1. Introduction
These days, butter products are becoming increasingly prevalent in people's life, such as the creams, cookies, ice creams, and other foods that we consume on a regular basis. While people are trying the meal, they are also considering whether or not products made with butter are healthy for our bodies and the appropriate amount to consume on a daily basis. The author did all of this research in order to arrive at this conclusion. The author was able to learn how to improve the conditions of soybean oil hydrogenation in order to reduce the production of trans fatty acids in hydrogenated vegetable oil by reading articles such as "Research on the hydrogenation of soybean oil by high-pressure hydrogenation" and "Current status and development trend of margarine research." In addition, the author was able to learn about the current status and future development prospects of margarine in the market. The former, on the other hand, did not discuss the effects that an excessive use of hydrogenated vegetable oil would have on humans, and the latter had not developed a solution for how to enhance the production process at the time that the publication was written. Therefore, in order to detail the process of making hydrogenated vegetable oil, the ways in which the products can be improved, and the relationship between consumption and human health, this paper conducts a literature review in order to incorporate the benefits that have been discussed in other articles. The objective of this paper is to provide a clear understanding of the goods that contain hydrogenated vegetable oil and to give readers the confidence to consume items that contain hydrogenated vegetable oil at levels that are within the defined values.
2. Hydrogenated vegetable oil
Butter is a solid fat derived from milk. It is the result of agitating fresh milk and removing some of the water from the layer of thick milk that has formed on top of it. Typically employed in the role of a condiment, nutritive. However, the cost of this procedure is high, and it generates a significant amount of waste: on average, around 100 grams of butter are required for every 30,000 grams of milk. People hydrogenated vegetable oil to manufacture vegetable butter, which is also known as margarine. This allowed them to obtain butter at a lower cost while maintaining the same flavor.
For instance, soybean oil is one of the world's most productive plant oils. It is also one of the most important edible oils that we use in our day-to-day lives. However, due to the fact that it contains 5% to 9% linolenic acid, soybean oil is prone to developing an unpleasant aftertaste and oxidation. This makes it difficult to transport and preserve soybean oil, and it also makes it susceptible to rancidity during long-term storage. The process of oil hydrogenation, which is a type of oil modification, has the potential to remedy the drawbacks mentioned above about soybean oil and expand its range of potential uses. In the process of hydrogenating grease, costly metals like reducing nickel are used as catalysts to add hydrogen to the double bonds of unsaturated fatty acids. The changed grease that is created through this process is referred to as hydrogenated oil [1].
It is possible for the color, flavor, and taste of soybean oil to be improved by using hydrogenated vegetable oil that has been produced in this manner, such as improving the oil's flexibility and boost its antioxidant activity [2]; Altering the physicochemical properties of oils and fats so that they are simpler to transport and store.
Despite these benefits, the production of hydrogenated vegetable oils results in the production of trans fatty acids as a byproduct. The first step, which is depicted visually in the picture, is diffusion, which is the process by which H2 in the oil phase diffuses towards the surface of the catalyst. The second stage involves the adsorption of hydrogen onto the surface of the catalyst. In order to create metal-hydrogen intermediates, electrons from H2 must first be transported to the "D-band holes" of metal atoms present in the catalyst. After that, a chemical reaction takes place on the surface of the catalyst between the hydrocarbon oil and the hydrogen. When the bond is coordinated with an active metal hydrogen intermediate, an activated and unstable metal- complex, also known as a hydrogenated intermediate, is produced. The hydrogenated intermediate either loses a hydrogen atom to form a new double bond or unites with an additional hydrogen atom to finish the double bond hydrogenation reaction. In the final step, the result of the reaction is removed from the surface of the catalyst, as displayed in figure 1. When hydrogen is adsorbed by a metal-carrier, the saturated alkanes are desorbed at the same time. As shown in figure 2, the link between carbon and carbon on the intermediate can spin to generate trans isomers, or double bond displacement may occur [3].
