1. Introduction
As an infectious and hematologic disease that is widespread with a high fatality rate, malaria is generally recognized as one of the most prominent protist diseases. Studies have been intensely carried out throughout the last decades, investigating and developing new medicines containing newly discovered compounds that exhibit antimalarial actions for the more efficient and effective treatment of malaria [1-3]. The recent discovery of dihydrolucilactaene as a secondary metabolite in fungus Fusarium sp. RK97-94 [4] led to the comparison of its function to lucilactaene, which is a cell cycle inhibitor with robust antimalarial activity [5,6] analogous to the structure of dihydrolucilactaene. The comparison resulted in the realization that the efficacy of the antimalarial activity of dihydrolucilactaene is 100-fold more active than lucilactaene while having inconsequential cytotoxic effect on cells other than malaria-causing pathogenic plasmodium [1], making it an ideal drug lead for the development of new effective antimalarial medicines. However, the metabolic pathway for the biosynthesis of dihydrolucilactaene is still to be determined due to an unknown enzyme for the catalytic reduction of a cyclic ether on the predecessor of dihydrolucilactaene [1], making it difficult for synthetic biological modifications to be made for optimized production of the compound via biological processes thus far. Mass production of dihydrolucilactaene for potential medical applications in industries that requires procedure optimization can be alternatively achieved by designing chemical synthesis routes. In this work, we propose a theoretical chemical synthetic pathway for dihydrolucilactaene using commercially available reagents that may be applied for the production of dihydrolucilactaene.
2. Synthesis of dihydrolucilactaene
2.1. Materials & synthetic methods
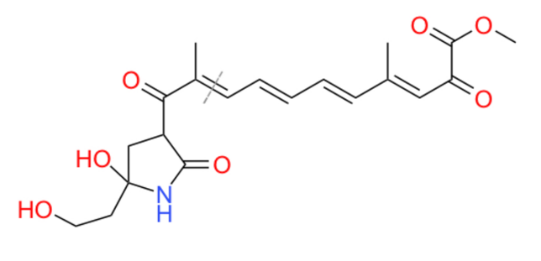
Our synthetic approach begins with a key disconnection, (Figure 1) to sever the carbon-carbon double bond beside the first methyl group of the chain. This disconnection is essential because it allows us to analyze the synthesis of the cyclic amide and the polyene chain with an ester end, respectively, which is the key to reducing the complexity of the molecule. Thus, the following explanation of the synthetic method will be bifurcated, the synthesis of cyclic amide and the synthesis of polyene chain.
|
Figure 1. Structure of dihydrolucilactaene and the position of key disconnection. |
