1. Introduction
1.1. Hepatocellular carcinoma
Liver cancer is currently one of the most common malignant tumors, ranking sixth in terms of incidence and third in terms of mortality [1]. Among them, hepatocellular carcinoma (HCC) is the most prevalent type, mainly affecting China, Southeast Asia, and sub-Saharan Africa (Figure 1) [2].
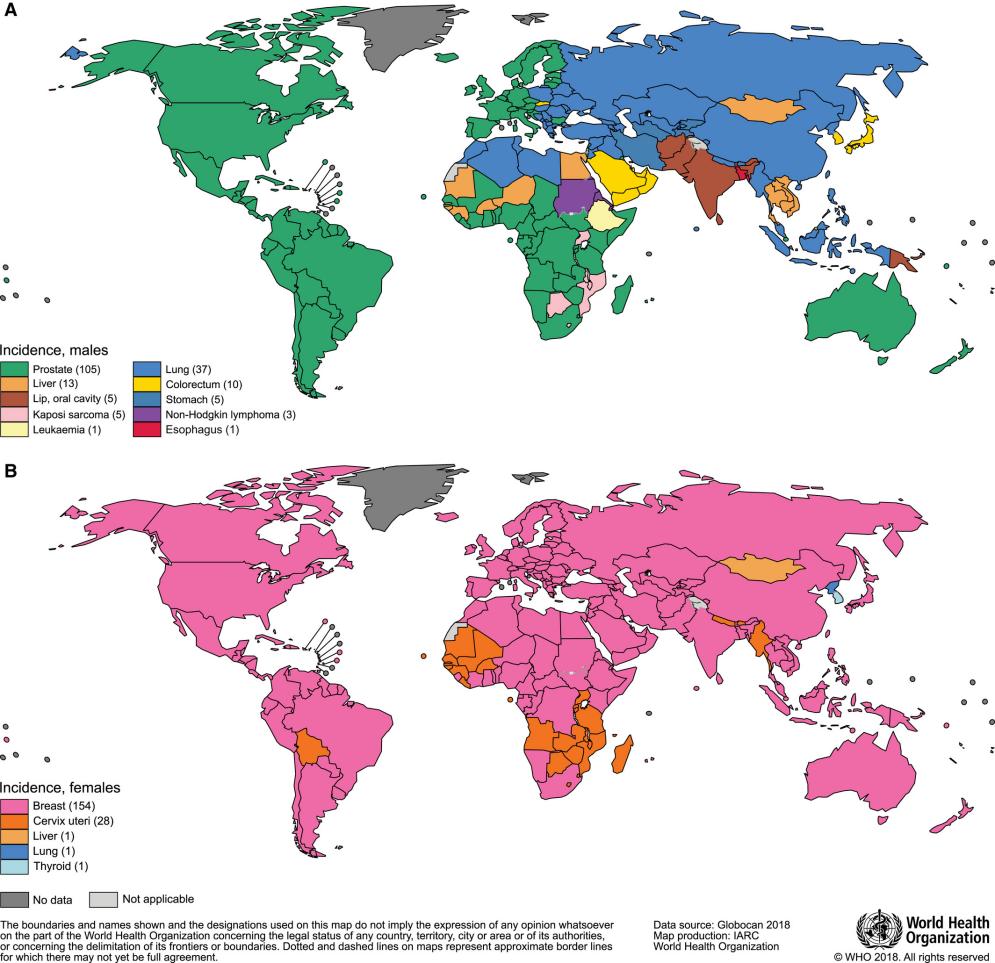
The pathogenesis of HCC is rather complex and is usually driven by multiple steps. Before the formation of malignant clonal proliferation in HCC, it generally goes through several stages such as chronic liver injury, liver cirrhosis, and hyperplasia [3]. And HCC usually has a latent onset and progresses rapidly [4].As a common disease, HCC has unique biological characteristics that directly affect its occurrence, development and treatment response. The frequency of gene mutations in most HCC is very high, especially mutations in the P53 and Insulin-like growth factor 2 [5]. HCC also has a relatively special tumor microenvironment such as abnormal vasculature, including hyper vascular and high interstitial pressure. These features collectively pose challenges in the treatment of HCC.
Since HCC has no specific symptoms in the early stage, it is difficult for diagnosis and treatment. Although millions of people lose their lives due to HCC every year, current treatment methods are still mainly based on hepatic resection [6]. However, these methods exhibit many side effects, making patients suffer greatly during the process, meanwhile, the delivery of the free drug to tumour is comparatively limited, leading to poor bioavailability. Therefore, it is important and urgent to find effective and less harmful treatment methods, such as some systems that can achieve targeted drug delivery.
1.2. Targeted drug delivery systems for hepatocellular carcinoma
Over the past few decades, efforts have been made to research and develop new targeted drug delivery systems. Among them, s series of treatment schemes achieved through nanotechnology are considered promising.
