1. Introduction
In organic synthesis, solvents not only serve as reaction media, but are also key factors affecting reaction rates, selectivity, and product yields. They not only dilute the reactants to react at a reasonable concentration, but also provide a homogeneous environment for reagents, dissolve catalysts, sometimes provide a proton transfer environment to drive the reaction, and help control the temperature and diffusion of the reactants, and of course sometimes participate in the reaction.
However, most conventional solvents such as dichloromethane, toluene, and N,N-dimethylformamide (DMF) are volatile, toxic, and environmentally persistent. Their use contributes significantly to air pollution, occupational health risks, and hazardous waste generation. In many industrial and laboratory processes, solvents account for the majority of material input and post-reaction waste. As a result, solvent management has become one of the primary environmental challenges in organic chemistry. In light of these concerns, the development and adoption of green solvents—those that are safer, more sustainable, and less harmful to human health and the environment—has become a central goal in the advancement of sustainable chemistry. This review highlights the potential hazards associated with traditional organic solvents and aims to identify recent studies on green solvents that align with the arguments presented in this article and have demonstrated practical applications. this study aims to provide an overview of the recent advances in the use of green solvents in organic synthesis.
2. Standards and selection of green solvents
2.1. Problems faced by traditional organic solvents
It is estimated that solvents account for over 50%–90% of the total mass used in many chemical processes, especially in pharmaceutical and fine chemical industries [1]. The choice of solvent often determines not only the feasibility of a synthesis but also its scalability. Table 1 lists the usage of several common solvents in organic synthesis I used to carry on in the laboratory.
|
Reaction type |
Substrates |
Solvents (*: Solvent participates reaction) |
Suitable concentration of reactants (mol/L) |
Solvent ratio (mass ratio, %) |
|
Lithium aluminum hydride reduction of esters |
diethyl 1H-pyrrole-3,4-dicarboxylate |
THF |
0.1-0.3 |
93-97 |
|
Skraup Reaction |
o-Aminophenol |
Glycerol + H2SO4* |
2.08 |
86.1 |
|
Oxidation of alcohols |
Benzoin |
Acetic Acid |
0.95 |
84.0 |
|
Nitration of aromatic compounds |
Acetanilide |
Acetic Acid + H2SO4 |
1.8 |
82.5 |
|
Vilsmeier-Haack Reaction |
Styrene |
DMF* |
0.18 |
73.2 |
|
Grignard Reaction |
Bromobenzene |
THF |
2.04 |
60.0 |
However, many traditional solvents such as dichloromethane, toluene, or N,N-dimethylformamide (DMF) are volatile, toxic, and difficult to recycle. These solvents pose serious risks to human health and the environment, contributing to air pollution, flammability hazards, and the generation of hazardous waste. Table 2 lists the general hazards of some common organic solvents [2].
|
Solvent |
VOC Emission |
Flammable |
Carcinogenic |
Reproductive Toxicity |
Acute Toxicity |
Hard to Recycle |
Environmental Persistence |
|
Ethanol |
✔️ |
✔️ |
✖️ |
✖️ |
✖️ |
✖️ |
✖️ |
|
Acetone |
✔️ |
✔️ |
✖️ |
✖️ |
✖️ |
✖️ |
✖️ |
|
Tetrahydrofuran |
✔️ |
✔️ |
✖️ |
✖️ |
✖️ |
✖️ |
✖️ |
|
Dichloromethane (DCM) |
✔️ |
✖️ |
✔️ |
✖️ |
✔️ |
✔️ |
✔️ |
|
Ethyl Acetate (EA) |
✔️ |
✔️ |
✖️ |
✖️ |
✖️ |
✖️ |
✖️ |
|
N,N-Dimethylformamide (DMF) |
✔️ |
✖️ |
✖️ |
✔️ |
✔️ |
✔️ |
✔️ |
|
Toluene |
✔️ |
✔️ |
✖️ |
✔️ |
✔️ |
✖️ |
✔️ |
|
Benzene |
✔️ |
✔️ |
✔️ |
✔️ |
✔️ |
✔️ |
✔️ |
|
Petroleum ether (PE) |
✔️ |
✔️ |
✖️ |
✖️ |
✔️ |
✖️ |
✔️ |
|
Acetonitrile |
✔️ |
✔️ |
✖️ |
✖️ |
✔️ |
✖️ |
✖️ |
√: The solvent has a significant risk in this hazard dimension.