2.2. Synthesis of cyclic amide
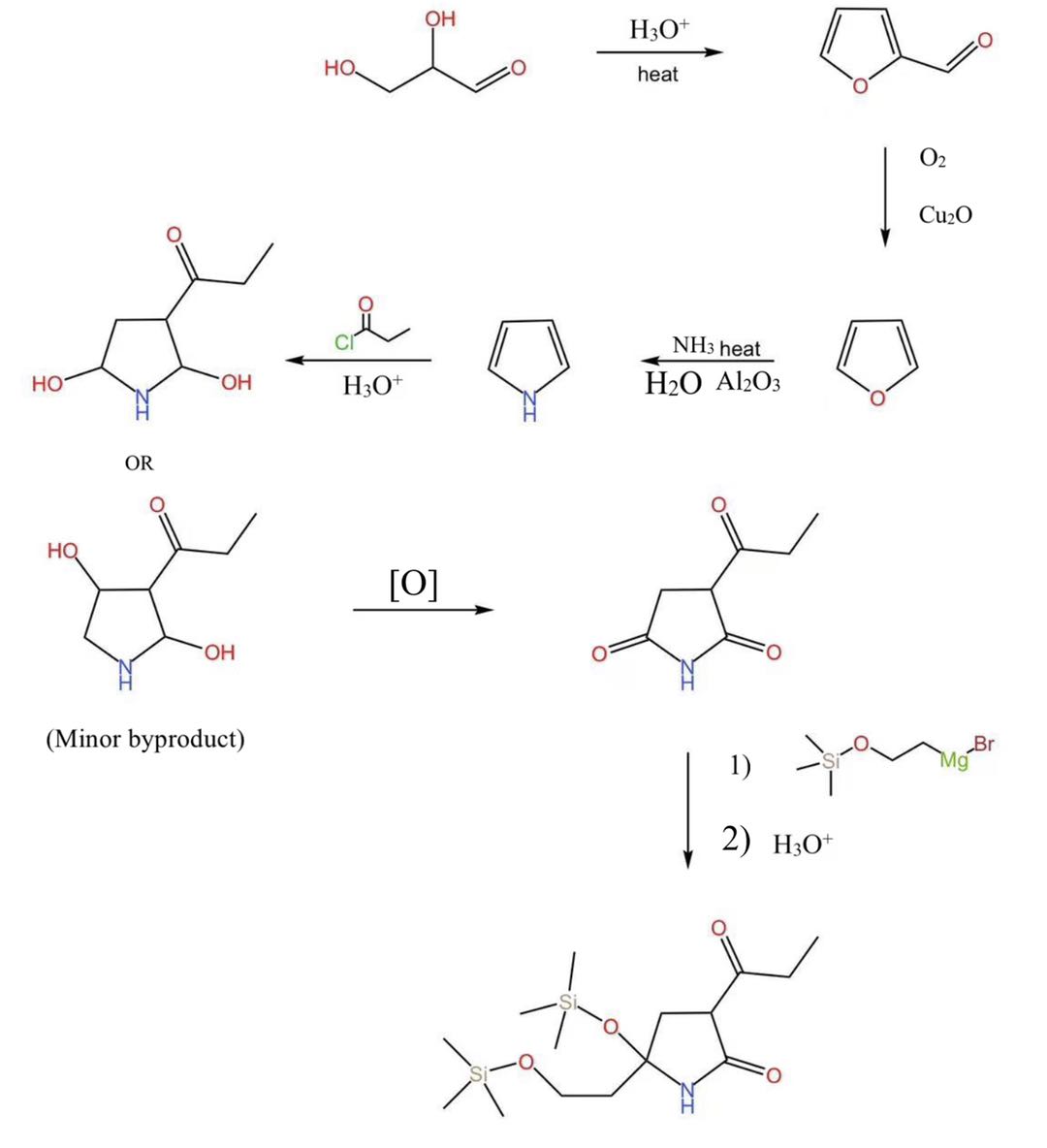
We start the synthesis of cyclic amide (Figure 2) from 2,3-dihydroxypropanal [7], the daughter compound of glyceraldehyde. Above all, we adopted a typical furan synthesis method [8], heating the solution after the addition of acid to obtain furfural, and continuing heating the mixture with tubing-in oxygen and cuprous oxide to obtain furan. So far, we have synthesized the essential five-membered ring component, except we are still one step from the ideal pyrrole derivative compound. In order to convert the furan to pyrrole, nitrogen in replacement of oxygen, we applied a simple reaction, heating the mixture in presence of ammonia, water, and aluminum oxide catalyst.
After this step, palladium-catalyzed hydrogenation, converting pyrrole to pyrrolidine, seems to be the choice getting us closer to the structure of the target molecule. However, since additions on sigma bonds are relatively rarer and require more rigid conditions, we erred on the double bonds and at utilized the delocalized pi-electron cloud to enable our further attack. In the following step, we applied simple acylation under acidic conditions. It is a one-step reaction with two intermediates, one appears after the addition of acyl chloride, and one immediately takes place with the addition of water. Supported by the resonance structure from the lone pair on the nitrogen in the cyclic amide, this reaction prefers a major product that is desirable (Figure 2), with additional thermodynamic control, we will get closer to the target molecule. Lastly, we adopt a Grignard reaction. With an additional silicon protection group, more alcohol will be produced without self-neutralizing the magnesium base.
|
Figure 2. Synthetic pathway of cyclic amide subunit. |
2.3. Synthesis of polyene chain
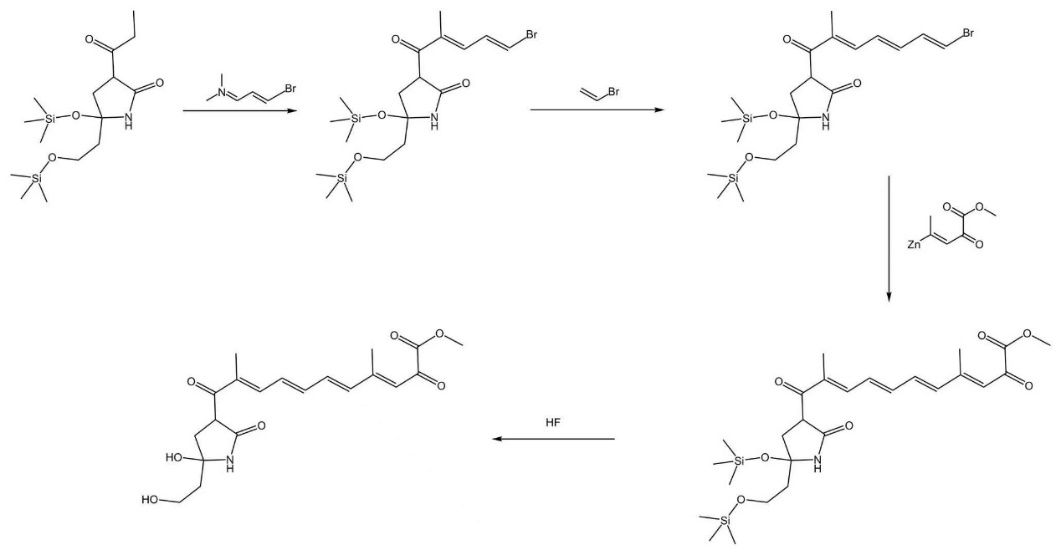
Throughout the synthesis of the polyene chain group, we adopt the combination of alkene building blocks in general (Figure 3). Our major concerns towards a ring-opening synthesis, although the initial steps were quite compatible with the ester group of the target molecule, were that the produced ether chain is chemically inert, unable to undergo an efficient rearrangement reaction, especially when the chain is rather long, and also that it is difficult to ensure the site selectivity and efficiency of elimination reaction, considering the rigid condition to eliminate hydrogen from alkane chains [9].
