1. Introduction
Alzheimer's disease (AD) is a highly age-related degenerative disorder of the central nervous system, primarily characterized by cognitive dysfunction [1]. The current understanding of the pathological mechanisms of AD includes the excessive aggregation of extracellular β-amyloid (Aβ) forming senile plaques, the excessive phosphorylation of intracellular Tau proteins leading to neurofibrillary tangles, synaptic dysfunction, neuroinflammation, and ultimately, neuronal death. The progression of AD can be divided into three main phases: the AD presymptomatic phase, mild cognitive impairment (MCI), and dementia [2]. Key pathological symptoms include memory impairment, language difficulties, decreased visuospatial ability, and emotional changes. AD primarily affects people aged 65 and older [3]. In China, the number of patients is projected to exceed 20 million by 2030, with an estimated increase of approximately 5 million per decade after 2050. Notably, nearly half of these cases are expected to involve people aged 80 years and older [4]. Despite growing insights into the pathophysiology of AD, there is no effective drug therapy that has been successful in combating the disease, and current treatments have proven incapable of halting or preventing its progression.
Current mainstream anti-AD drugs include cholinesterase inhibitors (e.g., tacrine, donepezil, and rivastigmine), NMDA receptor antagonists (e.g., memantine), Aβ immunotherapies, and modulators of gut microbiota. Recent advancements in targeting Aβ aggregation have resulted in novel agents such as aducanumab (2021) [5], lecanemab (2023) [6], and donanmab (2024) [7]. In China, the National Medical Products Administration (NMPA) conditionally approved GV-971 in 2019, a glycoligosaccharidic acid that targets the brain-gut axis, gut microbiota, and Aβ and Tau proteins [8-10]. Despite these developments, a definitive cure for AD remains elusive. Research continues to focus on single-target disease-modifying therapies, which face high failure rates, a lack of effective early diagnostic markers, and a need for innovative therapeutic strategies. Both Western and traditional Chinese medicine (TCM) have explored new avenues [11], including natural herbs like Huperzine A, tanshinones, and puerarin, which target multiple pathways to treat or delay AD. Notably, Huperzine A, derived from Huperzia serrata, has been approved in China for AD by elevating brain acetylcholine levels to enhance memory [12]. Herbal formulations such as Tonic Kidney Inhibiting Granules and Tranquilizing Brain Tonic are also used to improve sleep and mental state, thereby alleviating cognitive function [13,14].
Artemisia annua, a natural compound with anti-tumor [15] and antimalarial [16] properties, is extracted using ether at low temperatures to preserve its chemical integrity, as its sesquiterpene lactone structure with a unique peroxo-bridge is sensitive to high temperatures [17,18]. Sesquiterpene lactones, such as artemisinin, deoxyartemisinin, and their derivatives, are not only the primary active ingredients in Artemisia annua but have also recently been investigated for their neuroprotective effects, showing potential for the treatment of neurodegenerative diseases [19]. Emerging research indicates that artemisinin may improve brain function by inhibiting neuroinflammation, a common feature in AD patients [20]. The studies suggest that artemisinin can enhance cognitive function in vivo through promoting nerve growth factor secretion or influencing neuronal regeneration [21]. Additionally, artemisinin may mitigate Aβ accumulation, a hallmark of AD, by reducing Aβ deposition or enhancing its clearance [22]. However, the exact therapeutic mechanisms and targets of Artemisia annua and its derivatives for AD are still not fully understood, which limits their clinical applications. Further research on the mechanism of action of Artemisia annua and its derivatives and optimisation of their therapeutic approach could greatly improve the therapeutic efficacy of AD.
In this study, we employed a network pharmacology approach to predict and analyze the active components of Artemisia annua and their associated disease-related targets. Our findings indicate that Artemisia annua and its derivatives may improve neuronal function and slow the progression of AD through antioxidant, anti-inflammatory, and anti-apoptotic mechanisms. These insights provide a theoretical foundation for developing novel clinical treatments for AD.
2. Method
2.1. Prediction of main active components of Artemisia annua and its targets
Using “Artemisia annua” as the keyword, all chemical components were retrieved in the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP, http://tcmspw.com/tcmsp.php). According to pharmacokinetics (ADME) standards, oral bioavailability (OB) ≥ 30% and drug-likeness (DL) ≥ 0.18 were set as screening conditions to identify potentially active compounds of Artemisia annua. For each compound, its InChIKey was collected and the corresponding SMILES format was searched through the PubChem database. These SMILES structures were then imported into the SwissTargetPrediction (http://www.swisstargetprediction.ch/) database, with the species limited to "Human (Homo sapiens)". Potential targets were selected based on a probability threshold of >0.93.
2.2. Collection of disease-related genes
Two databases, GeneCards and DisGeNet, were utilized to collect AD-related genes using “Alzheimer’s disease” as the keyword. In ensure the reliability of the data, the top 500 genes were selected based on the scoring mechanism of the respective databases. If a database had fewer than 500 records, all available genes were included. Ultimately, 500 and 46 AD-related genes were obtained from GeneCards and DisGeNet, respectively. After removing duplicates, the two sets of data were merged to generate a comprehensive list of 530 AD-related genes.
2.3. Screening of common targets of Artemisia annua active ingredients and AD and construction of Protein-Protein Interaction (PPI) network
The targets of Artemisia annua active ingredient and AD-related genes were imported into the SRplot platform at https://www.bioinformatics.com.cn (last accessed on 10 Nov 2023) [23]. The intersection targets of the two were identified, and Venn diagrams were generated to visualiz the results. Subsequently, the String online platform (https://cn.string-db.org/) was used to analyze the intersecting targets. The 'multiple proteins’ mode of analysis was selected, and the species was limited to 'Homo sapiens’. The preliminary PPI network was constructed to obtain the interaction information between proteins. Given the limited number of intersecting genes, the minimum required interaction score was adjusted to the maximum confidence level (0.900), and the maximum number of interaction partners was set to ≤20 in the first tier and ≤50 in the second tier to expand the interaction range of the intersecting genes. The updated protein-protein interaction information was then extracted to construct an optimised PPI network. Finally, the TSV file of this network was imported into Cytoscape 3.10.1 software to further analyse the PPI network. The importance of the nodes in the network was assessed based on their degree (Degree) size, allowing for the selection of key targets.
