1. Introduction
At present, many studies are devoted to exploring methods to treat or slow down disease progression by regulating the phagocytic ability of microglia. Tinospora cordifolia (TC), as a plant that has been proven to have immune regulatory ability, is a promising candidate for phagocytic activity regulation [1]. Therefore, this study investigated the effect of TC extract on microglial phagocytosis to evaluate its potential as an inflammation regulating agent.
Microglia, an immune cell in the central nervous system, is tightly associated with various neurological diseases [2]. After detecting stimuli in the environment, microglia adopt a mobile cellular state and can move around in search for 'eat-me’ signals exposed on the membrane of target cells and then recognize and bind to the targets using various immune receptors expressed on its membrane to activate the process of phagocytosis [3].
During the developmental progress of some diseases, microglia appear to be beneficial at certain stages, then progress into a dysfunction and deleterious state [4]. Initially, microglia may show a moderate phagocytic ability for normal clearance of myelin debris and contribute to a suitable environment for the growth of oligodendrocyte precursor cells (OPCs), thus promoting remyelination [5]. OPCs will develop into oligodendrocytes, cells that form myelin sheathes that cover and insulate the axons of neurons in the CNS for faster signal transduction rate, which is crucial for maintaining normal effective brain activity [6]. Nevertheless, microglia may progress into a detrimental state under persistent infections at later stages of diseases and possess exaggerated phagocytic ability that can destroy the originally healthy axons, thus reducing the rate of signal processing and leading to the development of neurodegenerative diseases [4] [7]. In this context, pro-inflammatory cytokines produced by microglia may activate the full cytokine cascade, which can disrupt the function of the blood brain barrier by loosening endothelial tight junctions and contribute to massive cell death with elevated oxidative stress [8].
According to observations of a fluctuating microglial activity in certain diseases, determining the current stage of disease of a patient and then elevating or ameliorate the phagocytic activity of microglia accordingly may be a plausible treatment.
Tinospora cordifolia, a traditional herb in Ayurveda, has proved to have beneficial effects in treating neurodegenerative diseases [9]. Various extractions (e.g., ethanolic extract, aqueous extract) of its leaf, stem, and root were shown to contain validated components that have anti-oxidant and anti-cytotoxic effects [10]. Moreover, research supported that TC could inhibit the production of cytokines released by microglial NF-kB activation, a process where the transcription factor NF-kB recognizes and binds to the promotor on target genes that regulate the production of future cytokines, thus having a neuroprotective effect [11] [12]. In addition, experiments validated that ethyl acetate and water extract of the stem of TC at concentration of 0.1-2.5 μg/ml can significantly increase the percentage of phagocytosis of human neutrophils, a type of white blood cells on the frontline of the innate immune system in the peripheral immune system [13]. Since microglia and neutrophils have similar mechanisms of recognizing target cells, for instance, they both express Mer tyrosine kinase (MerTK) receptors on their membranes that can bind to phosphatidylserine (PS) exposed on the plasma membrane of target cells via the ligand GAS6 or Protein S [14] [15], it can be hypothesized that these extracts of TC stem within this range of concentrations may also promote the phagocytic ability of microglia, thus potentially elevating microglial performance in the stage of diseases with impaired phagocytic activity of microglia. However, the current paper covers experiments with mainly TC seed extracts due to issues with resource availability.
The Trypan Blue exclusion test was adopted as it could provide straightforward and easy-to-analyze data and is time efficient. pH-sensitive fluorescent microspheres were used to evaluate microglial phagocytic activity since their signals increase strengths at lower pH as were engulfed by microglia and they exhibit low background signals at relatively neutral pH as were not engulfed, thus reducing the dependence on washing out unengulfed microspheres.
Few studies have discussed TC extract’s impact on microglial phagocytosis, and previous researchers have not paid much attention to the effect of TC dry seed water extract on microglial phagocytosis. Thus, this research aims to explore the impact of different concentrations of TC seed water extract on the phagocytic activity of microglia and may provide additional insights.