The transition to the synthesis of the polyene chain group starts with a Mannich reaction. Without alternative carbonyl groups having a beta-secondary carbon, we are able to connect the Mannich reagent upon the target site. Next, we design two consecutive palladium-catalysed, cross-coupling reactions [10] to elongate the alkene chain. Surely there are a plethora of coupling reactions applicable, considering the beneficiaries of evading reactions with other reactive centers present, we adopted cross-coupling reactions and backlogged couplings like the Wittig reaction and Horner-Wadsworth-Emmons reaction as potential alternatives. Although one of the cross-coupling reagents in the second to last step (Figure 3) is relatively complicated and may lead to potential steric hinderance for the reaction, the electron-absorbing effect of carbonyl and ester group renders the reagents more reactive, balancing the decrease in reactivity brought by steric hinderance. By building the polyene chain block by block, we are able to gain more control over the process of the reaction and take advantage of the stability of the elongated conjugation system. With an additional acid workup by hydrofluoric acid [11], the sily ethers are converted back to alcohols, marking the finishing of our complete synthesis of dihydrolucilactaene.
|
Figure 3. Synthetic pathway of the polyene chain. |
2.4. Discussion
Above all, our reaction scheme is only in the stage of the proposal. Although we carefully selected and designed the reaction for the sake of higher yield, it would be probable that under laboratory conditions, the reaction might go awry with unexpected rearrangements and impurities. Our first concern is about the nitrogen atom in the oxidation reaction. The oxidative reagent can be carefully selected and controlled most certainly, nevertheless, since this reaction happens under a semi-alcoholic environment, which leaves room for the secondary amine to be oxidized into hydroxylamine.
3. Conclusion
The 10-step, anime-directed synthesis of dihydrolucilactaene provides a theoretically feasible synthetic pathway for the production of dihydrolucilactaene. Once proven viable experimentally, it can be applied for efficient industrial synthesis, as reagents used are all commercially available and easy to obtain, aiding in the further research for the activities of dihydrolucilactaene and the development of potent antimalarial drug involving dihydrolucilactaene. However, the proposed reaction has not been carried out experimentally and may need modifications in reaction conditions in real-life cases. This reaction pathway is also not stereoisomerically controlled; products of certain steps in the pathway may result in enantiomeric mixtures with less-than-ideal ratios between enantiomers. Stereoselective reactions can be used in further studies to modify this pathway for a higher yield of the desired dihydrolucilactaene.
Acknowledgment
Zijian Wei and Tian Gao contributed equally to this work and should be considered co-first authors.