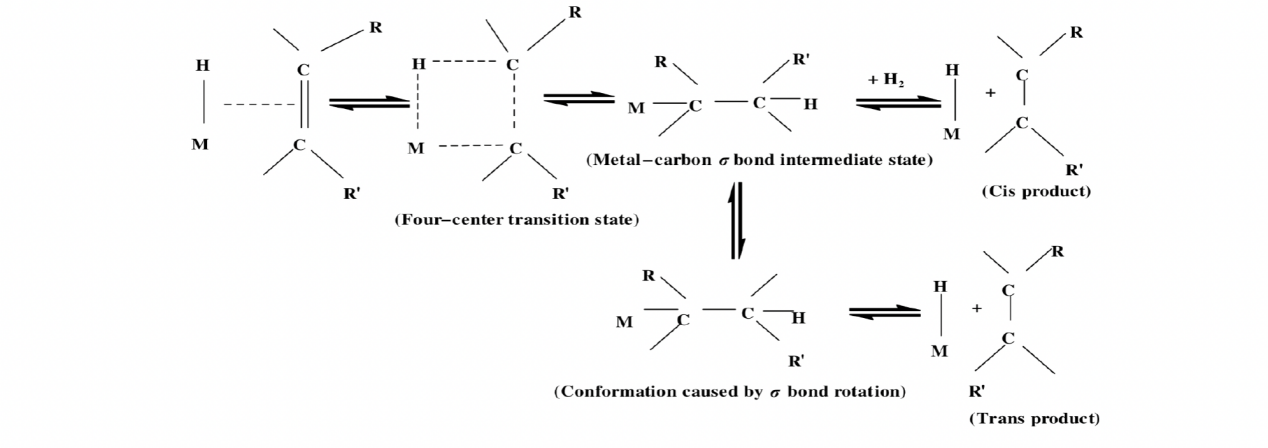
Figure 1. The reaction mechanism of hydrogenation [4].
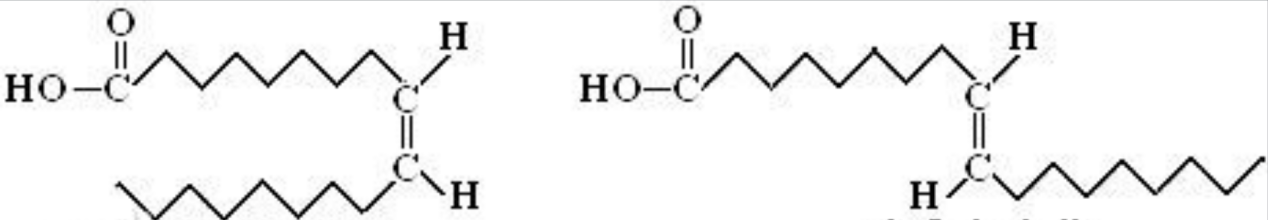
Figure 2. The cisisomerism and trans isomerism of fatty acid [5].
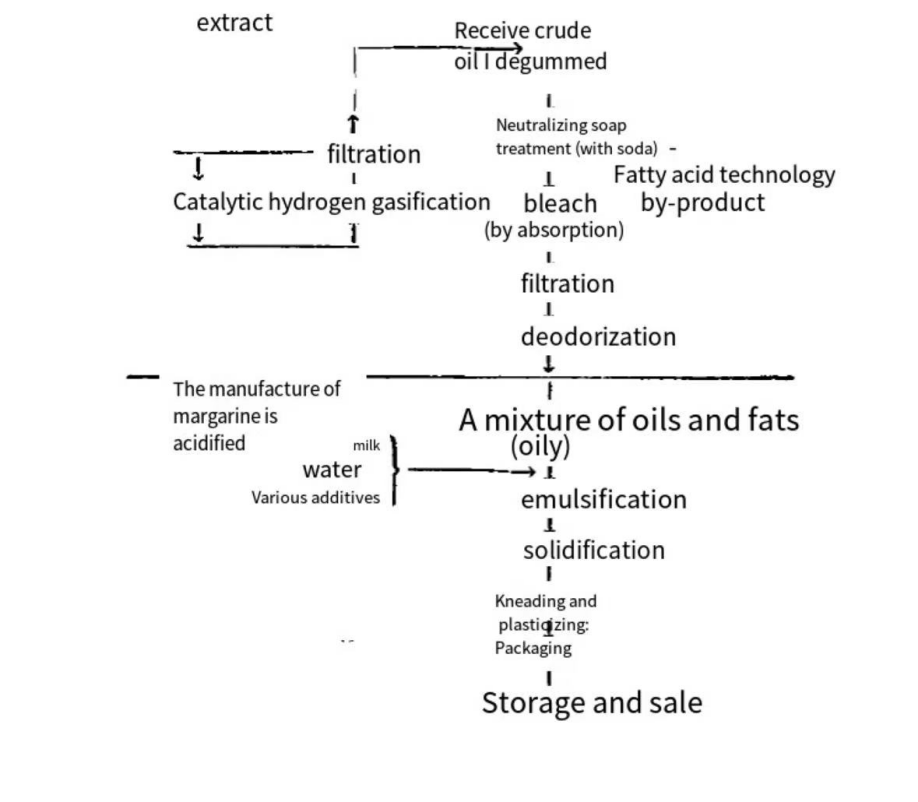
Figure 3. The production methods of vegetable butter [6].
