1. Introduction
Attention Deficit/Hyperactivity Disorder is a neurodevelopmental disorder with persistent pattern of inattention, hyperactivity or impulsivity that is shown to interfere with development or functioning, which can be regarded as an earlier criterion.[1] In autism spectrum disorders, attentional abnormalities such as this (more detail more focussed or hyperactive) are very common. When further concerns or hyperactivity are beyond what is normally anticipated in individuals of similar mental capacity, a diagnosis developed for ADHD is among the most common neurodevelopmental disorders, affecting 5–10% of children and affects between 2% to 5% for adults globally.[2] This ubiquity underscores the severely debilitating influence this disorder can have on those affected, often leading to dysfunction in scholastic progression, work performance and interpersonal relationships. The hallmark symptoms of ADHD are problems with executive functions, i.e., attention deficiencies (which presents as restlessness), hyperactivity or impulsiveness; inattentive type together these form the 3 presentations. Pharmacological intervention is vital for the successful management of ADHD, being effective in reducing symptoms and enhancing quality of life. There are primarily two types of drugs in the market that fall under these categories, which include stimulants and non-stimulants. Medications —Central Nervous System (CNS) stimulant medications, such as methylphenidate (MPH), dexmethylphenidate (d-MPH), lisdexamfetamine work by increasing the activity of specific neurotransmitters in the brain to improve attention and reduce hyperactive and impulsive behaviors which are key symptoms of ADHD.[3] In contrast, non-CNS stimulant drugs such as atomoxetine (ATX) can act through other mechanisms and often affect norepinephrine pathways to achieve similar effects.
A comparative analysis of stimulant and non-stimulant medications reveals distinct differences in their mechanisms, modes of delivery, efficacy, and associated side effects. Specific comparisons between d-MPH and MPH, MPH and LDX, MPH and ATX are discussed based on several studies; however, they might imply some limitations in design of studies which hinder general applicability of results in real-word scenarios.
2. Description of chemical structures of drugs
2.1. Stimulant medication
2.1.1. Methylphenidate
Methyl phenyl(piperidin-2-yl) acetate is an ester derived from amino acids, methyl phenylacetate, where one of the hydrogen alpha next to the carbonyl group is substituted with a piperidin-2-yl group. It belongs to the piperidines, methyl ester, and beta-amino acid groups.[4]
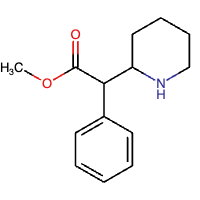
Figure 1: Chemical structure of MPH
2.1.2. Dexmethylphenidate
Methyl phenyl(piperidin-2-yl) acetate has R-configured stereocenters. It is the active enantiomer in the racemic drug MPH and functions as an adrenergic agent. It is an enantiomer of methyl (S)-phenyl[(S)-piperidin-2-yl]acetate. [5]
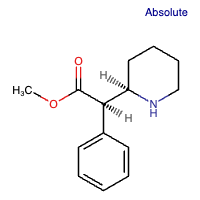
Figure 2: Chemical structure of d-MPH
2.1.3. Lisdexamfetamine
Lisdexamfetamine is an amino acid amide. Lisdexamfetamine is a prodrug of dextroamphetamine, covalently attached to the naturally occurring amino acid L-lysine.[6][7]
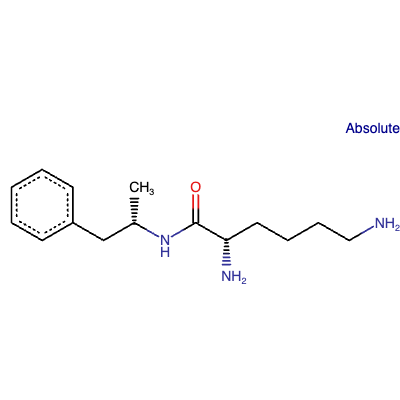
Figure 3: Chemical structure of LDX
2.2. Non stimulant medication
Atomoxetine is a secondary amino compound characterized by methyl and 3-(2-methylphenoxy)-3-phenylpropan-1-yl substituents.[8][9]
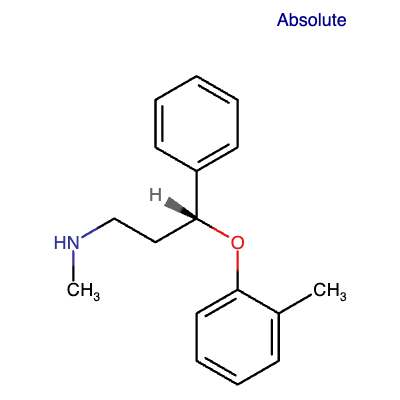
Figure 4: Chemical structure of ATX
3. Discussion of drug pharmacology
MPH and d-MPH are common stimulants that affect the central nervous system (CNS). They are widely used in treating ADHD. Their primary mode of action is believed to involve inhibition of dopamine transporter (DAT) and norepinephrine transporter (NET).[10][11][12] By virtue of blocking DAT, MPH and d-MPH elevate the levels of extracellular dopamine, which in turn enhances dopaminergic neurotransmission, a process that improves attention and executive functioning in people with ADHD.[13] In a paralleling way, when MPH and D-MPH inhibit NET, the level of norepinephrine in the synapse increases, hence aiding in more control of hyperactivity and impulsivity.[14] Recent research also indicates that MPH may work as an epigenetic modifier and lower the levels of some genes that belong to the human endogenous retrovirus family, HERV-H in particular, which are highly expressed in patients with ADHD.[15] Nonetheless, there is not enough precise evidence presently regarding the suspicion that d-MPH shares a similar epigenetic action. MPH and d-MPH are offered both in immediate and extended dosage forms. The IR dosage forms of MPH and d-MPH necessitate several administrations within a day to control the symptoms, whereas the ER dosage forms use various delivery technologies such as osmotic-controlled release oral delivery system (OROS) and multi-layer bead technology in order to regulate the release of the drug for more than 8 to 12 hours.[16] LDX is a prodrug, which is also contiguous to non-stimulant medication. Once taken orally, it is eventually converted into dextroamphetamine, its active component, which ensures slow, steady release throughout the day.[17] It acts by blocking the reuptake of these neurotransmitters; thus, it raises the levels of dopamine and norepinephrine outside of the neuron.[18]