2.4. KEGG pathway and GO biological process enrichment analysis of key target genes of Artemisia annua and construction of Artemisia compound-target-pathway network
To further investigate the anti-AD effect of Artemisia annua, the intersection gene set was imported into the Metascape online platform (https://metascape.org/gp/index.html#/main/step1), with the species limited to "Home sapiens". GO and KEGG analysiswere performed to screen out the molecular functions and signaling pathways related to AD and the active ingredients of Artemisia annua. The files of Artemisia annua compounds, key targets and pathways were then imported into Cytoscape 3.10.1 to construct the Artemisia annua compound-target-pathway network.
3. Method
3.1. Main active ingredients of Artemisia annua and their targets
The active compounds of Artemisia annua were identified using TCMSP and screened on the basis of OB ≥ 30% and DL ≥ 0.18, obtaining a total of 23 compounds and 403 targets. The basic information of these compounds was summarized in Table 1. Based on their structural and physicochemical properties, the active molecules identified in this study were classified into four main groups: sesquiterpenes, volatile oils, flavonoids and other compounds. (a) Sesquiterpenes: This class includes artemisinin (MOL007424), dihydroartemisinin (MOL007425), deoxyartemisinin (MOL007426), and artemisitene (MOL007389). These compounds are known for their complex ring structures and biological activities. (b) Volatile Oils: The volatile oils category comprises sitosterol (MOL000359), stigmasterol (MOL000449), areapillin (MOL004609), and skrofulein (MOL007274). These molecules are characterized by their low molecular weight and high volatility, which contribute to their unique pharmacological profiles. (c) Flavonoids: Flavonoids represent a diverse group of phytochemicals with antioxidant and anti-inflammatory properties. In this study, the following flavonoids were identified: isorhamnetin (MOL000354), tamarixetin (MOL004083), patuletin (MOL004112), kaempferol (MOL000422), luteolin (MOL000006), quercetin (MOL000098), and cirsiliol (MOL007401). (d) Other compounds: Several additional compounds that do not fit neatly into the above categories were also identified. These included 6,8-di-C-glucosylapigenin (MOL007423), vicenin-2 (MOL007400), vitexin (MOL007404), eupatin (MOL002235), DMQT (MOL007412), artemetin (MOL005229), and [(2S)-2-[[(2S)-2-(benzoylamino)-3-phenylpropanoyl]amino]-3-phenylpropyl]acetate (MOL007415).
|
Mol ID |
Molecule Name |
MW |
