2.7. Future of treatments for H. pylori-induced peptic ulcers
Current antibiotic treatments are already highly effective in eradicating H. pylori and healing peptic ulcers; it is a very classical case of using antibiotics to solve bacterial infections. As long as there is enough supply to be distributed to needy individuals, H. pylori should not be as much of a concern as it is currently. Figure 4 below demonstrates the prevalence of peptic ulcers from 1990 to 2019, and the total number of prevalences has been reduced by 1 million over 29 years.
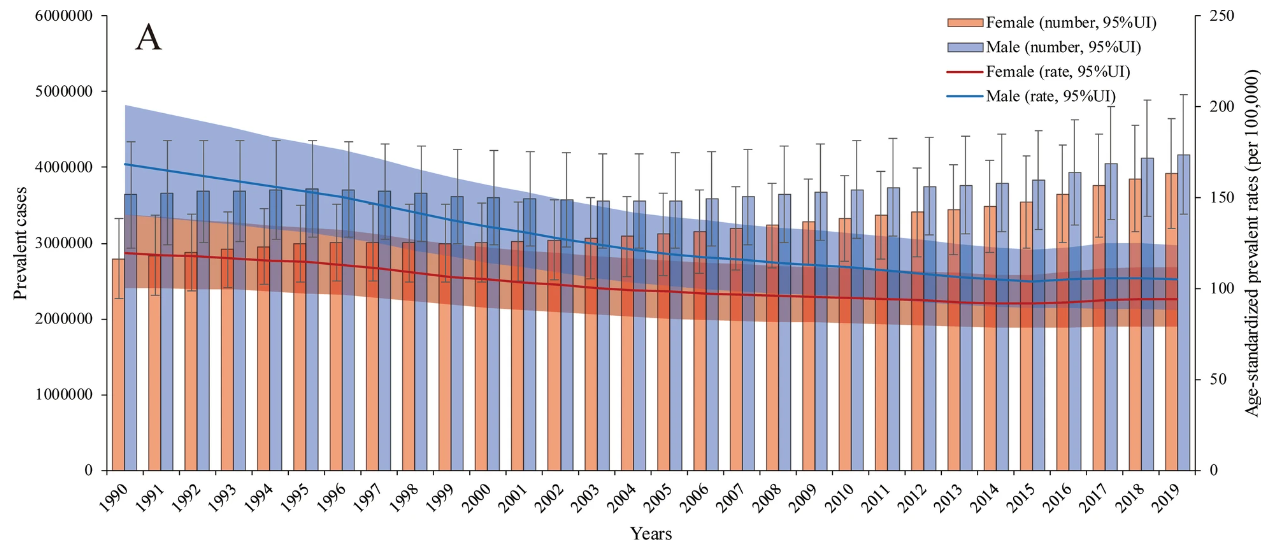
Figure 4: The numbers of prevalent cases and age-standardized prevalence rates in males and females [23]
3. Conclusion
In conclusion, effectively treating H. pylori-induced peptic ulcers depends on a well-structured therapeutic approach considering the patient’s circumstances, including antibiotic resistance, tolerance, and underlying conditions. Amoxicillin remains a reliable choice at the initial stage of bacterial infection since it is broadly accessible, low-cost, and has minimal side effects.
On the other hand, clarithromycin offers a superior eradication function when H. pylori has already successfully colonized deep into the gastric epithelial cells. Clarithromycin’s ability to inhibit bacterial protein synthesis and reach dormant bacteria makes it highly effective in triple therapy regimens. Yet clarithromycin faces burdens such as increasing global resistance rates and more severe side effects, especially concerning interactions with other drugs due to its impact on cytochrome P450 enzymes.
The combination treatment of the two above antibiotics and a PPI in triple therapy offers the highest eradication rate for H. pylori among the population. For patients experiencing initial contact with bacteria or side effect concerns, clarithromycin-based triple therapy may offer the best outcomes, especially in more severe cases where it removes future concerns of recurrence. In contrast, amoxicillin remains a highly viable option for widespread public use with high resistance rates. Close adherence to treatment regimens, drug interactions, and the use of updated local resistance data are critical factors in achieving optimal eradication results and minimizing side effects. As a parting thought, while amoxicillin and clarithromycin are effective against H. pylori, they are also broadly effective in treating various bacterial infections.