Unlike stimulant medications, ATX is a selective norepinephrine reuptake inhibitor.[19] It binds to the NET and promotes the retention of norepinephrine in the brain.[20] However, it produces similar effects as stimulant medications: such inhibition results in increased norepinephrine levels in the synaptic cleft and, therefore, promotes noradrenergic transmission.[21] Also, ATX inhibits dopamine reuptake in the prefrontal cortex (PFC), which plays an important role in attention and executive function.[22] In this manner, by acting on these systems and other neurotransmitters, ATX helps to ameliorate the key symptoms of ADHD, that is, inattention, hyperactivity, and impulsivity. This aspect of the drug action has been confirmed by neuroimaging studies which also showed elevated prefrontal cortex activity and other brain regions related to better cognitive and behavioral control in ADHD patients.[23] ATX is given orally in the form of a capsule.[24] ATX may be given either as a single loading dose or two divided doses of equal amounts.[25] It is also subjected to metabolism by cytochrome P450 2D6, its polymorphic enzyme, which determines the atomoxetine pharmacokinetics.[26] Because of this metabolism, the serum elimination half-life of atomoxetine is as short as 5.2 hours in extensive metabolizers, and 21.6 hours in poor metabolizers.[27] This mode of drug delivery ensures that there is a gradual rise in levels of norepinephrine in the prefrontal cortical region, which assists in the improvement of attention and reduction of hyperactive and impulsive behavior without the risk of misuse as with stimulant drugs. The accessibility of ATX as a non-stimulant substance and the possibility of flexible dosing allows for controlling ADHD symptoms even in patients comorbid with other psychiatric illnesses or having a high potential for substance use disorder.[28]
4. Comparison of MPH and d-MPH
Quinn et al. [29] conducted studies to compare the effectiveness of acute equimolar of d-MPH and d,l-MPH in reducing ADHD symptoms over an 8-hour period in a laboratory school setting. The aim of the investigation is to find out the effects of plasma levels of MPH on the efficacy of two formulations. By examining these studies, the authors indicate that the pharmacological effects of MPH are mediated exclusively by the d-isomer.
4.1. Methods
In a double-blinded, active-controlled, crossover study, 32 children with diagnosed ADHD participated, with 31 completing the study. On seven separate occasions, spaced at least 6 days apart, the children received a single morning dose of d-MPH (2.5, 5, or 10 mg), d,l-MPH (5, 10, or 20 mg), or placebo. Participants were observed in a laboratory classroom setting for 8 hours. At predetermined intervals, blinded observers rated the participant's behavior, and the participants performed a computerized math test. The study shows that the plasma levels of MPH were related to the response to the study medication while also comparing the safety profiles of two formulations.[29]
4.2. Results
Responses to both formulations were dose-related. Plasma concentrations of l-MPH were minimal, while those of d-MPH were similar between the two treatments. Clinical efficacy shows a strong correlation with plasma concentrations of d-MPH. The study concluded that single doses of d-MPH provided comparable efficacy to equimolar doses of d,l-MPH over a 6-hour period, based on repeated evaluations of academic performance and behavior ratings. Both formulations were well-tolerated, with no notable differences in side effects for equimolar doses.[29]
4.3. Discussion
The efficacy and safety profiles of d-MPH are suggested to be on par with that of d, l MPH. Efficacy measures for similar doses indicated no better improvements compared to placebo up to and including 4 hours post-dose in this study, although d-MPH appears to extend longer. This longer duration of action is not explained by the higher plasma level but by the intrinsic pharmacodynamics of d-MPH. Results sum up that qualitative and quantitative effects of d-MPH when used to treat children with ADHD symptoms is equal to that of racemic MPH in a dose half its strength. This suggests that those forms of MPH that contain both d-MPH and l-MPH (i.e. racemic formulations) are not better than pure d-MPH in terms of clinical effectiveness.