2. Materials and methods
2.1. Cell line
Bv2 cells were obtained from Sunncell® (Sunncell Biotech, Wuhan, China) and maintained in Pricella® DMEM/F12 complete culture medium with 10% FBS + 1% P/S solution (Pricella Biotechnology, Wuhan, China) in T25 flasks at 37℃ with 5% CO2. Cells were passed after the density reached approximately 80%. During cell passage, previous culture medium was discarded and 3 mL Labshark® phosphate buffer saline (PBS; Bikeman Bio, Hunan, China) was added to cells. The flask was gently swirled for 1 min. PBS was then discarded and 2 mL 0.25% Servicebio® Trypsin (previously stored under -20℃; thawed under 37℃ in water bath; dissolved in D-Hank’s; contain EDTA; phenol red free) was added. The system was incubated under 37℃ and 5% CO2 for 2 min. Cells were observed under 100X and 5 mL complete culture medium was added to stop digestion if most cells detached. Supernatant was collected into 15 mL centrifuge tubes and was centrifuged under 1000 rmp for 3 min. Supernatant was discarded and cells were resuspended with 4 mL complete culture medium. Cells were typically passed in a ratio of 1:2 or 1:4 into new T25 flasks with 10 mL complete culture medium.
2.2. Extract preparation
TC fresh seeds were left at RT to dry for two weeks, then grinder is used to obtain fine powders of dry seeds. 0.2 g powders were measured with analytical balance and added into 100 ml distilled water (thus achieved an initial concentration of 2 mg/ml) and the mixture was soaked at RT overnight. 12 hours later the mixture was boiled for 30 min with alcohol lamp and cooled to RT before the supernatant was purified with needle filters (aperture = 2μm; Tianjin keyilonglab Equipment, Tianjin, China). Prepared solutions were used immediately.
2.3. Cytotoxicity measurement
Bv2 cells were planted in TC-treated 12-well plate (50000 cells per well) in 1.5 mL complete culture medium and incubated for three nights under 37℃ and 5% CO2. Before the experiment, culture medium is removed from each well and 1.5 mL extract with planned concentrations diluted in complete culture medium is added to wells. The system was then incubated for 2 h under 37℃ and 5% CO2 with enough moisture. Liquid is removed and 2 mL pre-cold (4℃) PBS solution was added to each well. Cell scraper was used to scrap up cells. Liquid in each well was mixed uniformly and 250 μL liquid from each well was collected into respective 1.5 mL EP tubes. 250 μL 0.4% Solarbio® Trypan Blue stain solution (Beijing, China) is added and the number of total and viable cells were determined with cell counting chamber. The percentage of viable cells was then calculated by dividing the number of viable cells with the number of total cells.
2.4. Phagocytic ability measurement
5000 Bv2 cells were planted in 200 μL complete culture medium in each well of the 96-well plate (TC treated) and were incubated for 3 nights for adhesion. Each empty well in margins were filled with 200 μL PBS to ameliorate the influence of evaporation.
On the day of the experiment, the prepared extract was diluted with pre-warmed (37℃) complete culture medium into desired concentrations (e.g., 0.1 μg/ml, 0.5 μg/ml, 1.0 μg/ml, 2.0 μg/ml). 100 μL diluted extract were added for each well after the original medium was removed and the system was incubated for 2 h under 37℃ and 5% CO2. pH-sensitive fluorescent microspheres (pHrodoTM E.coli BiopariclesTM, Thermo Fisher scientific, Waltham, MA, USA; stored at -20℃) were pre-warmed in water bath to 25℃ and were diluted in gibco® Hank’s balanced salt solution (Grand Island, NY, USA) [16]. The extract was not removed and 100 μL microspheres prepared at 50 μg/ mL were added on the wall of each well to avoid washing away the cells [17]. The system was then incubated for 2 h under 37℃ and 5% CO2. Relative fluorescent units (RFU) were measured at 560nm directly after the incubation with Spark® fluorescent plate reader (Tecan Trading AG, Switzerland).