×: The solvent has a low risk or no significant risk in this hazard dimension.
Therefore, the development and application of greener solvents—such as water, bio-based solvents, ionic liquids, and supercritical fluids—has become a critical direction in modern organic synthesis. These alternative solvents aim to reduce environmental impact while maintaining or even improving synthetic efficiency.
Green solvents are solvents that are used to reduce the impact on human health and the environment during chemical synthesis and industrial processes [3]. They embody the core concept of green chemistry, which focuses on reducing or eliminating the use and generation of hazardous substances throughout the entire chemical life cycle, from raw material acquisition and production to usage and final disposal.
2.2. Selection criteria for green solvents
The selection criteria for green solvents are highly consistent with the principles of green chemistry. Green chemistry aims to reduce or eliminate the hazards of chemicals and processes to the environment and human health from the source. The "12 Principles of Green Chemistry" proposed by the US Environmental Protection Agency (EPA) and Anastas et al. are widely regarded as an important framework for guiding green chemistry practices Among them, the principles most closely related to green solvents include the following:.
Principle 4 shows the most direct relevance to green solvents, emphasizing the importance of selecting solvents that are safer for human health and the environment, while avoiding those that are toxic, highly volatile, or difficult to degrade. Principle 1 highlights the need to prevent waste, which supports the use of recyclable and reusable solvents to reduce overall solvent consumption and waste generation. Principle 3 supports the design of less hazardous syntheses, where replacing traditional solvents with greener alternatives plays a key role. Principle 5 encourages improved energy efficiency, as some green solvents such as water or supercritical CO₂ enable reactions to proceed under milder conditions. Principle 6 promotes the use of renewable feedstocks, which aligns with bio-based solvents derived from biomass. Principle 7 focuses on designing degradable chemicals, implying that green solvents should be environmentally benign and readily biodegradable [4].
Therefore, the so-called "green solvents" should generally have the following core characteristics: low toxicity, low volatility, biodegradability, renewable sources, low impact on the environment, good reaction compatibility and economic feasibility.
2.3. Introduction to green solvent screening guide
The Solvent Sustainability Guide (GSK), proposed and updated multiple times by pharmaceutical companies such as GlaxoSmithKline, has undergone significant revisions, with the latest update in 2016. This update added 44 new solvents, including those identified as "green" in various literatures. The scoring method was refined, and the toxicology and health hazard data were updated, adopting the GHS classification standard to replace the EU risk phrases (R phrases). In this guideline, GSK evaluates solvents based on four major categories: waste, environmental impact, health, and safety, each containing multiple subcategories. The Composite Score is calculated using the geometric mean, classifying solvents into three categories: green (>7.5), amber (3.5-7.5), and red (<3.5) [5].
This guide offers scientists an intuitive solvent selection tool through systematic scoring and color coding, making it more convenient and accurate than relying solely on physical and chemical properties. Future directions include improving life cycle assessment (LCA) data, expanding applications to other fields like analytical chemistry and biology, and promoting international cooperation to standardize industry practices.
However, challenges remain, including inconsistencies in physical data, limited practical use of some new solvents, the need to adapt to evolving regulations, and the risk that overall scores may overlook specific strengths or weaknesses of individual solvents [5].
3. The application progress of several typical green solvents
3.1. Water as solvent
Water is an ideal green solvent due to its non-toxicity, abundance, non-flammability, and minimal environmental impact. Despite early concerns about the incompatibility of water with many organic reagents and catalysts, recent advances have demonstrated that water can support a wide range of organic transformations with improved sustainability. Notably, transition metal-catalyzed coupling reactions—once thought to require strictly anhydrous conditions—can now be carried out efficiently in aqueous media under optimized conditions.