Nevertheless, a considerable drawback of this research is the laboratory school protocol used in its design. Settlements such as only children with ADHD present and staff-pupils proportions being greater than those in conventional classrooms may affect the behavior and interactions within the classes and, as such, make the results less applicable in the real world. In addition, the length and repetitiveness of the sessions are starkly different from the regular school experience, which is long and offers varied activities. The sample size, although it is sufficient for large effect sizes, is inadequate for the detection of smaller yet clinically meaningful differences; thus, further studies are warranted to fully understand the difference between d-MPH and d, l-MPH. Also, the relatively small age range of the participants skews the findings where such treatments are also practiced for a wider age group population like teenagers and toddlers who are often placed on stimulants.
There are also certain restrictions on the study design. It is noteworthy that the lack of a non-ADHD control group diminishes the strength of data pertaining to the effects of the medication. Also, the varied follow-up durations ranging from 2 to 12 weeks may affect the uniformity and dependability of the outcome. Moreover, the use of a computerized math test for measuring academic performance differs from the written tests used in other studies Hence, there are challenges in making direct comparisons. Confirming these limitations is a conclusion that more research with bigger and more representative samples and involved appropriate methods is needed to fully see the benefits of using pure enantiomer (d-MPH) rather than d,l MPH in the finished drug product.
5. Comparison of MPH and LDX
Newcorn et al. [30] conducted two extensive, randomized, double-blinded, active-controlled studies to compare the effectiveness, safety, and tolerability of LXD and OROS-MPH in adolescents with ADHD. These studies are among the largest head-to-head trials between LXD and MPH conducted to date. As measured by the ADHD Rating Scale IV (ADHD-RS-IV) total score and the dichotomized Clinical Global Impression (CGI) scale, LDX showed superior results to osmotic-release oral system-MPH (OROS-MPH) in the forced-dose study but not in the flexible-dose study, suggesting that certain doses of LXD may be more beneficial than others.
5.1. Methods
Adolescents (13-17 years) diagnosed with ADHD as per Diagnostic and Statistical Manual of Mental Disorders criteria were recruited for two studies. The 8-week flexible-dose study included participants randomized to LDX at doses ranging from 30-70 mg/day, OROS-MPH at doses of 18 to 72 mg/day, or placebo. The 6-week forced-dose study included participants randomized to LDX (70 mg/day), OROS-MPH (72 mg/day), or placebo. The primary outcome measured was the change in ADHD-RS-IV total score from baseline. Secondary outcomes included the CGI-I scale and changes in ADHD-RS-IV subscale scores. Safety evaluations covered comparable rates of treatment-emergent adverse events (TEAEs) and vital signs.[30]
5.2. Results
In the forced-dose study, least squares (LS) mean changes in ADHD-RS-IV total scores were -17.0 for placebo, -25.4 for LDX, and -22.1 for OROS-MPH. LDX was significantly superior to OROS-MPH (-3.4, p = 0.0013, ES -0.33). In the flexible-dose study, LS mean changes were -13.4 for placebo, -25.6 for LDX, and -23.5 for OROS-MPH, with LDX showing a numerical but not statistically significant advantage (-2.1, p = 0.0717, ES -0.20). CGI-I scales were significantly greater for LDX compared to OROS-MPH in the forced-dose study (81.4% vs. 71.3%, p = 0.0188) but not in the flexible-dose study (83.1% vs. 81.0%, p = 0.6165). Both LDX and OROS-MPH outperformed placebo on all efficacy-related outcomes. TEAEs occurred in 66.5% (LDX) and 58.9% (OROS-MPH) of participants in the forced-dose study and 83.2% (LDX) and 82.1% (OROS-MPH) in the flexible-dose study, with notable differences in side effects like decreased appetite and increased heart rate.[30]
5.3. Discussion
These results align with previous findings as well as meta-analyses insinuating the slight superiority of amphetamine derivatives (LXD) at the given dosages compared with methylphenidate derivatives (OROS-MPH).
In terms of safety and tolerability, both medications were generally well-tolerated with comparable rates of TEAEs. In the flexible-dose study, overall TEAEs were similar for both agents, but the discontinuation and severe TEAE rates were modestly higher for LXD. On the contrary, in the forced-dose study, LXD showed higher overall TEAEs but lower severe TEAEs when compared with OROS-MPH. Importantly, it was observed that LXD led to more weight loss, while OROS-MPH caused a greater increase in heart rate.
The clinical take-home message from these studies is that LXD may prove to be more effective in some circumstances but with a higher risk of side effects. However, both drugs have shown a substantial improvement over placebo in trials, indicating their usefulness in the treatment of adolescents with Attention Deficit Hyperactivity Disorder. To clinicians, this indicates that it is reasonable to use either class of stimulant as a first-line agent and switch to another class if there is no adequate response or if side effects are intolerable.
6. Comparison of MPH and ATX
Eleven eligible randomized-controlled trials were included in the studies from Liu et al. (2017), showing that MPH has a significantly higher response rate compared to ATX in treating ADHD in children and adolescents, and MPH relative to ATX was more effective in reducing inattention. This better efficacy might be due to the mechanism of MPH, which not only inhibits the reuptake transport of dopamine but also facilitates the efflux of monoamines from presynaptic terminals, resulting in excessive levels of catecholamines in the synapse.