2.5. Statistical analysis
Data were expressed as means ± standard error of measurement (SEM). GraphPad Prism version 10.1.2 for Windows (GraphPad Software, Boston, MA, USA, www.graphpad.com) was used to identify outliers with ROUT method (Q = 1%) and perform one-way analysis of variance (ANOVA) and Tukey’s test to compare the statistical significance of data between control and experimental groups. The level of significance (∗) was determined at P < 0.05.
3. Results
3.1. Cytotoxicity measurement
Since previous study revealed that TC stem hot water extract within the concentration range of 0.01 – 2.5 μg/ml resulted in a viability of at least 90% for human neutrophil cells [13], TC dry seed water extract with concentrations of 1.0 μg/ml, 2.0 μg/ml, and 5.0 μg/ml were selected for cytotoxicity analysis.
The mean percentage of viable cells under treatments with different concentrations of TC dry seed water extract and SEM are plotted with bar graph (Fig. 1). No outlier was identified.
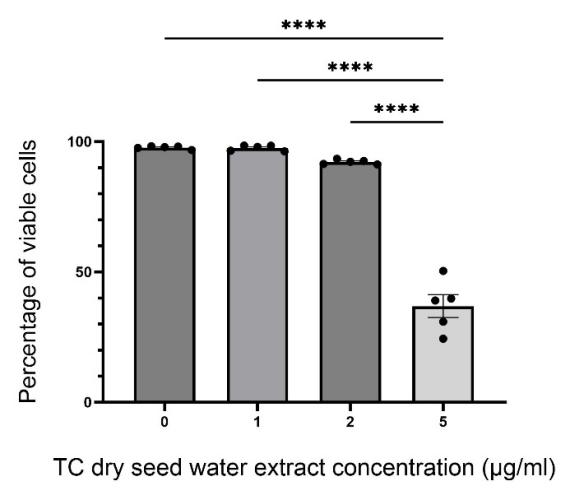
According to results, 1.0 μg/ml extract did not exhibit detectible cytotoxicity under 2 h incubation, as suggested by the insignificant difference of results as compared with the control group (p > 0.9999). 2.0 μg/ml extract showed certain visible cytotoxicity (average cell viability ≈ 92.23%) as reflected by reduced percentage of viable cells counted after 2 h incubation though the difference with the control group is still insignificant (p = 0.3309). 5.0 μg/ml extract eliminated about half of the cells during the period of incubation, indicating a significant cytotoxic effect towards Bv2 cells (p < 0.0001). The approximate range of concentration of extract (between 0.0 μg/ml and 2.0 μg/ml) that leads to the resulted percentage of viable cells that are larger than 90% is chosen for the following experiments of measuring phagocytic activity.
3.2. Measuring phagocytic activity
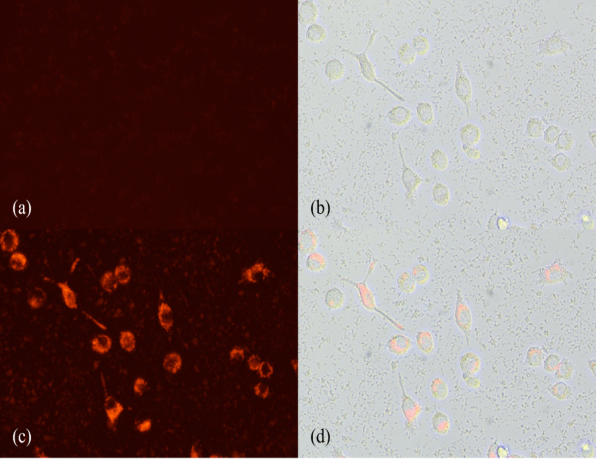
Results were measured with Spark® multimode microplate reader at 560nm. pH-sensitive fluorescent microspheres were excited to emit fluorescence when being engulfed by microglia into an acidic environment. No washing is required before the measurement since non-engulfed microspheres emit negligible signals in weak acidic environment of the culture medium (see Fig. 2). Washing could result in cell loss, especially due to the mobile nature of the 'active’ microglia. Emitted fluorescence at
585nm were captured. Results were recorded as RFU.
Mean RFU values detected from samples treated with different concentrations of TC dry seed water extract and SEM were plotted (see Fig. 3).