AlogP |
Hdon |
Hacc |
OB(%) |
Caco-2 |
BBB |
DL |
FASA- |
HL |
|
|
MOL002235 |
EUPATIN |
360.34 |
1.98 |
3 |
8 |
50.80 |
0.53 |
-0.25 |
0.40 |
118.58 |
13.93 |
|
|
MOL000354 |
isorhamnetin |
316.28 |
1.75 |
4 |
7 |
49.60 |
0.30 |
-0.54 |
0.30 |
120.36 |
14.33 |
|
|
MOL000359 |
sitosterol |
414.79 |
8.08 |
1 |
1 |
36.91 |
1.32 |
0.87 |
0.75 |
20.22 |
5.37 |
|
|
MOL004083 |
Tamarixetin |
316.28 |
1.75 |
4 |
7 |
32.85 |
0.11 |
-0.44 |
0.30 |
120.36 |
14.59 |
|
|
MOL004112 |
Patuletin |
332.28 |
1.48 |
5 |
8 |
53.11 |
0.01 |
-0.70 |
0.33 |
140.58 |
14.31 |
|
|
MOL000422 |
kaempferol |
286.25 |
1.77 |
4 |
6 |
41.88 |
0.26 |
-0.55 |
0.24 |
111.12 |
14.74 |
|
|
MOL000449 |
Stigmasterol |
412.77 |
7.64 |
1 |
1 |
43.82 |
1.44 |
1.00 |
0.75 |
20.22 |
5.57 |
|
|
MOL004609 |
Areapillin |
360.34 |
2.28 |
3 |
8 |
48.96 |
0.60 |
-0.28 |
0.41 |
118.58 |
16.52 |
|
|
MOL005229 |
Artemetin |
388.40 |
2.30 |
1 |
8 |
49.55 |
0.81 |
-0.08 |
0.47 |
96.58 |
15.00 |
|
|
MOL000006 |
luteolin |
286.25 |
2.06 |
4 |
6 |
36.16 |
0.18 |
-0.84 |
0.24 |
111.12 |
15.94 |
|
|
MOL007274 |
Skrofulein |
314.31 |
2.56 |
2 |
6 |
30.35 |
0.71 |
-0.34 |
0.29 |
89.12 |
15.80 |
|
|
MOL007389 |
artemisitene |
280.35 |
3.15 |
0 |
5 |
54.36 |
0.54 |
0.59 |
0.30 |
53.99 |
-0.74 |
|
|
MOL007400 |
vicenin-2_qt |
270.25 |
2.82 |
3 |
5 |
45.84 |
0.24 |
-0.17 |
0.21 |
89.05 |
16.75 |
|
|
MOL007401 |
Cirsiliol |
330.31 |
2.30 |
3 |
7 |
43.46 |
0.55 |
-0.50 |
0.33 |
109.36 |
15.04 |
|
|
MOL007404 |
vitexin_qt |
270.25 |
1.16 |
3 |
5 |
52.18 |
0.28 |
-0.39 |
0.21 |
89.05 |
3.40 |
|
|
MOL007412 |
DMQT |
346.31 |
1.55 |
4 |
8 |
42.60 |
0.30 |
-0.68 |
0.36 |
129.58 |
15.15 |
|
|
MOL007415 |
[(2S)-2-[[(2S)-2-(benzoylamino)-3-phenylpropanoyl]amino]-3-phenylpropyl]acetate |
444.57 |
4.01 |
2 |
6 |
58.01 |
0.32 |
-0.26 |
0.51 |
84.5 |
6.03 |
|
|
MOL007423 |
6,8-di-c-glucosylapigenin_qt |
270.25 |
1.16 |
3 |
5 |
59.85 |
0.37 |
-0.48 |
0.21 |
89.05 |
3.38 |
|
|
MOL007424 |
artemisinin |
282.37 |
3.14 |
0 |
5 |
49.87 |
0.50 |
0.49 |
0.30 |
53.99 |
-0.59 |
|
|
MOL007425 |
dihydroartemisinin |
284.39 |
2.76 |
1 |
5 |
50.75 |
0.39 |
0.26 |
0.30 |
57.15 |
-0.40 |
|
|
MOL007426 |
deoxyartemisinin |
266.37 |
2.15 |
0 |
4 |
54.47 |
0.65 |
0.69 |
0.26 |
44.75 |
4.65 |
|
|
MOL000098 |
quercetin |
302.25 |
1.50 |
5 |
7 |
46.43 |
0.04 |
-0.76 |
0.27 |
131.36 |
14.40 |
|
3.2. Identification of AD-related genes and intersection with Artemisia annua targets