The method of manufacturing margarine from scratch is broken down into steps and depicted in this figure 3. First, vegetable oil is hydrogenated, and then a number of processes, including degumming, neutralizing, bleaching, filtering, and deodorizing, are carried out in order to obtain a combination of different fats and oils. After that, the mixture is kneaded and plasticized, after which milk, water, and additives are added to it and it is emulsified and cured together with the mixture.
However, during the hydrogenation process, the reaction is active and prone to the formation of high amounts of trans fatty acids. Additionally, hydrogen is combustible and explosive, and the reaction that occurs during hydrogenation is exothermic, which makes it difficult to manage. The commercial hydrogenation of soybean oil often takes place in a condition of lower pressure at the moment; however, this process is not only slow but also more likely to result in the formation of a significant amount of trans fatty acids. In the single-factor test and the orthogonal test for "high-pressure hydrogenation method of hydrogenation of soybean oil research," the iodine value and the trans fatty acid content were used as the examination index. This was done by browsing the "high-pressure hydrogenation method of hydrogenation of soybean oil research." In order to obtain a hydrogenated soybean oil product with an iodine value of 42.18g L2/100g and a trans fatty acid content of 17.387% after hydrogenation, the optimal process parameters for high-pressure hydrogenation of soybean oil were as follows: catalyst Pd/C, catalyst addition of 1.5%, hydrogen pressure of 2.5 MPa in the reactor, reaction temperature of 110°C, reaction time of 120 min, and stirring speed of 300 r/min [7].
3. Comparison between natural butter and vegetable butter
According to the USDA, there are 100 calories, 11.4 grams of fat, 7.19 grams of saturated fat, 30.1 milligrams of cholesterol, and 0 grams of carbohydrates in one tablespoon of butter. In comparison, one tablespoon of margarine has the following nutritional breakdown: 100 calories, 11.3 grams of fat, 2.13 grams of saturated fat, 0 grams of cholesterol, and 0 grams of carbs [8]. According to the research, margarine has a much lower proportion of saturated fat compared to butter; as a result, eating margarine can help lower one's consumption of saturated fat and hence lower one's risk of developing cardiovascular disease. However, margarine that is derived from hydrogenated vegetable oils does include trans fatty acids, and consuming too much of it can cause the gastrointestinal burden to grow and lead to malfunction in the gastrointestinal tract. It also causes an increase in the amount of LDL cholesterol in the blood, which in turn causes an increase in the viscosity and cohesiveness of the blood. This can result in blood clots and an increased risk of cardiovascular disease. In addition to leading to abdominal obesity, which can lead to insulin resistance and, ultimately, diabetes [9], it is essential that we make thoughtful decisions regarding the foods that we eat. For instance, according to the recommendations made by the World Health Organization and the Food and Agriculture Organization of the United Nations in Diet, Nutrition and Chronic Diseases, a person who consumes 2000 kcal per day ought to have a daily intake of total fat that is 66 grams, with no more than 2.2 grams coming from trans fatty acids [10].