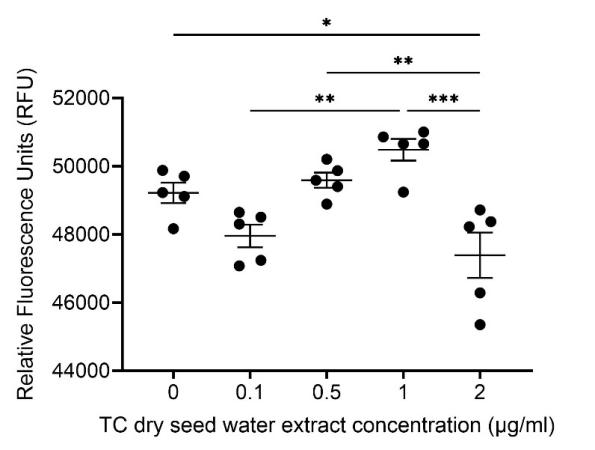
The value of RFU decreases first as the concentration of extract increases to 0.1 μg/ml and then increases till the concentration reaches 1.0 μg/ml, and finally decreases again as the concentration increases further to 2.0 μg/ml. A positive trend is shown for results of experimental groups treated with 0.5 μg/ml and 1.0 μg/ml extracts, and the means of RFU under these two conditions are larger than those obtained in the control group (0.0 μg/ml), indicating an increase of phagocytic activity.
Significant differences revealed between the control group and the group with the concentration of 2.0 μg/ml (p = 0.0289), groups with concentrations of 0.1 μg/ml and 1/0 μg/ml (p = 0.0018), groups with concentrations of 0.5 μg/ml and 2.0 μg/ml (p = 0.0068), and groups with concentrations of 1.0 μg/ml and 2.0 μg/ml (p = 0.0002).
4. Discussion
We found that 2.0 μg/ml TC dry seed water extract tends to reduce microglial phagocytosis without resulting in obvious cytotoxicity. Hence, the extract may be considered as a potential agent for mediating the phagocytic activity of microglia under inflammation, so as to protect healthy myelin sheathes and reduce negative influences caused by autoimmunity.
Moreover, the significant difference between groups with 1.0 μg/ml extract and 2.0 μg/ml extract reveals a changing effect of the extract on microglial phagocytosis with the tipping point lies between these concentrations. Extracts with increased concentrations tend to elevate the RFU value between concentrations of 0.1 μg/ml and 1.0 μg/ml.
Similar results were obtained from the research conducted by [13]for determining the effect of TC stem water extract on the phagocytic activity of human neutrophil cells. A generally positive trend was observed with the maximal effect at 0.5 μg/ml, and a generally negative trend was noted with higher doses.
One possible explanation for results obtained under 0.1 μg/ml extract is that the extract itself can slightly influence the intensity of fluorescent signals emitted by the microspheres, as revealed by a minuscule decrement of RFU value measured from the NC group with only 0.1 μg/ml extract and microspheres diluted in buffer solution in complete culture medium with Bv2 cells as compared with the control group with no extract. The extract may contribute to a more basic environment and thus reducing the 'sensitivity’ of pH-sensitive fluorescent microspheres since their signals are strengthened under acidic environment. In this case, even if 0.1 μg/ml extract can promote phagocytosis, the extent to which it elevates this ability may be unable to fight back the effect of increasing pH of the medium, thus resulting in weaker overall fluorescent signals (background signals are reduced in more basic environment) detected as compared with the control group. The other reasons may include that extract with this concentration reduces microglial phagocytosis due to cellular mechanisms which are currently unclear and that 0.1 μg/ml extract will not significantly influence the pH-environment of the medium. Also, since groups with lower extract concentrations are placed on the left side of the 96-well plate and are therefore prepared earlier, culture medium in these groups were exposed to the air for longer time than groups with larger concentrations on the right side. Extended exposure to air may resulting in more CO2 release in the culture medium, thus elevating the pH which may reduce the intensity of signals emitted by the microspheres in the background.