6.1. Methods
In order to identify head to head trials comparing ATX and MPH in children and adolescents, a literature review was conducted across databases to April 2016. Major outcomes, including response rate, ADHD Rating Scale score, and adverse events, were compared between the two treatments. Effect sizes for continuous and dichotomous data were shown by using standardized mean difference and risk ratio, along with their corresponding confidence intervals.[31]
6.2. Results
Eleven randomized-controlled trials met the eligibility criteria, with two being double-blind and the rest open-label. MPH showed a higher response rate (RR=1.14, 95% CI [1.09, 1.20]), better reduction in inattention (SMD=-0.13, 95% CI [-0.25, -0.01]), and fewer adverse events (drowsiness: RR=0.17, 95% CI [0.11, 0.26]; nausea: RR = 0.49; 95% CI [0.29, 0.85]; vomiting: RR = 0.41, 95% CI [0.27, 0.63]), comparing to ATX. However, MPH was associated with a higher risk of insomnia than ATX (RR = 2.27, 95% CI [1.63, 3.15], p<.01). These results suggest MPH as a potentially more effective first-line treatment option for the majority of patients with ADHD.[31]
6.3. Discussion
MPH not only has a better response rate but also enhances neuropsychological executive functions, working memory, and set-shifting abilities greatly. An event-related potential study carried out by Kratz et al. showed that treatment with MPH rather than with ATX helped patients with attention-deficit hyperactivity disorder to cut down reaction time, which in effect helped alleviate inattention and general cognition.
Considering the adverse events, ATX was found to cause more drowsiness (somnolence), nausea, and vomiting than MPH treatment. On the contrary, treatment with MPH resulted in more incidences of sleeplessness. Some side effects were less frequent because of the specific pharmacological characteristics of MPH, such as stimulating the arousal system in the brainstem and increasing dopaminergic activity. Still, this is an adverse effect that cannot be overlooked. This is consistent with the conclusion reached by Rostain.
However, most studies were conducted in Asian countries, suggesting a potential bias that MPH’s advantageous effects might be particularly pronounced in these populations. While this geographic aspect indicates a potential cultural and biological influence on the medication's efficacy and side effect profile, it also indicates these findings might not be entirely generalizable to non-Asian populations. Different genetic, cultural, and environmental factors could influence how patients respond to ADHD medications. Further studies, including diverse populations, are necessary to confirm the general applicability of the advantageous effects of MPH over ATX. Additionally, most studies were open-label rather than blinded, which could also introduce bias since the expectations of the participants and researchers may affect the outcomes, whether on purpose or by accident, and open-label designs are widespread in such studies, which compromise the findings’ reliability and validity.
7. Conclusion
This thorough review, synthesis, and evaluation of the studies show that there are marked differences between stimulant and non-stimulant medication efficacy and safety among children and adolescents suffering from ADHD. Most notably, Methylphenidate (MPH or d-METH) is particularly effective for the attentional-enhancing symptoms of ADHD when compared to ATX, which is a non-stimulant medication. The studies assert that the pharmacological effects of MPH in treatment are seen mainly from its d-isomer, which is also found to be effective as a racial mixture (d, l-MPH) in clinical areas.
To assess these treatments, a number of randomized, double-blind, placebo-controlled research endeavors were conducted. A total of thirty-two subjects entered into a crossover trial of d-MPH, d, l-MPH, and behavior; academic performance was then monitored. Literature reviews also included comparisons of ATX head-to-head with MPH, including response rates, ADHD Rating Scale (ADHD-RS) scores, and adverse events.
The results showed a consistently higher response rate and inattention reduction effect of MPH compared to ATX. Tolerated were both d-MPH and d, l-MPH, and the clinical effect was statistically significant correlated with plasma d-MPH concentrations. MPH was associated with a lower risk of adverse effects such as drowsiness, nausea, and vomiting but an increased risk of insomnia relative to ATX. In some dosing regimens, LXD was also superior to OROS-MPH but manifested increased weight loss and heart rate elevation consequences. General laws of pharmacology have proved that besides higher response rates, MPH also drastically enhances neuropsychological executive functions, especially working memory and set-shifting.
An event-related potential study, for example carried out by Kratz et al. showed that patients with attention deficit hyperactivity disorder and attention-deficit disorder with medical supervision while taking MPH will present lower reaction time measures than in patients managed with ATX, and this is an indication that MPH will enhance cognition and most especially attention as suffering focal ADHD patients. Talking about the side effects, it was shown that although patients taking d-MPH or d, l-MPH experienced lower levels of drowsiness, nausea and vomiting compared to patients given ATX, they still experienced incidences of insomnias. The unique properties of MPH that make it effective for treating ADHD include its ability to activate the arousal system in the brain through the tegmental system as well as stimulation of dopaminergic firing within certain brain areas. Still, even though the incidence of some side effects was reduced, the increased risk of developing insomnia is another issue that should not be underestimated, as noted by Rostain.