Using “Alzheimer's disease” as the keyword, AD-related genes were collected by GeneCards and DisGeNeT databases. The top 500 genes in each database were collected according to their respective scoring rules, resulting in a combined total of 546 genes. After removing 16 duplicate entries, 530 unique AD-related targets were retained. The 393 Artemisia annua targets identified in Section 2.1 and the 530 AD-related targets from Section 2.2 were visualized and intersected using the online SRplot platform. This analysis revealed five key targets: Presenilin 2(PSEN2),Nitric Oxide Synthase 3 (NOS3),Presenilin 1 (PSEN1),Myeloperoxidase (MPO),Amyloid Beta Precursor Protein (APP), and the results were presented in a Venn diagram (Figure 1).
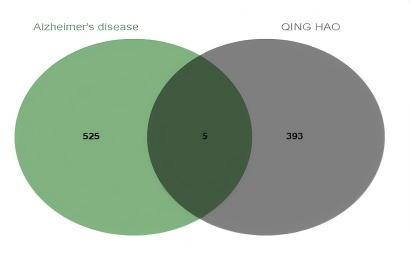
3.3. Screening of active ingredients and AD common targets and construction of PPI network of Artemisia annua
The PPI network was constructed using the STRNG database with 271 edges and 75 nodes, as shown in Figure 2. The PPI network was analysed with “Degree” as the parameter, the larger the Degree, the more important the function of the node in the network, and 64 key targets were screened, drgree >20,including Amyloid Beta Precursor Protein (APP), Inositol 1,4,5-Trisphosphate Receptor Type 1 (ITPR1),Inositol 1,4,5-Trisphosphate Receptor Type 2 (ITPR2),Inositol 1,4,5-Trisphosphate Receptor Type 3 (ITPR3).
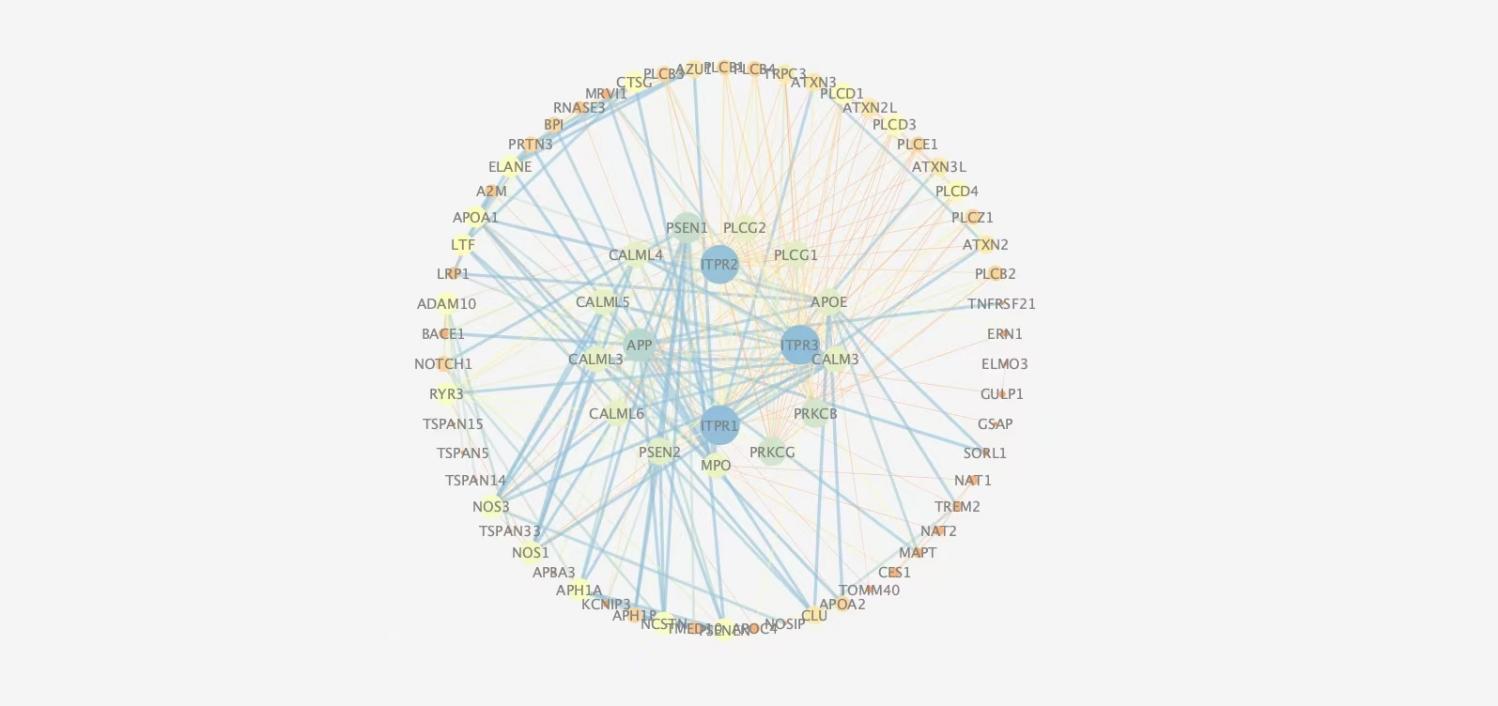
3.4. Functional annotation of key targets and pathway enrichment
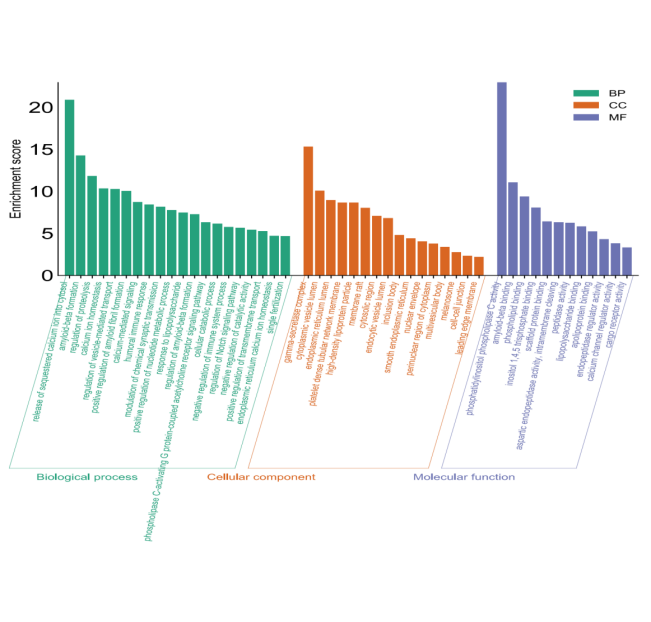
The Metascape database was used to analyze the 64 intersection targets of Artemisia annua in treating AD in terms of biological processes (BP), cellular components (CC), and molecular functions (MF). Through visual processing on the SRplot platform, the GO analysis histogram was produced according to the count value from high to low (Figure 3). Among them, the main processes related to BP include release of sequestered calcium ion into cytosol, amyloid-beta formation, regulation of proteolysis, etc.; the main locations related to CC include gamma-secretase complex, cytoplasmic vesicle lumen, endoplasmic reticulum lumen, etc., and MF Relevant main functions include phosphatidylinositol phospholipase C activity, amyloid-beta binding, phospholipid binding, etc.
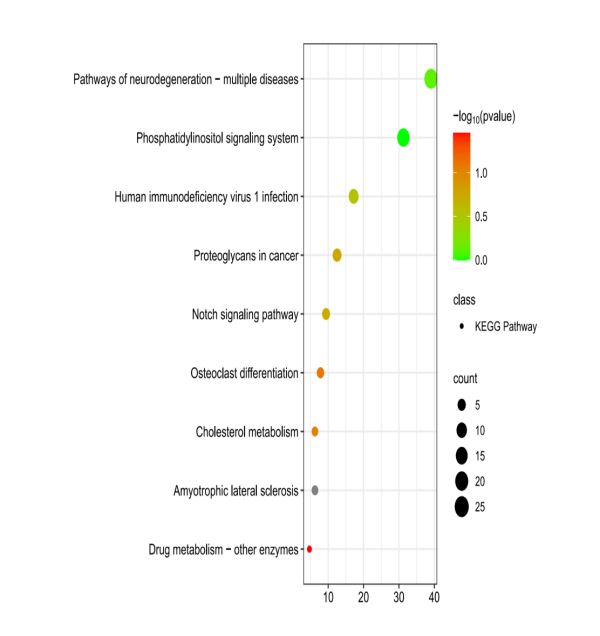
The KEGG enrichment analysis results showed 9 pathways, which were displayed in order from high to low according to -log(p) value (Figure 4). The main related pathways of Artemisia annua against AD include Notch signaling pathway, Phosphatidylinositol signaling system, pathways of neurodegeneration - multiple diseases, etc.