The development of more efficient catalysts suitable for aqueous reactions is the key to adapting classic organic synthesis reactions to aqueous systems. One of the most noteworthy research results is a new type of Pd-C3N4 at titanate nanotube (TNT) catalyst reported by Velpula, Venkata Ramana Kumar and others in 2019, which is believed to have a satisfactory catalytic effect on aqueous Suzuki-Miyaura coupling and Heck coupling [6]. This novel heterogeneous catalyst, Pd-C₃N₄@TNT, features palladium anchored on a triazine-structured C₃N₄ layer supported by protonated titanate nanotubes (H-TNT). It is synthesized in four steps: hydrothermal treatment of TiO₂ to Na-TNT, acid treatment to form H-TNT, thermal calcination with urea to grow C₃N₄, and palladium loading using palladium acetate. The structure and synthetic route of the catalyst are shown in Figure 1. This catalyst demonstrates excellent activity in aqueous-phase Heck and Suzuki–Miyaura couplings. For the Mizoroki–Heck reaction, 1 mmol acrylate and iodobenzene, 5 mol% TBAI, 10 mg catalyst, and 3 mL water yield nearly 100% conversion in 45 minutes at 75–80 °C. Similarly, Suzuki-Miyaura coupling using phenylboronic acid and iodobenzene in 3 mL water–ethanol (2:1) at 80 °C for 1 hour achieves almost quantitative yield [6]. The catalyst tolerates various substituents and retains activity after five cycles with no significant Pd leaching, indicating high stability and recyclability.
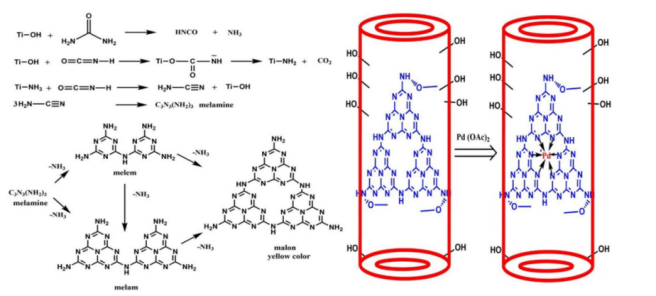
In addition, modifying water by physical methods is also a common method. For example, Mohammad Bakherad et al. reported in 2020 a metal-free, alkali-free organic synthesis method using magnetized distilled water (MDW) for the synthesis of aromatic amine compounds.
Magnetized distilled water (MDW) is prepared by placing a test tube of 5 mL of ordinary distilled water in a 0.8 T magnetic field for 20 minutes. It is used in a metal-free, base-free N-arylation reaction, that is, the synthesis of aromatic amine compounds through the reaction of aromatic halides with secondary amines. This reaction is carried out in magnetized water, and a variety of aromatic halides (including iodobenzene, bromobenzene, chlorobenzene, etc.) and secondary amines (such as morpholine, piperidine, etc.) can react efficiently, with reaction yields ranging from 60% to 98% [7].
Compared with the traditional Buchwald-Hartwig coupling reaction, it does not require transition metal catalysts and toluene solvents, and has the advantages of high efficiency, environmental protection, and low cost, providing a green chemistry alternative for the synthesis of aromatic amine compounds.
3.2. Ionic liquid
Ionic liquids (ILs), typically composed of organic cations and inorganic or organic anions, have attracted considerable attention as alternative solvents due to their unique physicochemical properties—such as negligible vapor pressure, high thermal stability, and tunable polarity. Unlike conventional volatile organic solvents, ionic liquids significantly reduce the risk of air pollution and flammability. Their structural flexibility also allows the design of task-specific media for various reactions. These features make ionic liquids promising candidates in the context of green chemistry.
Ionic liquids exhibit excellent properties in enzymatic reactions which is a promising field in organic synthesis. For instance, a study from Sichuan University in 2014 utilized 1-butyl-3-methylimidazole hexafluorophosphate ([BMIM][PF6]) as the reaction medium to conduct an asymmetric cross-aldol condensation reaction of aromatic aldehydes or heteroaromatic aldehydes with various ketones catalyzed by lipase. This study achieved an impressive yield of 99%, an enantiomeric excess (ee) of 90%, and a diastereomeric ratio (dr) greater than 99:1. [8].