1.2.1. Nano system
A nano-based drug delivery system refers to drug delivery carriers designed through nanotechnology (such as liposomes, polymer nanoparticles, inorganic nanomaterials, etc.). The carrier not only protects the drugs from degradation but also enhances their solubility. Due to their unique size effect, nano-scale carriers exhibit significant advantages in cancer treatment. For example, the abnormal blood vessels and absent lymphatic drainage in HCC enable nanoparticles to selectively accumulate in tumor tissues through the EPR effect, increasing drug concentration. In addition, in the context of HCC, nano drugs can be actively transported by the liver’s reticuloendothelial system, thus naturally accumulating in the liver tumor area. Another advantage of nanocarrier is the possibility of manipulating and combining the functional design of different carriers, thus achieving more precise cellular and subcellular targeting, which could significantly improve efficacy while reducing side effects.
1.2.2. Different carriers for the treatment of HCC
By selecting appropriate materials and optimizing the design of the structure, nano carrier can enable controlled release and targeted delivery of the drug. Currently, a large number of nanomedicines with promising development have been developed. These materials could be classified into organic and inorganic categories.
In the treatment of HCC, organic nano carriers mainly include liposomes, polymer-based particles, etc. Their core advantage lies in excellent biocompatibility and degradability. Through the EPR effect and the natural uptake effect of the reticuloendothelial system, they can effectively achieve the enrichment of drugs at the liver tumor site. These carriers are easy to be functionalized for prolonged circulation and enhanced targeting, and the preparation process is relatively mature. The structure of these organic carriers enables them to not only carry hydrophilic drugs (such as doxorubicin), but also hydrophobic drugs (such as sorafenib), showing certain flexibility. Controlled release can be achieved through responsive design of the carriers, significantly improving efficacy and reducing systemic toxicity.
Inorganic nano carriers are mainly represented by gold nanoparticles and mesoporous silica, etc. Their characteristics include stable structure, high drug loading capacity and strong functional expansion ability. This kind of carriers possess unique electrical and magnetic properties, enabling multimodal diagnosis and treatment integration. Through precise control of size and surface chemistry, inorganic carriers can integrate different functional components and, while deliver drugs, may also achieve synergistic treatment with different therapy. The two types of carriers complement advantages of each other, jointly promoting the development of precise treatment for HCC.
1.2.3. Methods for enhancing targeting
The excellent performance of nanocarriers in experiment has revealed their potential. To achieve more efficient drug delivery by carriers, ultrasound-enhanced drug delivery has been proposed, which is a delivery technology that utilizes the physical effects of ultrasound to enhance the targeting and efficacy of drugs. Its core lies in achieving precise positioning and controlled release through the interaction between ultrasound and the carriers.
1.3. Ultrasound-enhanced drug delivery system
1.3.1. Ultrasound cavitation
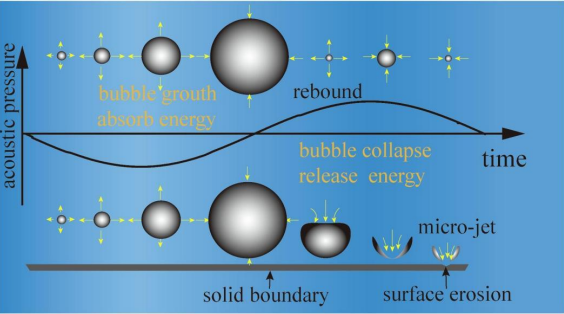
Ultrasonic cavitation effect refers to the mechanical movement of tiny bubbles under the action of ultrasound waves, the process of which includes bubble formation, oscillation, growth and collapse (Figure 2). According to the dynamic behavior of the bubbles, the cavitation can be divided into two types. Stable cavitation means that bubbles oscillate periodically in the ultrasonic field without rupturing. This process can enhance the permeability of blood vessels and cell membranes, then promote the slow release of drugs from carriers [7]. Inertial cavitation refers to the instantaneous collapse of bubbles after intense expansion, generating local high temperature and pressure. The phenomenon of ultrasonic cavitation has important applications in the medical field (such as drug delivery, tumor treatment, and tissue ablation.
1.3.2. Advantages
Ultrasound-enhanced drug delivery has demonstrated multiple significant advantages in the treatment of HCC due to its unique regulatory mechanism. Firstly, this therapy improves the accuracy of targeting and reduced side effects. Since ultrasound waves can precisely focus on the tumor area while avoiding systemic exposure, the toxicity to normal tissues can be reduced. Moreover, the carrier can selectively rupture under the action of ultrasound, achieving local release of the drug. Besides, the cavitation effect can open the intercellular spaces within blood vessels and increase the permeability of the cell membrane, thereby promoting the delivery and absorption of the drug.
In addition to improving the transportation efficiency of the drugs, this therapy also demonstrates high biocompatibility. The ultrasound probe can achieve targeted treatment of deep tumors outside the body, avoiding the risks of surgery or catheter insertion. At the same time, many carriers themselves can also be used as ultrasound contrast agents, enabling simultaneous treatment and effect monitoring, and having the potential for integrated diagnosis and treatment.