On the other hand, most of the studies have been carried out in Asian countries, indicating possible bias, which means that the benefits of MPH use may be more evident in these populations. Geographically, this adds a potential cultural and biological factor to the efficiency and safety profile of the drug, but it also suggests that such results may not fully apply to populations outside Asia. Other cultural, genetic, and environmental variables may also affect the response of patients to drugs used for treating ADHD. Further studies, including diverse populations, are necessary to confirm the general applicability of the advantageous effects of MPH over ATX.
Stimulants such as MPH or LDX are generally more effective in alleviating symptoms of ADHD compared to non-stimulants such as ATX. Both d-MPH and d, l-MPH are therapeutically similar; however, d-MPH may eliminate the necessity of using a higher ratio of the racemic d, l-MPH in order to achieve a similar therapeutic effect. The results also highlight the importance of tailoring treatment and taking into account the negative effects that might arise, such as sleeplessness. Medication that is not stimulant in nature may not be as effective on some measures but may be acceptable in patients who cannot take stimulants. There is a need for more research with larger and more representative sample sizes and unified approaches in order to really appreciate the long-term effects and improve the management of ADHD.
References
[1]. Arnsten, A. F. (2009). ADHD and the prefrontal cortex. The Journal of Pediatrics, 154(5), I-S43. https://doi.org/10.1016/j.jpeds.2009.01.018
[2]. Polanczyk, G. V., Willcutt, E. G., Salum, G. A., Kieling, C., & Rohde, L. A. (2014). ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. International Journal of Epidemiology, 43(2), 434–442. https://doi.org/10.1093/ije/dyt261
[3]. Nazarova, V. A., Sokolov, A. V., Chubarev, V. N., Tarasov, V. V., & Schiöth, H. B. (2022). Treatment of ADHD: Drugs, psychological therapies, devices, complementary and alternative methods as well as the trends in clinical trials. Frontiers in Pharmacology, 13. https://doi.org/10.3389/fphar.2022.1066988
[4]. Team, E. W. (n.d.). methyl phenyl(piperidin-2-yl)acetate (CHEBI:84276). https://www.ebi.ac.uk/chebi/searchId.do?chebiId=84276
[5]. Team, E. W. (n.d.-a). dexmethylphenidate (CHEBI:51860). https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:51860
[6]. Blick, S. K. A., & Keating, G. M. (2007). Lisdexamfetamine. Pediatric Drugs, 9(2), 129–135. https://doi.org/10.2165/00148581-200709020-00007
[7]. Team, E. W. (n.d.). lisdexamfetamine (CHEBI:135925). https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:135925
[8]. Team, E. W. (n.d.-a). atomoxetine (CHEBI:127342). https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:127342
[9]. PubChem. (n.d.). Atomoxetine. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/Atomoxetine
[10]. Dresel, S. H. J., Kung, M. T., Huang, X., Plössl, K., Hou, C., Shiue, C. Y., Karp, J., & Kung, H. F. (1999). In vivo imaging of serotonin transporters with [ 99m Tc]TRODAT-1 in nonhuman primates. European Journal of Nuclear Medicine and Molecular Imaging, 26(4), 342–347. https://doi.org/10.1007/s002590050396
[11]. Gamo, N. J., Wang, M., & Arnsten, A. F. (2010). Methylphenidate and atomoxetine enhance prefrontal function through Α2-Adrenergic and dopamine D1 receptors. Journal of the American Academy of Child & Adolescent Psychiatry, 49(10), 1011–1023. https://doi.org/10.1016/j.jaac.2010.06.015
[12]. Federici, M., Geracitano, R., Bernardi, G., & Mercuri, N. B. (2005). Actions of methylphenidate on dopaminergic neurons of the ventral midbrain. Biological Psychiatry, 57(4), 361–365. https://doi.org/10.1016/j.biopsych.2004.11.030
[13]. Jaeschke, R. R., Sujkowska, E., & Sowa-Kućma, M. (2021). Methylphenidate for attention-deficit/hyperactivity disorder in adults: a narrative review. Psychopharmacology, 238(10), 2667–2691. https://doi.org/10.1007/s00213-021-05946-0
[14]. Economidou, D., Theobald, D. E. H., Robbins, T. W., Everitt, B. J., & Dalley, J. W. (2012). Norepinephrine and dopamine modulate impulsivity on the Five-Choice serial reaction time task through opponent actions in the shell and core Sub-Regions of the nucleus accumbens. Neuropsychopharmacology, 37(9), 2057–2066. https://doi.org/10.1038/npp.2012.53