[BMIM][PF6] as media have excellent recyclability and reusability, avoiding the use of traditional organic solvents and reducing the emission of volatile organic compounds (VOCs), making it more environmentally friendly. While enzymatic reactions face limitations in organic synthesis—such as enzyme specificity, poor stability, and challenges in recycling—recent advancements, including protein engineering and enzyme immobilization, have addressed these issues to some extent [9].
Microwave-assisted organic synthesis has greatly promoted the innovation of organic synthesis due to its advantages such as short reaction time and high yield. Ionic liquids, with their low volatility, non-flammability, high thermal stability and versatility in solubility, complement microwave synthesis effectively. A study from Mohanlal Sukhadia University shows that the research team has successfully synthesized a variety of heterocyclic compounds in ionic liquids by microwave method, including imidazole, tetrazole, indole, quinoline, pyridine, etc. While significantly shortening the reaction time, good yields have been obtained [10].
Although ionic liquids have low volatility and recyclability, which can reduce the environmental impact of solvent use, many types of ionic liquids are significantly toxic to aquatic organisms and may have adverse effects on human cells. Moreover, many ionic liquids are difficult to degrade in the environment, so caution should be taken when using them. In the future, the development of ionic liquids with lower toxicity and degradability will remain a core issue in this regard.
3.3. Deep eutectic solvent (des)
Deep eutectic solvents (DESs), typically formed by mixing hydrogen bond donors and acceptors, have attracted growing attention as alternative green solvents. Compared with ionic liquids, DESs are generally cheaper, easier to prepare, and often more biodegradable. Although they share similar physicochemical properties, such as low volatility and tunable polarity, DESs offer improved sustainability profiles, making them an increasingly attractive option in green organic synthesis.
In 2024, a new study reported a sustainable and scalable method for synthesizing various functionalized sulfonamides, which are key structural features in many pharmacologically active compounds. Researchers have developed a new method for synthesizing sulfonamides using a switchable deep eutectic solvent (DESs) based on choline chloride (ChCl). Here, "switchable" refers to the control of the reaction process by simply changing the composition or conditions of the deep eutectic solvent to adjust its properties. The researchers selected two types of DESs: ChCl/ glycerol and ChCl/ urea, in which the amine and sulfonyl chloride were reacted. After optimizing the conditions, the reaction could be carried out at normal temperature and pressure, and a yield as high as 97% was achieved [11].
This method is applicable to the synthesis of various aromatic and aliphatic amines as well as sulfonyl chlorides, including halogenated sulfonamides, which are of great significance in medicinal chemistry. By employing non-volatile and non-flammable deep eutectic solvents (DESs), the method reduces the reliance on traditional organic solvents, thereby minimizing environmental impact.
However, DES generally has a relatively high viscosity, which may affect mass transfer. Moreover, its solubility for non-polar molecules and macromolecules is limited. Attention should be paid when using it. At present, there are no unified standards for the classification, purity, toxicological assessment and other aspects of DES, which limits its application to a certain extent. Nonetheless, as an environmentally friendly alternative to conventional organic solvents, DESs are gaining increasing attention in the field.
3.4. Supercritical fluid
Supercritical fluids (SCFs), characterized by gas-like diffusivity and liquid-like solvating power, have gained attention as alternative media for green chemistry. Among them, supercritical carbon dioxide (Sc-CO₂) is particularly attractive due to its low toxicity, non-flammability, and mild critical conditions. It has been successfully applied in various organic transformations. The following example illustrates an efficient organic synthesis performed in Sc-CO₂, highlighting its potential as a sustainable reaction medium.
A study published in 2024 demonstrated that the research team, in a supercritical carbon dioxide (Sc-CO₂) reaction medium, catalyzed the synthesis of benzyl n-butyrate from benzyl n-butyrate donors using steapsin (a type of lipase). Among the butyryl donors, the conversion rate of vinyl butyrate was the highest. After optimizing the reaction conditions and adding additives, it reached 99%, which was nearly four times higher than that of the reaction carried out in traditional organic solvents [12].