References
[1]. Shibeshi, M. A., Kifle, Z. D., & Atnafie, S. A. (2020). <p>Antimalarial Drug Resistance and Novel Targets for Antimalarial Drug Discovery</p> Infection and Drug Resistance, Volume 13, 4047–4060. https://doi.org/10.2147/idr.s279433
[2]. Tse, E. G., Korsik, M., & Todd, M. H. (2019). The past, present and future of anti-malarial medicines. Malaria Journal, 18(1). https://doi.org/10.1186/s12936-019-2724-z
[3]. Rosenthal, P. J. (2003). Antimalarial drug discovery: old and new approaches. The Journal of Experimental Biology, 206(21), 3735–3744. https://doi.org/10.1242/jeb.00589
[4]. Abdelhakim, I. A., Mahmud, F. B., Motoyama, T., Futamura, Y., Takahashi, S., & Osada, H. (2021). Dihydrolucilactaene, a Potent Antimalarial Compound from Fusarium sp. RK97-94. Journal of Natural Products, 85(1), 63–69. https://doi.org/10.1021/acs.jnatprod.1c00677
[5]. Kato, S., Motoyama, T., Futamura, Y., Uramoto, M., Nogawa, T., Hayashi, T., Hirota, H., Tanaka, A., Takahashi-Ando, N., Kamakura, T., & Osada, H. (2020). Biosynthetic gene cluster identification and biological activity of lucilactaene from Fusarium sp. RK97-94. Bioscience, Biotechnology, and Biochemistry, 84(6), 1303–1307. https://doi.org/10.1080/09168451.2020.1725419
[6]. Kakeya, H., Kageyama, S., Nie, L., Onose, R., Okada, G., Beppu, T., Norbury, C. C., & Osada, H. (2001). Lucilactaene, a New Cell Cycle Inhibitor in p53-Transfected Cancer Cells, Produced by a Fusarium sp. The Journal of Antibiotics, 54(10), 850–854. https://doi.org/10.7164/antibiotics.54.850
[7]. P. (n.d.). 2,3-Dihydroxypropanal; methane. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/118588631
[8]. Heterocyclic Chemistry. (n.d.). https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/heterocy.htm
[9]. Introduction to polyenes. (n.d.). https://etd.ohiolink.edu/apexprod/rws_etd/send_file/send?accession=osu1206036742&disposition=inline
[10]. LibreTexts. C−C cross-coupling reactions. https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Map%3A_Inorganic_Chemistry_%28Housecroft%29/25%3A_Catalysis_and_some_industrial_processes/25.05%3A_Homogeneous_Catalysis_-_Industrial_Applications/25.5F%3A_CC_cross-coupling_reactions
[11]. S. (2018, September 23). Silyl Protective Groups. Chem-Station Int. Ed. https://en.chem-station.com/reactions-2/2014/03/silyl-protective-groups.html
Cite this article
Wei,Z.;Gao,T. (2023). Synthesis of dihydrolucilactaene: a newly discoveredantimalarial compound. Theoretical and Natural Science,6,376-380.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the International Conference on Modern Medicine and Global Health (ICMMGH 2023)
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Shibeshi, M. A., Kifle, Z. D., & Atnafie, S. A. (2020). <p>Antimalarial Drug Resistance and Novel Targets for Antimalarial Drug Discovery</p> Infection and Drug Resistance, Volume 13, 4047–4060. https://doi.org/10.2147/idr.s279433
[2]. Tse, E. G., Korsik, M., & Todd, M. H. (2019). The past, present and future of anti-malarial medicines. Malaria Journal, 18(1). https://doi.org/10.1186/s12936-019-2724-z
[3]. Rosenthal, P. J. (2003). Antimalarial drug discovery: old and new approaches. The Journal of Experimental Biology, 206(21), 3735–3744. https://doi.org/10.1242/jeb.00589
[4]. Abdelhakim, I. A., Mahmud, F. B., Motoyama, T., Futamura, Y., Takahashi, S., & Osada, H. (2021). Dihydrolucilactaene, a Potent Antimalarial Compound from Fusarium sp. RK97-94. Journal of Natural Products, 85(1), 63–69. https://doi.org/10.1021/acs.jnatprod.1c00677
[5]. Kato, S., Motoyama, T., Futamura, Y., Uramoto, M., Nogawa, T., Hayashi, T., Hirota, H., Tanaka, A., Takahashi-Ando, N., Kamakura, T., & Osada, H. (2020). Biosynthetic gene cluster identification and biological activity of lucilactaene from Fusarium sp. RK97-94. Bioscience, Biotechnology, and Biochemistry, 84(6), 1303–1307. https://doi.org/10.1080/09168451.2020.1725419
[6]. Kakeya, H., Kageyama, S., Nie, L., Onose, R., Okada, G., Beppu, T., Norbury, C. C., & Osada, H. (2001). Lucilactaene, a New Cell Cycle Inhibitor in p53-Transfected Cancer Cells, Produced by a Fusarium sp. The Journal of Antibiotics, 54(10), 850–854. https://doi.org/10.7164/antibiotics.54.850
[7]. P. (n.d.). 2,3-Dihydroxypropanal; methane. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/118588631
[8]. Heterocyclic Chemistry. (n.d.). https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/heterocy.htm
[9]. Introduction to polyenes. (n.d.). https://etd.ohiolink.edu/apexprod/rws_etd/send_file/send?accession=osu1206036742&disposition=inline
[10]. LibreTexts. C−C cross-coupling reactions. https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Map%3A_Inorganic_Chemistry_%28Housecroft%29/25%3A_Catalysis_and_some_industrial_processes/25.05%3A_Homogeneous_Catalysis_-_Industrial_Applications/25.5F%3A_CC_cross-coupling_reactions
[11]. S. (2018, September 23). Silyl Protective Groups. Chem-Station Int. Ed. https://en.chem-station.com/reactions-2/2014/03/silyl-protective-groups.html