More future experiments may be performed for replications and more replicates for each concentration may be designed. The concentration gradient of 1.5 μg/ml may be added to evaluate the more precise tipping point at which the effect of extract on phagocytosis changes since current experiments reveal that the change occurs in a certain point between 1.0 μg/ml and 2.0 μg/ml. In addition, experiments to explore the possible underlying mechanisms of changing phagocytic ability may be performed. For instance, western blot may be adopted for assessing the expression of mer receptor tyrosine kinase (MerTK), a sensor for apoptotic phosphatidylserine which links directly to phagocytic activity.
5. Conclusion
To study the impact of TC dry seed water extract on the phagocytic activity of microglia, the Trypan Blue exclusion assay was used in this research to determine the extract’s cytotoxicity, and therefore confirmed the suitable concentration range of 0.0 – 2.0 μg/ml. Afterwards, pH-sensitive fluorescent microspheres were used to evaluate the extract’s impact on microglial phagocytosis, as indicated by RFU values. Results indicate that 2.0 μg/ml TC dry seed water extract can significantly reduce the phagocytic activity of microglia without triggering an obvious decrease in cell viability. Therefore, TC dry seed water extract with a concentration of 2.0 μg/ml may function as a potential therapeutic agent that can modulate microglial activity and thereby protect the healthy myelin sheath, slowing down the progression of diseases associated with abnormal microglial phagocytosis.
References
[1]. Yates, C. R., Bruno, E. J., & Yates, M. E. D. (2022). Tinospora Cordifolia: A review of its immunomodulatory properties. Journal of Dietary Supplements, 19(2), 271–285. https: //doi.org/10.1080/19390211.2021.1873214
[2]. Song, W. M., & Colonna, M. (2018). The identity and function of microglia in neurodegeneration. Nature Immunology, 19(10), 1048–1058. https: //doi.org/10.1038/s41590-018-0212-1
[3]. Richard M. Ransohoff & Melissa A. Brown. (2012). Innate immunity in the central nervous system. Journal of Clinical Investigation, 122(4), 1164–1171. https: //doi.org/10.1172/JCI58644
[4]. Suzanne Hickman, Izzy, S., Sen, P., Morsett, L., & El Khoury, J. (2018). Microglia in neurodegeneration. Nature Neuroscience, 21(10), 1359–1369. https: //doi.org/10.1038/s41593-018-0242-x
[5]. Amy F. Lloyd, & Miron, V. E. (2019). The pro-remyelination properties of microglia in the central nervous system. Nature Reviews Neurology, 15(8), 447–458. https: //doi.org/10.1038/s41582-019-0184-2
[6]. Sarah A. Kent, & Miron, V. E. (2024). Microglia regulation of central nervous system myelin health and regeneration. Nature Reviews Immunology, 24(1), 49–63. https: //doi.org/10.1038/s41577-023-00907-4
[7]. Salter, M. W., & Stevens, B. (2017). Microglia emerge as central players in brain disease. Nature Medicine, 23(9), 1018–1027. https: //doi.org/10.1038/nm.4397
[8]. Siamon Gordon, & Martinez, F. O. (2010). Alternative Activation of Macrophages: Mechanism and Functions. Immunity, 32(5), 593–604. https: //doi.org/10.1016/j.immuni.2010.05.007
[9]. Vipin V. Dhote, Kilor, V. A., Mohan Maruga Raja, M. K., Singhai, A., Mandloi, A. S., & Upaganlawar, A. B. (2023). Effect of Tinospora cordifolia on neuroinflammation. In Treatments, Nutraceuticals, Supplements, and Herbal Medicine in Neurological Disorders (pp. 601–621). Elsevier. https: //doi.org/10.1016/B978-0-323-90052-2.00019-6