In Sc-CO₂, the activity and stability of steapsin were better maintained, and it could be recycled multiple times, improving the economy of the catalyst. Furthermore, Sc-CO₂ is non-flammable and safer to use. After the reaction is completed, through a simple pressure reduction operation, Sc-CO₂ can also be rapidly separated from the reaction system without the need for a complex post-treatment process, reducing the production cost [12]. Due to the above advantages, this reaction has been able to achieve continuous industrial production to a certain extent, reflecting the emerging research topic of the industrial application of enzymatic reactions.
3.5. Bio-based solvents
Bio-based solvents, derived from renewable feedstocks such as cellulose, sugars, and fatty acids, have emerged as promising alternatives to petroleum-derived solvents. These solvents often offer reduced toxicity, improved biodegradability, and a lower carbon footprint. Among them, Cyrene, a dihydrolevoglucosenone-based solvent, has attracted considerable interest for its excellent solvating ability and benign environmental profile. The following study demonstrates its effectiveness in promoting sustainable organic transformations.
Cyrene can be simply prepared from biomass through a two-step reaction. First, levoglucosenone (LGO) is generated through the pyrolysis of biomass, and then LGO is reduced to Cyrene through hydrogenation.
Cyrene is used as a solvent for a variety of reactions, including but not limited to: the Sonogashira reaction, the Suzume-Miyaura reaction, the Bayli-Hillman reaction, and the synthesis of metal-organic frameworks (MOF), all achieving reaction effects similar to those of traditional solvents. As a renewable bio-based solvent, cyrene is non-toxic, non-mutagenic and biodegradable. These characteristics make it have a relatively small impact on the environment and meet the requirements of sustainable development. Cyrene is miscible with water and can be separated from the reaction mixture through simple aqueous phase extraction, which is convenient for recovery and reuse. These properties make cyrene a satisfactory green solvent choice [13].
However, due to the presence of ketone groups in the chemical structure of Cyrene, it may participate in certain reactions, which limits its application range as a solvent. In some reactions, the solubility of Cyrene for reactants or products may be lower than that of traditional solvents, and it is necessary to further optimize the reaction conditions or add cosolvents. Although Cyrene is derived from biomass, its production cost may be higher than that of some traditional solvents, which may limit its application in large-scale industrial production [14]. In fact, these problems also represent the general issues of bio-based solvents. With the development of green chemistry, further developing new bio-based solvents that can solve these problems will be an attractive topic.
4. Conclusion
In recent years, significant progress has been made in the development and application of green solvents for organic synthesis. This review has summarized the characteristics, design principles, and typical applications of several major categories of green solvents, including water, ionic liquids, deep eutectic solvents (DESs), supercritical fluids, and bio-based solvents. These solvents offer diverse pathways to reduce the environmental and health impacts associated with traditional organic solvents, aligning with the broader goals of green chemistry.
Despite their many advantages, the widespread adoption of green solvents still faces several challenges. Many green solvents are associated with higher costs, limited availability, or compatibility issues with certain reactions. Moreover, some candidates, such as ionic liquids and DESs, may pose concerns regarding toxicity, viscosity, or standardization. Nonetheless, with the global emphasis on sustainable development, the pursuit of safer, cleaner, and more renewable solvent systems is not only desirable but inevitable.
Regrettably, due to time constraints, laboratory arrangements and other reasons, several confirmatory experiments arranged by the author could not be carried out, so this research remains at the paper stage. Further reasearch should focus on conducting verification experiments, and the understanding and conclusions of green solvents may be further deepened.
Looking ahead, the search for greener solvents will remain a critical and evolving area of research in organic chemistry. Continued efforts in solvent design, life cycle assessment, and multidisciplinary collaboration are essential to overcome current limitations and accelerate practical applications. Solvent selection must increasingly incorporate environmental, health, and safety (EHS) data in a systematic and transparent manner to guide future innovation. Ultimately, the quest for truly sustainable solvent systems is not just a chemical challenge—but a scientific responsibility.
References
[1]. Cai, L. Z. (Ed. ), Yu, Y. , Wang, Y. R. , & Xiong, Y. (2019). Daxue jichu huaxue shiyan (II) [College Basic Chemistry Experiments (II)] (3rd ed. ). Chemical Industry Press.