[15]. Chiara, C., Bernanda, P. M., Claudia, M., Elisa, D., Tony, M. M., Valentina, R., Sandro, G., Paolo, C., Paola, S., Augusto, P., & Emanuela, B. (2018). The Decrease in Human Endogenous Retrovirus-H Activity Runs in Parallel with Improvement in ADHD Symptoms in Patients Undergoing Methylphenidate Therapy. International Journal of Molecular Sciences, 19(11), 3286. https://doi.org/10.3390/ijms19113286
[16]. Steingard, R., Taskiran, S., Connor, D. F., Markowitz, J. S., & Stein, M. A. (2019). New formulations of stimulants: an update for clinicians. Journal of Child and Adolescent Psychopharmacology, 29(5), 324–339. https://doi.org/10.1089/cap.2019.0043
[17]. Ermer, J. C., Pennick, M., & Frick, G. (2016). Lisdexamfetamine dimesylate: prodrug delivery, amphetamine exposure and duration of efficacy. Clinical Drug Investigation, 36(5), 341–356. https://doi.org/10.1007/s40261-015-0354-y
[18]. Quintero, J., Gutiérrez-Casares, J. R., & Álamo, C. (2022). Molecular Characterisation of the mechanism of action of stimulant drugs lisdexamfetamine and methylphenidate on ADHD Neurobiology: a review. Neurology and Therapy, 11(4), 1489–1517. https://doi.org/10.1007/s40120-022-00392-2
[19]. Corona, J. C., Carreón-Trujillo, S., González-Pérez, R., Gómez-Bautista, D., Vázquez-González, D., & Salazar-García, M. (2019). Atomoxetine produces oxidative stress and alters mitochondrial function in human neuron-like cells. Scientific Reports, 9(1). https://doi.org/10.1038/s41598-019-49609-9
[20]. Logan, J., Wang, G., Telang, F., Fowler, J. S., Alexoff, D., Zabroski, J., Jayne, M., Hubbard, B., King, P., Carter, P., Shea, C., Xu, Y., Muench, L., Schlyer, D., Learned-Coughlin, S., Cosson, V., Volkow, N. D., & Ding, Y. (2007). Imaging the norepinephrine transporter in humans with (S,S)-[11C]O-methyl reboxetine and PET: problems and progress. Nuclear Medicine and Biology, 34(6), 667–679. https://doi.org/10.1016/j.nucmedbio.2007.03.013
[21]. Hussain, L. S., Reddy, V., & Maani, C. V. (2023, May 1). Physiology, noradrenergic synapse. StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK540977/
[22]. Sugimoto, A., Suzuki, Y., Yoshinaga, K., Orime, N., Hayashi, T., Egawa, J., Ono, S., Sugai, T., & Someya, T. (2021). Influence of atomoxetine on relationship between ADHD symptoms and prefrontal cortex activity during task execution in adult patients. Frontiers in Human Neuroscience, 15. https://doi.org/10.3389/fnhum.2021.755025
[23]. Arnsten, A. F. T. (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews. Neuroscience, 10(6), 410–422. https://doi.org/10.1038/nrn2648
[24]. Atomoxetine: MedlinePlus drug information. (n.d.). https://medlineplus.gov/druginfo/meds/a603013.html
[25]. Clemow, D. B., Nyhuis, A. W., & Robinson, R. L. (2016). Clinical Impact of Not Achieving Recommended Dose on Duration of Atomoxetine Treatment in Adults with Attention‐Deficit/Hyperactivity Disorder. CNS Neuroscience & Therapeutics, 22(12), 970–978. https://doi.org/10.1111/cns.12595
[26]. Brown, J., Abdel‐Rahman, S., Van Haandel, L., Gaedigk, A., Lin, Y., & Leeder, J. (2015). Single dose, CYP2D6 genotype‐stratified pharmacokinetic study of atomoxetine in children with ADHD. Clinical Pharmacology & Therapeutics, 99(6), 642–650. https://doi.org/10.1002/cpt.319
[27]. Sauer, J., Ring, B. J., & Witcher, J. W. (2005). Clinical pharmacokinetics of atomoxetine. Clinical Pharmacokinetics, 44(6), 571–590. https://doi.org/10.2165/00003088-200544060-00002
[28]. Perugi, G., & Vannucchi, G. (2015). The use of stimulants and atomoxetine in adults with comorbid ADHD and bipolar disorder. Expert Opinion on Pharmacotherapy, 16(14), 2193–2204. https://doi.org/10.1517/14656566.2015.1079620
[29]. Quinn, D., Wigal, S., Swanson, J., Hirsch, S., Ottolini, Y., Dariani, M., Roffman, M., Zeldis, J., & Cooper, T. (n.d.). Comparative Pharmacodynamics and Plasma Concentrations of d-threo-Methylphenidate Hydrochloride After Single Doses of d-threo-Methylphenidate Hydrochloride and d,l-threo-Methylphenidate Hydrochloride in a Double-Blind, Placebo-Controlled, Crossover Laboratory School Study in Children With Attention-Deficit/Hyperactivity Disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 43(11), 1422–1429. https://doi.org/10.1097/01.chi.0000140455.96946.2b
[30]. Quinn, D., Wigal, S., Swanson, J., Hirsch, S., Ottolini, Y., Dariani, M., Roffman, M., Zeldis, J., & Cooper, T. (n.d.). Comparative Pharmacodynamics and Plasma Concentrations of d-threo-Methylphenidate Hydrochloride After Single Doses of d-threo-Methylphenidate Hydrochloride and d,l-threo-Methylphenidate Hydrochloride in a Double-Blind, Placebo-Controlled, Crossover Laboratory School Study in Children With Attention-Deficit/Hyperactivity Disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 43(11), 1422–1429. https://doi.org/10.1097/01.chi.0000140455.96946.2b