References
[1]. Malik TF, Gnanapandithan K, Singh K. Peptic Ulcer Disease. [Updated 2023 Jun 5]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534792/
[2]. Molaoa S. Z. (2021). Prevalence of Helicobacter pylori infection and the incidence of the associated malignant and peptic ulcer disease (PUD) at Nelson Mandela Academic Hospital: a retrospective analysis. Journal of Drug Assessment, 10(1), 57–61. https://doi.org/10.1080/21556660.2020.1854560
[3]. Shah, S., Cappell, K., Sedgley, R. et al. Diagnosis and treatment patterns among patients with newly diagnosed Helicobacter pylori infection in the United States 2016–2019. Sci Rep 13, 1375 (2023). https://doi.org/10.1038/s41598-023-28200-3
[4]. Abbasi-Kangevari, M., Ahmadi, N., Fattahi, N., et al. (2022). Quality of care of peptic ulcer disease worldwide: A systematic analysis for the global burden of disease study 1990-2019. PloS One, 17(8), e0271284. https://doi.org/10.1371/journal.pone.0271284
[5]. Quach, D. T., Vilaichone, R. K., Vu, K. V., et al. (2018). Helicobacter pylori Infection and Related Gastrointestinal Diseases in Southeast Asian Countries: An Expert Opinion Survey. Asian Pacific journal of cancer prevention, 19(12), 3565–3569. https://doi.org/10.31557/APJCP.2018.19.12.3565
[6]. Kim, S. Y., Choi, D. J., & Chung, J. W. (2015). Antibiotic treatment for Helicobacter pylori: Is the end coming?. World Journal of Gastrointestinal Pharmacology and Therapeutics, 6(4), 183–198. https://doi.org/10.4292/wjgpt.v6.i4.183
[7]. Doohan, D., Rezkitha, Y. A. A., et al. (2021). Helicobacter pylori BabA-SabA key roles in the adherence phase: The synergic mechanism for successful colonization and disease development. Toxins, 13(7), 485. https://doi.org/10.3390/toxins13070485
[8]. Lee, K. E., Khoi, P. N., Xia, Y., et al. (2013). Helicobacter pylori and interleukin-8 in gastric cancer. World Journal of Gastroenterology, 19(45), 8192–8202. https://doi.org/10.3748/wjg.v19.i45.8192
[9]. Alzahrani, S., Lina, T. T., Gonzalez, J., et al. (2014). Effect of Helicobacter pylori on gastric epithelial cells. World Journal of Gastroenterology, 20(36), 12767–12780. https://doi.org/10.3748/wjg.v20.i36.12767
[10]. Kim, A., Servetas, S. L., Kang, J., et al. (2015). Helicobacter pylori bab paralog distribution and association with cagA, vacA, and homA/B genotypes in american and south korean clinical isolates. PloS One, 10(8), e0137078. https://doi.org/10.1371/journal.pone.0137078
[11]. Medakina, I., Tsapkova, L., Polyakova, V., et al. (2023). Helicobacter pylori antibiotic resistance: molecular basis and diagnostic methods. International Journal of Molecular Sciences, 24(11), 9433. https://doi.org/10.3390/ijms24119433
[12]. National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 33613, Amoxicillin. Retrieved October 15, 2024 from https://pubchem.ncbi.nlm.nih.gov/compound/Amoxicillin.
[13]. Takemori, N., Ooi, H. K., Imai, G., et al. (2020). Possible mechanisms of action of clarithromycin and its clinical application as a repurposing drug for treating multiple myeloma. Ecancermedicalscience, 14, 1088. https://doi.org/10.3332/ecancer.2020.1088
[14]. National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 84029, Clarithromycin. Retrieved October 15, 2024 from https://pubchem.ncbi.nlm.nih.gov/compound/Clarithromycin.
[15]. Gené, E., Calvet, X., Azagra, R., & Gisbert, J. P. (2003). Triple vs. quadruple therapy for treating Helicobacter pylori infection: a meta-analysis. Alimentary Pharmacology & Therapeutics, 17(9), 1137–1143. https://doi.org/10.1046/j.1365-2036.2003.01566.x
[16]. Peretz, A., Paritsky, M., Nasser, O., et al. Resistance of Helicobacter pylori to tetracycline, amoxicillin, clarithromycin and metronidazole in Israeli children and adults. J Antibiot 67, 555–557 (2014). https://doi.org/10.1038/ja.2014.38
[17]. Akhavan, B. J., Vijhani, P., & Khanna, N. (2023, November 17). Amoxicillin. Nih.gov; StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK482250/
[18]. Champney WS. Antibiotics targeting bacterial ribosomal subunit biogenesis. J Antimicrob Chemother. 2020 Apr 1;75(4):787-806. doi: 10.1093/jac/dkz544. PMID: 31942624; PMCID: PMC8453389.