4. Conclusion
The author integrated the prior research by going back over the published articles and looking them over. Even though hydrogenated vegetable oil products contain a low amount of saturated fat, they still produce trans fatty acids because the carbon-carbon double bond inside breaks down into a single bond. This causes the hydrogen molecule that is combined with it to rotate, which results in the production of trans fatty acids. It is recommended that the consumption of trans fatty acids not exceed 2.2 grams per day. Because of this, items like wafer cookies and cream cakes should not be consumed in excessive amounts on a daily basis. During this time, a number of experiments have been carried out in an effort to lower the amount of TFA that is present in hydrogenated vegetable oil products. The results of these experiments have led researchers to the conclusion that it is possible to obtain TFA using a catalyst composed of Pd/C, with a catalyst addition of 1.5%, hydrogen pressure in the reactor of 2.5 MPa, reaction temperature of 110 °C, reaction time of approximately 120 minutes, and stirring speed of 300 r/min. It was possible to obtain the hydrogenated vegetable oil with a concentration of 17.387%. This has significantly contributed to the improvement of the overall safety of margarine products currently available on the market. As a result of the ongoing research, it is hoped that in the not-too-distant future, new catalysts or conditions that are more effective for reducing the TFA concentration will be identified. This would enable individuals to continue to enjoy the meal while experiencing fewer adverse effects.
References
[1]. Chang yunhe, Feng hongxia. Study on hydrogenation of soybean oil by high pressure hydrogenation [A]. Guiyang Guiyang University, 2019, 05b-0001-04.
[2]. Zhou xiaoli, Liu xiaoyan, Ma lizhi. Study on hydrogenation of soybean oil by high pressure hydrogenation [A]. Guiyang Guiyang University, 2019, 5.
[3]. Gu tingting, Song huanling. The research progress of oil hydrogenation catalyst [J]. Green Chemistry Review, 2020, 6.
[4]. Chou lingjun. The research progress of oil hydrogenation catalyst [J]. Modern Chemical Industry, 2020, 6.
[5]. Mechanisms of Action of trans Fatty Acids, 2020. https://pubmed.ncbi.nlm.nih.gov/31782488/
[6]. Hou kaizong. The Research of Vegetable Butter [J] Modern Chemical Industry, 2006, 36.
[7]. Feng yuhong. Study on hydrogenation of soybean oil by high pressure hydrogenation [J]. Research Institute of Hangzhou Wahaha Group Co. LTD, 2019, 5.
[8]. Yang wenping, Chen dongli. Is butter or margarine Healthier? 2022. Butter vs. margarine: Which is most healthful? (medicalnewstoday.com)
[9]. Fang yuming, Liu zengwu, Han wei. Research status and development trend of margarine [A]. Animal Husbandry and Feed Science, 2010, 84-85.
[10]. Li tao, Zhao ming. Hydrogenated vegetable oils are not equal to trans fatty acids [J]. China Food Quality News, 2010.
Cite this article
Qiu,H. (2023). Overview of hydrogenated vegetable oil. Theoretical and Natural Science,6,132-135.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the International Conference on Modern Medicine and Global Health (ICMMGH 2023)
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Chang yunhe, Feng hongxia. Study on hydrogenation of soybean oil by high pressure hydrogenation [A]. Guiyang Guiyang University, 2019, 05b-0001-04.
[2]. Zhou xiaoli, Liu xiaoyan, Ma lizhi. Study on hydrogenation of soybean oil by high pressure hydrogenation [A]. Guiyang Guiyang University, 2019, 5.
[3]. Gu tingting, Song huanling. The research progress of oil hydrogenation catalyst [J]. Green Chemistry Review, 2020, 6.
[4]. Chou lingjun. The research progress of oil hydrogenation catalyst [J]. Modern Chemical Industry, 2020, 6.
[5]. Mechanisms of Action of trans Fatty Acids, 2020. https://pubmed.ncbi.nlm.nih.gov/31782488/
[6]. Hou kaizong. The Research of Vegetable Butter [J] Modern Chemical Industry, 2006, 36.
[7]. Feng yuhong. Study on hydrogenation of soybean oil by high pressure hydrogenation [J]. Research Institute of Hangzhou Wahaha Group Co. LTD, 2019, 5.
[8]. Yang wenping, Chen dongli. Is butter or margarine Healthier? 2022. Butter vs. margarine: Which is most healthful? (medicalnewstoday.com)
[9]. Fang yuming, Liu zengwu, Han wei. Research status and development trend of margarine [A]. Animal Husbandry and Feed Science, 2010, 84-85.
[10]. Li tao, Zhao ming. Hydrogenated vegetable oils are not equal to trans fatty acids [J]. China Food Quality News, 2010.