[31]. Liu, Q., Zhang, H., Fang, Q., & Qin, L. (2017). Comparative efficacy and safety of methylphenidate and atomoxetine for attention-deficit hyperactivity disorder in children and adolescents: Meta-analysis based on head-to-head trials. Journal of Clinical and Experimental Neuropsychology, 39(9), 854–865. https://doi.org/10.1080/13803395.2016.1273320
Cite this article
Lai,Y. (2025). Attention-Deficit/Hyperactivity Disorder Medication Use: Stimulant Medication and Non-stimulant Medication. Theoretical and Natural Science,116,96-105.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Arnsten, A. F. (2009). ADHD and the prefrontal cortex. The Journal of Pediatrics, 154(5), I-S43. https://doi.org/10.1016/j.jpeds.2009.01.018
[2]. Polanczyk, G. V., Willcutt, E. G., Salum, G. A., Kieling, C., & Rohde, L. A. (2014). ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. International Journal of Epidemiology, 43(2), 434–442. https://doi.org/10.1093/ije/dyt261
[3]. Nazarova, V. A., Sokolov, A. V., Chubarev, V. N., Tarasov, V. V., & Schiöth, H. B. (2022). Treatment of ADHD: Drugs, psychological therapies, devices, complementary and alternative methods as well as the trends in clinical trials. Frontiers in Pharmacology, 13. https://doi.org/10.3389/fphar.2022.1066988
[4]. Team, E. W. (n.d.). methyl phenyl(piperidin-2-yl)acetate (CHEBI:84276). https://www.ebi.ac.uk/chebi/searchId.do?chebiId=84276
[5]. Team, E. W. (n.d.-a). dexmethylphenidate (CHEBI:51860). https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:51860
[6]. Blick, S. K. A., & Keating, G. M. (2007). Lisdexamfetamine. Pediatric Drugs, 9(2), 129–135. https://doi.org/10.2165/00148581-200709020-00007
[7]. Team, E. W. (n.d.). lisdexamfetamine (CHEBI:135925). https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:135925
[8]. Team, E. W. (n.d.-a). atomoxetine (CHEBI:127342). https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:127342
[9]. PubChem. (n.d.). Atomoxetine. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/Atomoxetine
[10]. Dresel, S. H. J., Kung, M. T., Huang, X., Plössl, K., Hou, C., Shiue, C. Y., Karp, J., & Kung, H. F. (1999). In vivo imaging of serotonin transporters with [ 99m Tc]TRODAT-1 in nonhuman primates. European Journal of Nuclear Medicine and Molecular Imaging, 26(4), 342–347. https://doi.org/10.1007/s002590050396
[11]. Gamo, N. J., Wang, M., & Arnsten, A. F. (2010). Methylphenidate and atomoxetine enhance prefrontal function through Α2-Adrenergic and dopamine D1 receptors. Journal of the American Academy of Child & Adolescent Psychiatry, 49(10), 1011–1023. https://doi.org/10.1016/j.jaac.2010.06.015
[12]. Federici, M., Geracitano, R., Bernardi, G., & Mercuri, N. B. (2005). Actions of methylphenidate on dopaminergic neurons of the ventral midbrain. Biological Psychiatry, 57(4), 361–365. https://doi.org/10.1016/j.biopsych.2004.11.030
[13]. Jaeschke, R. R., Sujkowska, E., & Sowa-Kućma, M. (2021). Methylphenidate for attention-deficit/hyperactivity disorder in adults: a narrative review. Psychopharmacology, 238(10), 2667–2691. https://doi.org/10.1007/s00213-021-05946-0
[14]. Economidou, D., Theobald, D. E. H., Robbins, T. W., Everitt, B. J., & Dalley, J. W. (2012). Norepinephrine and dopamine modulate impulsivity on the Five-Choice serial reaction time task through opponent actions in the shell and core Sub-Regions of the nucleus accumbens. Neuropsychopharmacology, 37(9), 2057–2066. https://doi.org/10.1038/npp.2012.53
[15]. Chiara, C., Bernanda, P. M., Claudia, M., Elisa, D., Tony, M. M., Valentina, R., Sandro, G., Paolo, C., Paola, S., Augusto, P., & Emanuela, B. (2018). The Decrease in Human Endogenous Retrovirus-H Activity Runs in Parallel with Improvement in ADHD Symptoms in Patients Undergoing Methylphenidate Therapy. International Journal of Molecular Sciences, 19(11), 3286. https://doi.org/10.3390/ijms19113286
[16]. Steingard, R., Taskiran, S., Connor, D. F., Markowitz, J. S., & Stein, M. A. (2019). New formulations of stimulants: an update for clinicians. Journal of Child and Adolescent Psychopharmacology, 29(5), 324–339. https://doi.org/10.1089/cap.2019.0043