[10]. Anuradha Sharma, Bajaj, P., Bhandari, A., & Kaur, G. (2020). From ayurvedic folk medicine to preclinical neurotherapeutic role of a miraculous herb, Tinospora cordifolia. Neurochemistry International, 141, 104891. https: //doi.org/10.1016/j.neuint.2020.104891
[11]. Syed Afroz Ali, & Datusalia, A. K. (2024). Protective effects of Tinospora cordifolia miers extract against hepatic and neurobehavioral deficits in thioacetamide-induced hepatic encephalopathy in rats via modulating hyperammonemia and glial cell activation. Journal of Ethnopharmacology, 323, 117700. https: //doi.org/10.1016/j.jep.2023.117700
[12]. Hareram Birla, Rai, S. N., Singh, S. S., Zahra, W., Rawat, A., Tiwari, N., Singh, R. K., Pathak, A., & Singh, S. P. (2019). Tinospora cordifolia Suppresses Neuroinflammation in Parkinsonian Mouse Model. NeuroMolecular Medicine, 21(1), 42–53. https: //doi.org/10.1007/s12017-018-08521-7
[13]. Upendra Sharma, Bala, M., Kumar, N., Singh, B., Munshi, R. K., & Bhalerao, S. (2012). Immunomodulatory active compounds from Tinospora cordifolia. Journal of Ethnopharmacology, 141(3), 918–926. https: //doi.org/10.1016/j.jep.2012.03.027
[14]. Dorion, M.-F., Yaqubi, M., Senkevich, K., Kieran, N. W., MacDonald, A., Chen, C. X. Q., Luo, W., Wallis, A., Shlaifer, I., Hall, J. A., Dudley, R. W. R., Glass, I. A., Birth Defects Research Laboratory, Stratton, J. A., Fon, E. A., Bartels, T., Antel, J. P., Gan-or, Z., Durcan, T. M., & Healy, L. M. (2024). MerTK is a mediator of alpha-synuclein fibril uptake by human microglia. Brain, 147(2), 427–443. https: //doi.org/10.1093/brain/awad298
[15]. Guignant, C., Venet, F., Planel, S., Demaret, J., Gouel-Chéron, A., Nougier, C., Friggeri, A., Allaouchiche, B., Lepape, A., & Monneret, G. (2013). Increased MerTK expression in circulating innate immune cells of patients with septic shock. Intensive Care Medicine, 39(9), 1556–1564. https: //doi.org/10.1007/s00134-013-3006-9
[16]. Lee, J.-W. (2024). Enhanced phagocytosis associated with multinucleated microglia via Pyk2 inhibition in an acute β-amyloid infusion model.
[17]. Lindner, B., Burkard, T., & Schuler, M. (2020). Phagocytosis Assays with Different pH-Sensitive Fluorescent Particles and Various Readouts. BioTechniques, 68(5), 245–250. https: //doi.org/10.2144/btn-2020-0003
Cite this article
Liu,F. (2025). The Impact of Tinospora Cordifolia Dry Seed Water Extract on the Phagocytic Activity of Microglia. Theoretical and Natural Science,133,1-8.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: AI for Healthcare: Advanced Medical Data Analytics and Smart Rehabilitation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Yates, C. R., Bruno, E. J., & Yates, M. E. D. (2022). Tinospora Cordifolia: A review of its immunomodulatory properties. Journal of Dietary Supplements, 19(2), 271–285. https: //doi.org/10.1080/19390211.2021.1873214
[2]. Song, W. M., & Colonna, M. (2018). The identity and function of microglia in neurodegeneration. Nature Immunology, 19(10), 1048–1058. https: //doi.org/10.1038/s41590-018-0212-1
[3]. Richard M. Ransohoff & Melissa A. Brown. (2012). Innate immunity in the central nervous system. Journal of Clinical Investigation, 122(4), 1164–1171. https: //doi.org/10.1172/JCI58644
[4]. Suzanne Hickman, Izzy, S., Sen, P., Morsett, L., & El Khoury, J. (2018). Microglia in neurodegeneration. Nature Neuroscience, 21(10), 1359–1369. https: //doi.org/10.1038/s41593-018-0242-x