[19]. Lebel M. (1993). Pharmacokinetic properties of clarithromycin: A comparison with erythromycin and azithromycin. The Canadian Journal of Infectious Diseases = Journal Canadien des Maladies Infectieuses, 4(3), 148–152. https://doi.org/10.1155/1993/168061
[20]. Pateria, P., Chin, M., Goodheart, R., et al. (2020). Increasing secondary resistance to fluoroquinolones amongst Helicobacter pylori in Western Australia. Tasman Medical Journal, 2(1), 15–19. https://tasmanmedicaljournal.com/2020/03/increasing-secondary-resistance-to-fluoroquinolones-amongst-helicobacter-pylori-in-western-australia/
[21]. Shu, X., Ye, D., Hu, C., et al. (2022). Alarming antibiotics resistance of Helicobacter pylori from children in Southeast China over 6 years. Sci Rep 12, 17754. https://doi.org/10.1038/s41598-022-21661-y
[22]. Sun, W. H., Ou, X. L., Cao, D. Z., et al. (2005). Efficacy of omeprazole and amoxicillin with either clarithromycin or metronidazole on eradication of Helicobacter pylori in Chinese peptic ulcer patients. World Journal of Gastroenterology, 11(16), 2477–2481. https://doi.org/10.3748/wjg.v11.i16.2477
[23]. Xie, X., Ren, K., Zhou, Z., et al. (2022). The global, regional and national burden of peptic ulcer disease from 1990 to 2019: a population-based study. BMC Gastroenterology, 22(1), 58. https://doi.org/10.1186/s12876-022-02130-2
Cite this article
Tang,J. (2025). Comparative Analysis of Amoxicillin and Clarithromycin in Triple Therapy for Helicobacter Pylori-Induced Peptic Ulcers. Theoretical and Natural Science,115,118-124.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Malik TF, Gnanapandithan K, Singh K. Peptic Ulcer Disease. [Updated 2023 Jun 5]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534792/
[2]. Molaoa S. Z. (2021). Prevalence of Helicobacter pylori infection and the incidence of the associated malignant and peptic ulcer disease (PUD) at Nelson Mandela Academic Hospital: a retrospective analysis. Journal of Drug Assessment, 10(1), 57–61. https://doi.org/10.1080/21556660.2020.1854560
[3]. Shah, S., Cappell, K., Sedgley, R. et al. Diagnosis and treatment patterns among patients with newly diagnosed Helicobacter pylori infection in the United States 2016–2019. Sci Rep 13, 1375 (2023). https://doi.org/10.1038/s41598-023-28200-3
[4]. Abbasi-Kangevari, M., Ahmadi, N., Fattahi, N., et al. (2022). Quality of care of peptic ulcer disease worldwide: A systematic analysis for the global burden of disease study 1990-2019. PloS One, 17(8), e0271284. https://doi.org/10.1371/journal.pone.0271284
[5]. Quach, D. T., Vilaichone, R. K., Vu, K. V., et al. (2018). Helicobacter pylori Infection and Related Gastrointestinal Diseases in Southeast Asian Countries: An Expert Opinion Survey. Asian Pacific journal of cancer prevention, 19(12), 3565–3569. https://doi.org/10.31557/APJCP.2018.19.12.3565
[6]. Kim, S. Y., Choi, D. J., & Chung, J. W. (2015). Antibiotic treatment for Helicobacter pylori: Is the end coming?. World Journal of Gastrointestinal Pharmacology and Therapeutics, 6(4), 183–198. https://doi.org/10.4292/wjgpt.v6.i4.183
[7]. Doohan, D., Rezkitha, Y. A. A., et al. (2021). Helicobacter pylori BabA-SabA key roles in the adherence phase: The synergic mechanism for successful colonization and disease development. Toxins, 13(7), 485. https://doi.org/10.3390/toxins13070485
[8]. Lee, K. E., Khoi, P. N., Xia, Y., et al. (2013). Helicobacter pylori and interleukin-8 in gastric cancer. World Journal of Gastroenterology, 19(45), 8192–8202. https://doi.org/10.3748/wjg.v19.i45.8192
[9]. Alzahrani, S., Lina, T. T., Gonzalez, J., et al. (2014). Effect of Helicobacter pylori on gastric epithelial cells. World Journal of Gastroenterology, 20(36), 12767–12780. https://doi.org/10.3748/wjg.v20.i36.12767
[10]. Kim, A., Servetas, S. L., Kang, J., et al. (2015). Helicobacter pylori bab paralog distribution and association with cagA, vacA, and homA/B genotypes in american and south korean clinical isolates. PloS One, 10(8), e0137078. https://doi.org/10.1371/journal.pone.0137078
[11]. Medakina, I., Tsapkova, L., Polyakova, V., et al. (2023). Helicobacter pylori antibiotic resistance: molecular basis and diagnostic methods. International Journal of Molecular Sciences, 24(11), 9433. https://doi.org/10.3390/ijms24119433
[12]. National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 33613, Amoxicillin. Retrieved October 15, 2024 from https://pubchem.ncbi.nlm.nih.gov/compound/Amoxicillin.