[17]. Ermer, J. C., Pennick, M., & Frick, G. (2016). Lisdexamfetamine dimesylate: prodrug delivery, amphetamine exposure and duration of efficacy. Clinical Drug Investigation, 36(5), 341–356. https://doi.org/10.1007/s40261-015-0354-y
[18]. Quintero, J., Gutiérrez-Casares, J. R., & Álamo, C. (2022). Molecular Characterisation of the mechanism of action of stimulant drugs lisdexamfetamine and methylphenidate on ADHD Neurobiology: a review. Neurology and Therapy, 11(4), 1489–1517. https://doi.org/10.1007/s40120-022-00392-2
[19]. Corona, J. C., Carreón-Trujillo, S., González-Pérez, R., Gómez-Bautista, D., Vázquez-González, D., & Salazar-García, M. (2019). Atomoxetine produces oxidative stress and alters mitochondrial function in human neuron-like cells. Scientific Reports, 9(1). https://doi.org/10.1038/s41598-019-49609-9
[20]. Logan, J., Wang, G., Telang, F., Fowler, J. S., Alexoff, D., Zabroski, J., Jayne, M., Hubbard, B., King, P., Carter, P., Shea, C., Xu, Y., Muench, L., Schlyer, D., Learned-Coughlin, S., Cosson, V., Volkow, N. D., & Ding, Y. (2007). Imaging the norepinephrine transporter in humans with (S,S)-[11C]O-methyl reboxetine and PET: problems and progress. Nuclear Medicine and Biology, 34(6), 667–679. https://doi.org/10.1016/j.nucmedbio.2007.03.013
[21]. Hussain, L. S., Reddy, V., & Maani, C. V. (2023, May 1). Physiology, noradrenergic synapse. StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK540977/
[22]. Sugimoto, A., Suzuki, Y., Yoshinaga, K., Orime, N., Hayashi, T., Egawa, J., Ono, S., Sugai, T., & Someya, T. (2021). Influence of atomoxetine on relationship between ADHD symptoms and prefrontal cortex activity during task execution in adult patients. Frontiers in Human Neuroscience, 15. https://doi.org/10.3389/fnhum.2021.755025
[23]. Arnsten, A. F. T. (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews. Neuroscience, 10(6), 410–422. https://doi.org/10.1038/nrn2648
[24]. Atomoxetine: MedlinePlus drug information. (n.d.). https://medlineplus.gov/druginfo/meds/a603013.html
[25]. Clemow, D. B., Nyhuis, A. W., & Robinson, R. L. (2016). Clinical Impact of Not Achieving Recommended Dose on Duration of Atomoxetine Treatment in Adults with Attention‐Deficit/Hyperactivity Disorder. CNS Neuroscience & Therapeutics, 22(12), 970–978. https://doi.org/10.1111/cns.12595
[26]. Brown, J., Abdel‐Rahman, S., Van Haandel, L., Gaedigk, A., Lin, Y., & Leeder, J. (2015). Single dose, CYP2D6 genotype‐stratified pharmacokinetic study of atomoxetine in children with ADHD. Clinical Pharmacology & Therapeutics, 99(6), 642–650. https://doi.org/10.1002/cpt.319
[27]. Sauer, J., Ring, B. J., & Witcher, J. W. (2005). Clinical pharmacokinetics of atomoxetine. Clinical Pharmacokinetics, 44(6), 571–590. https://doi.org/10.2165/00003088-200544060-00002
[28]. Perugi, G., & Vannucchi, G. (2015). The use of stimulants and atomoxetine in adults with comorbid ADHD and bipolar disorder. Expert Opinion on Pharmacotherapy, 16(14), 2193–2204. https://doi.org/10.1517/14656566.2015.1079620
[29]. Quinn, D., Wigal, S., Swanson, J., Hirsch, S., Ottolini, Y., Dariani, M., Roffman, M., Zeldis, J., & Cooper, T. (n.d.). Comparative Pharmacodynamics and Plasma Concentrations of d-threo-Methylphenidate Hydrochloride After Single Doses of d-threo-Methylphenidate Hydrochloride and d,l-threo-Methylphenidate Hydrochloride in a Double-Blind, Placebo-Controlled, Crossover Laboratory School Study in Children With Attention-Deficit/Hyperactivity Disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 43(11), 1422–1429. https://doi.org/10.1097/01.chi.0000140455.96946.2b
[30]. Quinn, D., Wigal, S., Swanson, J., Hirsch, S., Ottolini, Y., Dariani, M., Roffman, M., Zeldis, J., & Cooper, T. (n.d.). Comparative Pharmacodynamics and Plasma Concentrations of d-threo-Methylphenidate Hydrochloride After Single Doses of d-threo-Methylphenidate Hydrochloride and d,l-threo-Methylphenidate Hydrochloride in a Double-Blind, Placebo-Controlled, Crossover Laboratory School Study in Children With Attention-Deficit/Hyperactivity Disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 43(11), 1422–1429. https://doi.org/10.1097/01.chi.0000140455.96946.2b
[31]. Liu, Q., Zhang, H., Fang, Q., & Qin, L. (2017). Comparative efficacy and safety of methylphenidate and atomoxetine for attention-deficit hyperactivity disorder in children and adolescents: Meta-analysis based on head-to-head trials. Journal of Clinical and Experimental Neuropsychology, 39(9), 854–865. https://doi.org/10.1080/13803395.2016.1273320