3.5. Construction of Artemisia annua compound-target-pathway network
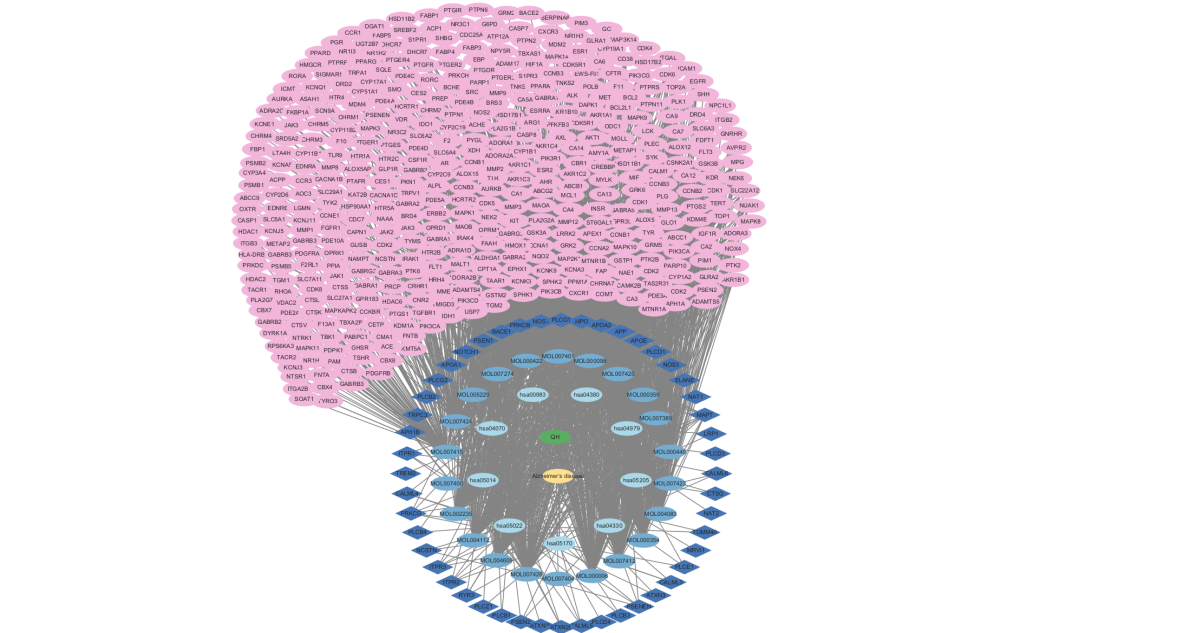
The 23 effective compounds, 64 key targets and 9 metabolic pathways were imported into Cytoscape 3.10.1 to construct the “Artemisia annua Compound-Target-Pathway Network”(Figure 5). The 64 dark blue diamonds represent the key targets of Artemisia annua, 23 sky blue ellipses represent the coding of effective compounds of Artemisia annua, 9 light blue ellipses represent the coding of metabolic pathways, the green ellipses represent Artemisia annua, the yellow ellipses represent AD, and 458 pink ellipses are the targets of the active ingredient, with a total of 2,129 edges and the interactions between the target and the chemical components.
4. Discussion
In this study, we screened 23 active ingredients of Artemisia annua, including sesquiterpenes, volatile oils, flavonoids, and other compounds. Among these, sesquiterpenes such as artemisinin and its derivatives (dihydroartemisinin, deoxyartemisinin) were noted for their anti-inflammatory and neuroprotective effects [24,25]. Flavonoids, including quercetin and luteolin, were recognized for their potent antioxidant properties, which may mitigate oxidative stress—a significant contributor to AD pathology [26]. Additionally, volatile oils like stigmasterol have been implicated in modulating neuroinflammation [27]. These active ingredients collectively demonstrate diverse pharmacological actions, suggesting their utility in targeting the multifactorial etiology of AD.
The study identified 530 AD-related targets and 9 associated signaling pathways. Network analysis revealed that Artemisia annua acted on key targets such as PSEN2, NOS3, PSEN1, MPO, and APP, elucidating its multi-target pharmacological mechanism. Research showed that PSEN1 and PSEN2 were crucial for γ-secretase activity, which was involved in the cleavage of APP to form Aβ peptides [28]. By modulating these targets, Artemisia annua may influence Aβ production and aggregation. Similarly, NOS3 was involved in nitric oxide (NO) production, contributing to neuroinflammation and oxidative stress [29-31], Artemisia annua's interaction with NOS3 could help reduce these pathological processes. The main findings suggested that Artemisia annua targeted inflammatory pathways and interacted with proteins involved in Aβ processing, potentially affecting the formation and aggregation of Aβ plaques. These results provide a comprehensive understanding of the therapeutic mechanisms of Artemisia annua in alleviating the pathogenesis of AD.
In this study, GO and KEGG enrichment analyses revealed that the targets of Artemisia annua were significantly involved in the Notch and phosphatidylinositol signaling pathways, providing valuable insights into its potential biological effects. The GO analysis demonstrated that Artemisia annua 's active ingredients were enriched in biological processes, including Aβ formation, protease regulation, and calcium release, all of which were directly associated with core pathological features of AD, such as neuroinflammation and synaptic dysfunction [32-34]. At the cellular component level, targets were distributed among subcellular structures like the endoplasmic reticulum lumen and cytoplasmic vesicles, indicating that Artemisia annua may regulate molecular events within these critical regions. At the molecular function level, significant enrichment was observed for functions such as phospholipase C activity and Aβ-protein binding, aligning with the role of Artemisia annua components in inhibiting Aβ production and promoting its clearance. The Notch signaling pathway, a highly conserved mechanism, played a critical role in the development and adult behavior of neural stem cells in specific brain regions, regulating cell proliferation, differentiation, and fate determination during neurodevelopment [35]. Both the activation of the Notch signaling pathway and the hydrolysis of APP depend on γ-secretase, suggesting that Notch signaling may be intricately involved in the pathological mechanisms of AD [36]. Additionally, the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) pathway, a classical signaling cascade, played a key role in cell growth, proliferation, and apoptosis, and had been implicated in the development of several neurodegenerative diseases, including AD [37]. Regulation of phosphatidylinositol signaling may affect synaptic plasticity and memory functions, which are typically impaired in AD. These findings emphasize the ability of Artemisia annua to act on multiple targets and pathways, reflecting the multidimensional nature of traditional Chinese medicine interventions. Unlike single-target therapies, Artemisia annua appears to modify disease progression through multifaceted pathway interactions, potentially offering a more comprehensive strategy for addressing the complex pathophysiology of AD. Future studies should aim to elucidate the detailed mechanisms of action and validate these findings in preclinical models, paving the way for potential clinical applications.
5. Conclusion
This study identified 23 active compounds from Artemisia annua targeting 403 genes, classified into sesquiterpenes, volatile oils, flavonoids, and other compounds. We pinpointed five critical targets ( PSEN2, NOS3,PSEN1,MPO,APP) for AD treatment. KEGG pathway enrichment highlighted the involvement of pathways such as the Notch signaling pathway and phosphatidylinositol signaling system in the therapeutic mechanism of Artemisia annua against AD. Ultimately, a comprehensive “Artemisia annua Compound-Target-Pathway Network" was constructed , providing insights into the potential efficacy and mechanisms of action of Artemisia annua in treating AD.