[2]. National Center for Biotechnology Information. (2024). PubChem Compound Summary. U. S. National Library of Medicine. https://pubchem. ncbi. nlm. nih. gov.
[3]. Winterton, N. (2021). The green solvent: a critical perspective. Clean Technologies and Environmental Policy, 23(1), 2499–2522. https://doi. org/10. 1007/s10098-021-02188-8.
[4]. U. S. Environmental Protection Agency. (2023). Basics of green chemistry. https://www. epa. gov/greenchemistry/basics-green-chemistry.
[5]. Alder, C. M. , Hayler, J. D. , Henderson, R. K. , Redman, A. M. , Shukla, L. , Shuster, L. E. , & Sneddon, H. F. (2016). Updating and further expanding GSK’s solvent sustainability guide. Green Chemistry, 18(13), 3879–3890. https://doi. org/10. 1039/c6gc00611f
[6]. Velpula, V. R. K. , Ketike, T. , Paleti, G. et al. (2020). A Facile Synthesis of Pd–C3N4@Titanate Nanotube Catalyst: Highly Efficient in Mizoroki–Heck, Suzuki–Miyaura C–C Couplings. Catal Lett 150, 95–105. https://doi. org/10. 1007/s10562-019-02955-9.
[7]. Bakherad, M. , Moosavi-Tekyeh, Z. , Rezaeifard, A. , Doosti, R. , Mehmandoost, N. , Goudarzi, N. , & Omara, S. (2020). Metal-free green synthesis of aryl amines in magnetized distilled water: Experimental aspects and molecular dynamics simulation. Green Chemistry. https://doi. org/10. 1039/d0gc01329c.
[8]. Zhang, Y. , Wang, N. , Xie, Z. -B. , Zhou, L. -H. , & Yu, X. -Q. (2014). Ionic liquid as a recyclable and efficient medium for lipase-catalyzed asymmetric cross aldol reaction. Journal of Molecular Catalysis B: Enzymatic, 110, 100–110. https://doi. org/10. 1016/j. molcatb. 2014. 10. 008.
[9]. Itoh, T. (2017). Ionic liquids as tool to improve enzymatic organic synthesis. Chemical Reviews, 117(10), 10567−10607. https://doi. org/10. 1021/acs. chemrev. 7b0017.
[10]. Pathak, A. K. , Ameta, C. , Ameta, R. , & Punjabia, P. B. (2016). Microwave-assisted organic synthesis in ionic liquids. Journal of Heterocyclic Chemistry, 53(11), 1697−1705. https://doi. org/10. 1002/jhet. 2515.
[11]. Simone, M. , Pulpito, M. , Perna, F. M. , Capriati, V. , & Vitale, P. (2024). Switchable deep eutectic solvents for sustainable sulfonamide synthesis. Chemistry - A European Journal, 30(e202402293). https://doi. org/10. 1002/chem. 202402293.
[12]. Badgujar, K. C. , Badgujar, J. K. , & Bhanage, B. M. (2024). Improved biocatalytic activity of steapsin lipase in supercritical carbon dioxide medium for the synthesis of benzyl butyrate: A commercially important flavour compound. Journal of Biotechnology, 384, 55–62. https://doi. org/10. 1016/j. jbiotec. 2024. 02. 010.
[13]. Stini, N. A. , Gkizis, P. L. , & Kokotos, C. G. (2022). Cyrene: a bio-based novel and sustainable solvent for organic synthesis. Green Chemistry, 24(24), 6435–6449. https://doi. org/10. 1039/d2gc02332f.
[14]. Musarurwa, H. , & Tavengwa, N. T. (2021). Emerging green solvents and their applications during pesticide analysis in food and environmental samples. Talanta, 223, 121507. https://doi. org/10. 1016/j. talanta. 2020. 121507
Cite this article
Zheng,B. (2025). A Review of the Application of Green Solvents in Organic Synthesis. Theoretical and Natural Science,119,16-24.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICEGEE 2025 Symposium: Sensor Technology and Multimodal Data Analysis 2025
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Cai, L. Z. (Ed. ), Yu, Y. , Wang, Y. R. , & Xiong, Y. (2019). Daxue jichu huaxue shiyan (II) [College Basic Chemistry Experiments (II)] (3rd ed. ). Chemical Industry Press.