[13]. Takemori, N., Ooi, H. K., Imai, G., et al. (2020). Possible mechanisms of action of clarithromycin and its clinical application as a repurposing drug for treating multiple myeloma. Ecancermedicalscience, 14, 1088. https://doi.org/10.3332/ecancer.2020.1088
[14]. National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 84029, Clarithromycin. Retrieved October 15, 2024 from https://pubchem.ncbi.nlm.nih.gov/compound/Clarithromycin.
[15]. Gené, E., Calvet, X., Azagra, R., & Gisbert, J. P. (2003). Triple vs. quadruple therapy for treating Helicobacter pylori infection: a meta-analysis. Alimentary Pharmacology & Therapeutics, 17(9), 1137–1143. https://doi.org/10.1046/j.1365-2036.2003.01566.x
[16]. Peretz, A., Paritsky, M., Nasser, O., et al. Resistance of Helicobacter pylori to tetracycline, amoxicillin, clarithromycin and metronidazole in Israeli children and adults. J Antibiot 67, 555–557 (2014). https://doi.org/10.1038/ja.2014.38
[17]. Akhavan, B. J., Vijhani, P., & Khanna, N. (2023, November 17). Amoxicillin. Nih.gov; StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK482250/
[18]. Champney WS. Antibiotics targeting bacterial ribosomal subunit biogenesis. J Antimicrob Chemother. 2020 Apr 1;75(4):787-806. doi: 10.1093/jac/dkz544. PMID: 31942624; PMCID: PMC8453389.
[19]. Lebel M. (1993). Pharmacokinetic properties of clarithromycin: A comparison with erythromycin and azithromycin. The Canadian Journal of Infectious Diseases = Journal Canadien des Maladies Infectieuses, 4(3), 148–152. https://doi.org/10.1155/1993/168061
[20]. Pateria, P., Chin, M., Goodheart, R., et al. (2020). Increasing secondary resistance to fluoroquinolones amongst Helicobacter pylori in Western Australia. Tasman Medical Journal, 2(1), 15–19. https://tasmanmedicaljournal.com/2020/03/increasing-secondary-resistance-to-fluoroquinolones-amongst-helicobacter-pylori-in-western-australia/
[21]. Shu, X., Ye, D., Hu, C., et al. (2022). Alarming antibiotics resistance of Helicobacter pylori from children in Southeast China over 6 years. Sci Rep 12, 17754. https://doi.org/10.1038/s41598-022-21661-y
[22]. Sun, W. H., Ou, X. L., Cao, D. Z., et al. (2005). Efficacy of omeprazole and amoxicillin with either clarithromycin or metronidazole on eradication of Helicobacter pylori in Chinese peptic ulcer patients. World Journal of Gastroenterology, 11(16), 2477–2481. https://doi.org/10.3748/wjg.v11.i16.2477
[23]. Xie, X., Ren, K., Zhou, Z., et al. (2022). The global, regional and national burden of peptic ulcer disease from 1990 to 2019: a population-based study. BMC Gastroenterology, 22(1), 58. https://doi.org/10.1186/s12876-022-02130-2