In conclusion, Artemisia annua and its active ingredient, artemisinin, show great promise for intervening in AD through a multi-target mechanism that effectively addresses neuroinflammation, amyloidogenesis, and synaptic pathways. While the clinical translation of these findings requires further exploration, this study lays a solid foundation for integrating traditional herbs with modern medicine to develop novel treatments for neurodegenerative diseases. Future research should focus on validating the predicted mechanisms through in vitro and in vivo experiments, as well as conducting detailed pharmacokinetic and pharmacodynamic studies to assess the safety and efficacy of Artemisia annua derivatives in preclinical and clinical settings.
References
[1]. Zhang, J., Zhang, H., Ding, H., et al. (2022) Progress of research on the treatment of Alzheimer's disease with Kaixin San. Journal of Tianjin University of Traditional Chinese Medicine, 41(4), 521-530.
[2]. Shen, Y., Zhang, Q., Zhao, J. Q., et al. (2018) Progress in the study of co-morbid pathological mechanisms of Alzheimer's disease and Parkinson's disease. Chinese Journal of Traditional Chinese Medicine, 36(2), 319-322.
[3]. Wang, W., & Song, C. (2019) Research progress on the pathogenesis of Alzheimer's disease and clinical medication. China Drug Evaluation, 36(3), 204.
[4]. Zhang, R., Zhang, J., Li, B., et al. (2020) Mechanism of anti-Alzheimer's disease of Gardenia jasminoides based on network pharmacology. Chinese Journal of Traditional Chinese Medicine, 45(11), 2601.
[5]. Knopman, D. S., Jones, D. T., & Greicius, M. D. (2021) Failure to demonstrate efficacy of aducanumab: An analysis of the EMERGE and ENGAGE trials as reported by Biogen, December 2019. Alzheimer's & Dementia, 17(4), 696–701.
[6]. Van Dyck, C. H., Swanson, C. J., Aisen, P., et al. (2023) Lecanemab in early Alzheimer’s disease. New England Journal of Medicine, 388(1), 9-21.
[7]. Mintun, M. A., Lo, A. C., Duggan, E. C., et al. (2024) Donanemab in early symptomatic Alzheimer’s disease: Results from the TRAILBLAZER-ALZ 2 trial. Journal of the American Medical Association (JAMA).
[8]. Deng, Q., & Ma, F. (2020) Progress of research on the pathogenesis and pharmacological treatment of Alzheimer's disease. Journal of Guizhou Normal University (Natural Science Edition), 38(1), 104-111.
[9]. Xie, Y., Gao, X., Dong, G., et al. (2018) Progress in the research of Alzheimer's disease pathogenic mechanism and novel therapeutic drugs. Biochemistry, 4(6), 116-119.
[10]. Lin, T., Zhan, Y., Fu, S., et al. (2018) Alzheimer's disease-related drug targets and clinical therapeutic advances. Journal of University of Science and Technology of China, 48(10), 825-837.
[11]. Wang, Y., Liang, J., Jia, R., et al. (2019) Predicting the prevalence of Alzheimer's disease in China from 2020 to 2050. Alzheimer's Disease and Related Diseases, 2(1), 289.
[12]. Fu, W. (2024) Prevention and treatment of Alzheimer's disease. Family Hundred, (6), 30-31.
[13]. Li, L., & Du, Y. (2017) Research progress on the treatment of Alzheimer's disease with the active ingredient of traditional Chinese medicine, Staphylococcus aureus. Practical Geriatrics, 31(7), 613-616.
[14]. Xiao, M., Zhang, X., Liu, W., et al. (2024) Progress of clinical and pharmacological mechanism of kidney tonic formula for Alzheimer's disease. New Chinese Medicines and Clinical Pharmacology, 35(10), 1628-1636.
[15]. Hu, X., Zhao, Y., Wang, E., et al. (2024) Study on nanodrug delivery system and anti-tumor mechanism of artemisinin and its derivatives. Chemical Reagents, 46(7), 11-19.
[16]. Ho, W. E., Peh, H. Y., Chan, T. K., & Wong, W. S. (2014) Artemisinins: Pharmacological actions beyond anti-malarial. Pharmacology & Therapeutics, 142, 126–139.
[17]. Tu, Y. (2016) Artemisinin - A gift from traditional Chinese medicine to the world (Nobel Lecture). Angewandte Chemie International Edition, 55(35), 10210-1026.
[18]. Li, T., Han, W., Ren, T., et al. (2024) Research progress of artemisinin and its nanoformulations against lung cancer. Chinese Medicine Pharmacology and Clinics, 1-15.
[19]. Kiss, E., Kins, S., Gorgas, K., et al. (2024) An alternative use for proven drugs: Experimental evidence for the therapeutic potential of artemisinin and its derivatives for Alzheimer's disease. International Journal of Molecular Science, 25(8).
[20]. Wang, H. Y., Li, H. Y., Luo, H. Y., et al. (2018) Artemisinin modulation of the NF-κB signaling pathway mediates the cellular model of inflammatory response in Alzheimer's disease. Journal of China Medical University, 47(6), 552–555, 561.
[21]. Xing, X., Fang, H., Tang, M., et al. (2018) Protective effects of artemisinin against oxidative stress in neurons and neuronal cells and its mechanism. Chinese Journal of Pharmacology and Toxicology, 32(9), 695.
[22]. Li, Y., Yang, X., Ma, C., Qiao, J., Zhang, C., Guo, Y., ... & Guo, Q. (2023). Anti-malaria drug artesunate prevents development of amyloid-β pathology in mice by upregulating PICALM at the blood–brain barrier. Molecular Neurodegeneration, 18, 4.