[5]. Amy F. Lloyd, & Miron, V. E. (2019). The pro-remyelination properties of microglia in the central nervous system. Nature Reviews Neurology, 15(8), 447–458. https: //doi.org/10.1038/s41582-019-0184-2
[6]. Sarah A. Kent, & Miron, V. E. (2024). Microglia regulation of central nervous system myelin health and regeneration. Nature Reviews Immunology, 24(1), 49–63. https: //doi.org/10.1038/s41577-023-00907-4
[7]. Salter, M. W., & Stevens, B. (2017). Microglia emerge as central players in brain disease. Nature Medicine, 23(9), 1018–1027. https: //doi.org/10.1038/nm.4397
[8]. Siamon Gordon, & Martinez, F. O. (2010). Alternative Activation of Macrophages: Mechanism and Functions. Immunity, 32(5), 593–604. https: //doi.org/10.1016/j.immuni.2010.05.007
[9]. Vipin V. Dhote, Kilor, V. A., Mohan Maruga Raja, M. K., Singhai, A., Mandloi, A. S., & Upaganlawar, A. B. (2023). Effect of Tinospora cordifolia on neuroinflammation. In Treatments, Nutraceuticals, Supplements, and Herbal Medicine in Neurological Disorders (pp. 601–621). Elsevier. https: //doi.org/10.1016/B978-0-323-90052-2.00019-6
[10]. Anuradha Sharma, Bajaj, P., Bhandari, A., & Kaur, G. (2020). From ayurvedic folk medicine to preclinical neurotherapeutic role of a miraculous herb, Tinospora cordifolia. Neurochemistry International, 141, 104891. https: //doi.org/10.1016/j.neuint.2020.104891
[11]. Syed Afroz Ali, & Datusalia, A. K. (2024). Protective effects of Tinospora cordifolia miers extract against hepatic and neurobehavioral deficits in thioacetamide-induced hepatic encephalopathy in rats via modulating hyperammonemia and glial cell activation. Journal of Ethnopharmacology, 323, 117700. https: //doi.org/10.1016/j.jep.2023.117700
[12]. Hareram Birla, Rai, S. N., Singh, S. S., Zahra, W., Rawat, A., Tiwari, N., Singh, R. K., Pathak, A., & Singh, S. P. (2019). Tinospora cordifolia Suppresses Neuroinflammation in Parkinsonian Mouse Model. NeuroMolecular Medicine, 21(1), 42–53. https: //doi.org/10.1007/s12017-018-08521-7
[13]. Upendra Sharma, Bala, M., Kumar, N., Singh, B., Munshi, R. K., & Bhalerao, S. (2012). Immunomodulatory active compounds from Tinospora cordifolia. Journal of Ethnopharmacology, 141(3), 918–926. https: //doi.org/10.1016/j.jep.2012.03.027
[14]. Dorion, M.-F., Yaqubi, M., Senkevich, K., Kieran, N. W., MacDonald, A., Chen, C. X. Q., Luo, W., Wallis, A., Shlaifer, I., Hall, J. A., Dudley, R. W. R., Glass, I. A., Birth Defects Research Laboratory, Stratton, J. A., Fon, E. A., Bartels, T., Antel, J. P., Gan-or, Z., Durcan, T. M., & Healy, L. M. (2024). MerTK is a mediator of alpha-synuclein fibril uptake by human microglia. Brain, 147(2), 427–443. https: //doi.org/10.1093/brain/awad298
[15]. Guignant, C., Venet, F., Planel, S., Demaret, J., Gouel-Chéron, A., Nougier, C., Friggeri, A., Allaouchiche, B., Lepape, A., & Monneret, G. (2013). Increased MerTK expression in circulating innate immune cells of patients with septic shock. Intensive Care Medicine, 39(9), 1556–1564. https: //doi.org/10.1007/s00134-013-3006-9
[16]. Lee, J.-W. (2024). Enhanced phagocytosis associated with multinucleated microglia via Pyk2 inhibition in an acute β-amyloid infusion model.
[17]. Lindner, B., Burkard, T., & Schuler, M. (2020). Phagocytosis Assays with Different pH-Sensitive Fluorescent Particles and Various Readouts. BioTechniques, 68(5), 245–250. https: //doi.org/10.2144/btn-2020-0003