[2]. National Center for Biotechnology Information. (2024). PubChem Compound Summary. U. S. National Library of Medicine. https://pubchem. ncbi. nlm. nih. gov.
[3]. Winterton, N. (2021). The green solvent: a critical perspective. Clean Technologies and Environmental Policy, 23(1), 2499–2522. https://doi. org/10. 1007/s10098-021-02188-8.
[4]. U. S. Environmental Protection Agency. (2023). Basics of green chemistry. https://www. epa. gov/greenchemistry/basics-green-chemistry.
[5]. Alder, C. M. , Hayler, J. D. , Henderson, R. K. , Redman, A. M. , Shukla, L. , Shuster, L. E. , & Sneddon, H. F. (2016). Updating and further expanding GSK’s solvent sustainability guide. Green Chemistry, 18(13), 3879–3890. https://doi. org/10. 1039/c6gc00611f
[6]. Velpula, V. R. K. , Ketike, T. , Paleti, G. et al. (2020). A Facile Synthesis of Pd–C3N4@Titanate Nanotube Catalyst: Highly Efficient in Mizoroki–Heck, Suzuki–Miyaura C–C Couplings. Catal Lett 150, 95–105. https://doi. org/10. 1007/s10562-019-02955-9.
[7]. Bakherad, M. , Moosavi-Tekyeh, Z. , Rezaeifard, A. , Doosti, R. , Mehmandoost, N. , Goudarzi, N. , & Omara, S. (2020). Metal-free green synthesis of aryl amines in magnetized distilled water: Experimental aspects and molecular dynamics simulation. Green Chemistry. https://doi. org/10. 1039/d0gc01329c.
[8]. Zhang, Y. , Wang, N. , Xie, Z. -B. , Zhou, L. -H. , & Yu, X. -Q. (2014). Ionic liquid as a recyclable and efficient medium for lipase-catalyzed asymmetric cross aldol reaction. Journal of Molecular Catalysis B: Enzymatic, 110, 100–110. https://doi. org/10. 1016/j. molcatb. 2014. 10. 008.
[9]. Itoh, T. (2017). Ionic liquids as tool to improve enzymatic organic synthesis. Chemical Reviews, 117(10), 10567−10607. https://doi. org/10. 1021/acs. chemrev. 7b0017.
[10]. Pathak, A. K. , Ameta, C. , Ameta, R. , & Punjabia, P. B. (2016). Microwave-assisted organic synthesis in ionic liquids. Journal of Heterocyclic Chemistry, 53(11), 1697−1705. https://doi. org/10. 1002/jhet. 2515.
[11]. Simone, M. , Pulpito, M. , Perna, F. M. , Capriati, V. , & Vitale, P. (2024). Switchable deep eutectic solvents for sustainable sulfonamide synthesis. Chemistry - A European Journal, 30(e202402293). https://doi. org/10. 1002/chem. 202402293.
[12]. Badgujar, K. C. , Badgujar, J. K. , & Bhanage, B. M. (2024). Improved biocatalytic activity of steapsin lipase in supercritical carbon dioxide medium for the synthesis of benzyl butyrate: A commercially important flavour compound. Journal of Biotechnology, 384, 55–62. https://doi. org/10. 1016/j. jbiotec. 2024. 02. 010.
[13]. Stini, N. A. , Gkizis, P. L. , & Kokotos, C. G. (2022). Cyrene: a bio-based novel and sustainable solvent for organic synthesis. Green Chemistry, 24(24), 6435–6449. https://doi. org/10. 1039/d2gc02332f.
[14]. Musarurwa, H. , & Tavengwa, N. T. (2021). Emerging green solvents and their applications during pesticide analysis in food and environmental samples. Talanta, 223, 121507. https://doi. org/10. 1016/j. talanta. 2020. 121507