[23]. Tang D, Chen M, Huang X, Zhang G, Zeng L, Zhang G, Wu S, Wang Y. SRplot: A free online platform for data visualization and graphing. PLoS One. 2023 Nov 9; 18(11): e0294236.
[24]. Tiratsuyan S, Hambardzumyan Y, Poghosyan M, et al. Neuroprotective Effect of Artemisinin in an Animal Model of Alzheimer's Disease. [J]. Current medicinal chemistry, 2024.
[25]. Zhao Yueyang. Study on the mechanism of action of dihydroartemisinin to improve cognitive impairment in Alzheimer's disease by promoting autophagosomal-lysosomal fusion and autophagic degradation to accelerate Aβ clearance [D]. Chongqing Medical University, 2020.
[26]. Yan Xiaona, Wang Ze, Li Rongfang. Progress of the mechanism of action of Garcinia cambogia flavonoids on Alzheimer's disease [J]. Modern Food Science and Technology, 2023, 39(8): 343-351.
[27]. Jieven. (2022) Study on the potential effect of soya sterol on neuroinflammation and regulatory mechanism in Alzheimer's disease. Zhejiang University.
[28]. WU Qiong, WEN Mimi, LI Na et al. Progress in the study of physiological functions of amyloid precursor protein [J]. Advances in Physiological Sciences, 2016, 47(2): 103-107.
[29]. Freitag, K., Obermayer, B., Heppner, F., & Jendrach, M. (2022) Spermidine reduces neuroinflammation and soluble amyloid beta in an Alzheimer’s disease mouse model. NCBI GEO, Accession GSE206202.
[30]. Oshima, T., Kater, M. S., Huffels, C. F., et al. (2023) Early amyloid-induced changes in microglia gene expression in APP/PS1 mice. NCBI GEO, Accession GSE226937.
[31]. Deng, Y., & Yu, G. (2014) Effect of DNA methylation on beta-amyloid in Alzheimer's disease. Genetics, 36(04), 295-300.
[32]. Jiang, M., Zhao, D., Zhou, Y., et al. (2024) Histone B regulates microglia migration and amyloid beta phagocytosis in patients with Alzheimer's disease through PI3K-Akt signalling. Neuropsychopharmacology.
[33]. Anand, P., & Raj, K. P. (2023) Mechanistic insights into the neuronal calcium and IP3 signalling systems regulating ATP release during ischemia in the progression of Alzheimer's disease. European Biophysical Journal: EBJ, 52(3).
[34]. Tate, M. D., Wijeratne, H., Kim, B., et al. (2023) Deletion of microRNA-33 ameliorates amyloid pathology in APP/PS1 mice. NCBI GEO, Accession GSE235179.
[35]. Sun, X. (2020) miR-34a is involved in apoptosis in early-onset Alzheimer's disease cell models by regulating Notch signalling pathway. Qingdao University.
[36]. Yan, M. (2016) The role of Reelin and Notch signalling pathway in the development of the nervous system and the pathogenesis of Alzheimer's disease. Henan University.
[37]. Guan, H. B., & Zhang, J. (2022) Research progress on the prevention and treatment of Alzheimer's disease based on the phosphatidylinositol 3-kinase/protein kinase B pathway in traditional Chinese medicine. Hebei Traditional Chinese Medicine, 44(3), 518-524.
Cite this article
Huang,X. (2025). Exploring the Mechanism of Action of Artemisia Annua in Alzheimer's Disease Based on Network Pharmacology. Theoretical and Natural Science,135,79-90.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: AI for Healthcare: Advanced Medical Data Analytics and Smart Rehabilitation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Zhang, J., Zhang, H., Ding, H., et al. (2022) Progress of research on the treatment of Alzheimer's disease with Kaixin San. Journal of Tianjin University of Traditional Chinese Medicine, 41(4), 521-530.
[2]. Shen, Y., Zhang, Q., Zhao, J. Q., et al. (2018) Progress in the study of co-morbid pathological mechanisms of Alzheimer's disease and Parkinson's disease. Chinese Journal of Traditional Chinese Medicine, 36(2), 319-322.
[3]. Wang, W., & Song, C. (2019) Research progress on the pathogenesis of Alzheimer's disease and clinical medication. China Drug Evaluation, 36(3), 204.
[4]. Zhang, R., Zhang, J., Li, B., et al. (2020) Mechanism of anti-Alzheimer's disease of Gardenia jasminoides based on network pharmacology. Chinese Journal of Traditional Chinese Medicine, 45(11), 2601.
[5]. Knopman, D. S., Jones, D. T., & Greicius, M. D. (2021) Failure to demonstrate efficacy of aducanumab: An analysis of the EMERGE and ENGAGE trials as reported by Biogen, December 2019. Alzheimer's & Dementia, 17(4), 696–701.
[6]. Van Dyck, C. H., Swanson, C. J., Aisen, P., et al. (2023) Lecanemab in early Alzheimer’s disease. New England Journal of Medicine, 388(1), 9-21.
[7]. Mintun, M. A., Lo, A. C., Duggan, E. C., et al. (2024) Donanemab in early symptomatic Alzheimer’s disease: Results from the TRAILBLAZER-ALZ 2 trial. Journal of the American Medical Association (JAMA).
[8]. Deng, Q., & Ma, F. (2020) Progress of research on the pathogenesis and pharmacological treatment of Alzheimer's disease. Journal of Guizhou Normal University (Natural Science Edition), 38(1), 104-111.
[9]. Xie, Y., Gao, X., Dong, G., et al. (2018) Progress in the research of Alzheimer's disease pathogenic mechanism and novel therapeutic drugs. Biochemistry, 4(6), 116-119.
[10]. Lin, T., Zhan, Y., Fu, S., et al. (2018) Alzheimer's disease-related drug targets and clinical therapeutic advances. Journal of University of Science and Technology of China, 48(10), 825-837.
[11]. Wang, Y., Liang, J., Jia, R., et al. (2019) Predicting the prevalence of Alzheimer's disease in China from 2020 to 2050. Alzheimer's Disease and Related Diseases, 2(1), 289.
[12]. Fu, W. (2024) Prevention and treatment of Alzheimer's disease. Family Hundred, (6), 30-31.
[13]. Li, L., & Du, Y. (2017) Research progress on the treatment of Alzheimer's disease with the active ingredient of traditional Chinese medicine, Staphylococcus aureus. Practical Geriatrics, 31(7), 613-616.
[14]. Xiao, M., Zhang, X., Liu, W., et al. (2024) Progress of clinical and pharmacological mechanism of kidney tonic formula for Alzheimer's disease. New Chinese Medicines and Clinical Pharmacology, 35(10), 1628-1636.
[15]. Hu, X., Zhao, Y., Wang, E., et al. (2024) Study on nanodrug delivery system and anti-tumor mechanism of artemisinin and its derivatives. Chemical Reagents, 46(7), 11-19.
[16]. Ho, W. E., Peh, H. Y., Chan, T. K., & Wong, W. S. (2014) Artemisinins: Pharmacological actions beyond anti-malarial. Pharmacology & Therapeutics, 142, 126–139.
[17]. Tu, Y. (2016) Artemisinin - A gift from traditional Chinese medicine to the world (Nobel Lecture). Angewandte Chemie International Edition, 55(35), 10210-1026.
[18]. Li, T., Han, W., Ren, T., et al. (2024) Research progress of artemisinin and its nanoformulations against lung cancer. Chinese Medicine Pharmacology and Clinics, 1-15.
[19]. Kiss, E., Kins, S., Gorgas, K., et al. (2024) An alternative use for proven drugs: Experimental evidence for the therapeutic potential of artemisinin and its derivatives for Alzheimer's disease. International Journal of Molecular Science, 25(8).
[20]. Wang, H. Y., Li, H. Y., Luo, H. Y., et al. (2018) Artemisinin modulation of the NF-κB signaling pathway mediates the cellular model of inflammatory response in Alzheimer's disease. Journal of China Medical University, 47(6), 552–555, 561.
[21]. Xing, X., Fang, H., Tang, M., et al. (2018) Protective effects of artemisinin against oxidative stress in neurons and neuronal cells and its mechanism. Chinese Journal of Pharmacology and Toxicology, 32(9), 695.
[22]. Li, Y., Yang, X., Ma, C., Qiao, J., Zhang, C., Guo, Y., ... & Guo, Q. (2023). Anti-malaria drug artesunate prevents development of amyloid-β pathology in mice by upregulating PICALM at the blood–brain barrier. Molecular Neurodegeneration, 18, 4.
[23]. Tang D, Chen M, Huang X, Zhang G, Zeng L, Zhang G, Wu S, Wang Y. SRplot: A free online platform for data visualization and graphing. PLoS One. 2023 Nov 9; 18(11): e0294236.
[24]. Tiratsuyan S, Hambardzumyan Y, Poghosyan M, et al. Neuroprotective Effect of Artemisinin in an Animal Model of Alzheimer's Disease. [J]. Current medicinal chemistry, 2024.
[25]. Zhao Yueyang. Study on the mechanism of action of dihydroartemisinin to improve cognitive impairment in Alzheimer's disease by promoting autophagosomal-lysosomal fusion and autophagic degradation to accelerate Aβ clearance [D]. Chongqing Medical University, 2020.
[26]. Yan Xiaona, Wang Ze, Li Rongfang. Progress of the mechanism of action of Garcinia cambogia flavonoids on Alzheimer's disease [J]. Modern Food Science and Technology, 2023, 39(8): 343-351.
[27]. Jieven. (2022) Study on the potential effect of soya sterol on neuroinflammation and regulatory mechanism in Alzheimer's disease. Zhejiang University.
[28]. WU Qiong, WEN Mimi, LI Na et al. Progress in the study of physiological functions of amyloid precursor protein [J]. Advances in Physiological Sciences, 2016, 47(2): 103-107.
[29]. Freitag, K., Obermayer, B., Heppner, F., & Jendrach, M. (2022) Spermidine reduces neuroinflammation and soluble amyloid beta in an Alzheimer’s disease mouse model. NCBI GEO, Accession GSE206202.
[30]. Oshima, T., Kater, M. S., Huffels, C. F., et al. (2023) Early amyloid-induced changes in microglia gene expression in APP/PS1 mice. NCBI GEO, Accession GSE226937.
[31]. Deng, Y., & Yu, G. (2014) Effect of DNA methylation on beta-amyloid in Alzheimer's disease. Genetics, 36(04), 295-300.
[32]. Jiang, M., Zhao, D., Zhou, Y., et al. (2024) Histone B regulates microglia migration and amyloid beta phagocytosis in patients with Alzheimer's disease through PI3K-Akt signalling. Neuropsychopharmacology.
[33]. Anand, P., & Raj, K. P. (2023) Mechanistic insights into the neuronal calcium and IP3 signalling systems regulating ATP release during ischemia in the progression of Alzheimer's disease. European Biophysical Journal: EBJ, 52(3).
[34]. Tate, M. D., Wijeratne, H., Kim, B., et al. (2023) Deletion of microRNA-33 ameliorates amyloid pathology in APP/PS1 mice. NCBI GEO, Accession GSE235179.
[35]. Sun, X. (2020) miR-34a is involved in apoptosis in early-onset Alzheimer's disease cell models by regulating Notch signalling pathway. Qingdao University.
[36]. Yan, M. (2016) The role of Reelin and Notch signalling pathway in the development of the nervous system and the pathogenesis of Alzheimer's disease. Henan University.
[37]. Guan, H. B., & Zhang, J. (2022) Research progress on the prevention and treatment of Alzheimer's disease based on the phosphatidylinositol 3-kinase/protein kinase B pathway in traditional Chinese medicine. Hebei Traditional Chinese Medicine, 44(3), 518-524.