1.3.3. Research pipeline of ultrasound-enhanced drug delivery system for HCC
Before the clinical trials, the experiments will be conducted in cell lines and animal models. The following will briefly introduce the two most common models, which will be mentioned later. The HepG2 cell line, used for in vitro cultivation, is derived from the hepatoblastoma cell line and has the feature of easy proliferation. This cell line is often used for drug screening and toxicity testing. The VX2 animal model is derived from rabbits. It can simulate the angiogenesis, necrosis and microenvironment of solid tumors, and can be used for interventional treatment research.
2. Carriers
At present, in the field of ultrasound-targeted drug delivery research, a variety of different carriers have been studied and developed. In the main context of this review, we will focus on introducing three common and typical carriers that have been extensively researched: bubbles, liposomes and particles. Additionally, some of their current application examples will be provided.
2.1. Bubbles
Bubbles are currently the most common drug carriers and have already achieved preliminary clinical applications. According to their sizes, bubbles can be divided into two types: microbubbles (2-5μm) and nanobubbles (50-500nm). Due to their difference size, these two exhibit different properties. In contrast to nanobubbles which can penetrate deep into tumor tissues, the penetration of microbubbles is limited by their size and mainly remains in the blood vessels. However, microbubbles have higher ultrasonic responsiveness and can rupture with low frequency ultrasound. Both can be triggered by ultrasound, which could induce cavitation effects, rupture, and further enable enhanced drug release in the target area.
2.1.1. Microbubbles
Based on its potential as a non-invasive delivery mechanism, ultrasound-targeted microbubble destruction has been used in various organ systems and tumors to successfully deliver drugs and genes. Liver is an ideal target organ for this method because it is accessible for ultrasound. Studies have revealed surprising uses of ultrasound-targeted delivery with microbubbles for treating liver cancer in vitro and vivo.
Recently, a kind of ultrasound microbubbles modified with the nano graphene oxide (GO) is regarded as a promising strategy for the treatment of liver cancer [9].Graphene is a single-layer, two-dimensional material with exceptional physical and chemical properties, which is ideal for drug carrying because of its large surface area. In this study, the team use L-theanine (TH), a natural polyphenol with antitumor and ant antioxidant effects, as an ideal reducing agent for GO to prepare nanoscale reduced graphene oxide (rGO-TH). The rGO which retains a portion of hydrophilic groups can be easier to attach to and modify microbubbles, thus SV@rGO-TH MBs capable of carrying drugs can be obtained (Figure 3). Then researchers evaluated the cytotoxicity of composite microbubbles under ultrasound irradiation on HepG2 cells in vivo and in vitro environments. After detecting the production of ROS in tumor cells as the measure of cytotoxicity, results showed that in contrast to PBS+US groups which were set as no-treatment groups and revealed no noticeable fluorescence, rGO+US groups exhibited weak green fluorescence, proving that rGO nanosheets generated a certain level of ROS, which resulted in the strongest cytotoxic effect on HepG2 cells. This offers a new method for the treatment of liver cancer.
![Figure 3. Transmission electron microscope images: f1:GO; f2:rGO-TH; f3:SV MBs; f4:SV@rGO-TH MBs [9].](https://file.ewadirect.com/press/media/markdown/document-image3_0iyNbyQ.png)
Apart from using microbubbles to deliver drugs, another therapy using sonoporation by microbubbles to deliver gene also showed promising results and is seen as a treatment option for patients with advanced HCC [10].In this study, researchers used microbubbles to deliver gene plasmids, activating the apoptosis pathway that was silenced in liver cancer. The team recorded an enhanced effect of pro-apoptotic events when gene sonoporation and epigenetic treatment were combined. Results showed that among several condition tested, 3 MHz, 51% Duty Cycle, and 5W/cm2, 60s were the best parameter to achieve a good gene transfection efficacy and lesser toxicity to body. Under these parameters, a transfection efficacy of 30%-50% was achieved and the expression of recombinant gene induced apoptotic effects. This study showed that it is possible to induce the expression of the pro-apoptotic gene in liver cancer cells through sonoporation.
2.1.2. Nanobubbles
With the advancement of technology, nanobubbles, which is smaller in size have also been used as one of the carriers for ultrasound-targeted delivery. Due to their smaller size, nanobubbles can directly penetrate tumors, enabling more efficient drug delivery. Currently, they are still in the stage of laboratory research. Both in vitro and in vivo experiments demonstrated outstanding performance respectively in early- and late-stage. Besides ultrasound targeting, different studies have explored additional alternative approaches.
Mingming Meng, et al took advantage of the EPR effect existing in blood vessels for passive tumor targeting in addition to ultrasound targeting [11]. This strategy is especially used for the early stage of cancer as the tumor structure is still loose. In this study, a doxorubicin nanobubble (DOX-NB) wrapping carbon tetrafluoride gas was prepared in double emulsion method. The team observed the ability to target drug delivery to tumors of loading DOX-NB in VX2 tumor model. It can be seen from the result that the total drug release rate of doxorubicin under ultrasound vibration was more rapid than nanobubbles alone. It can be confirmed that ultrasound has controlled the effect of drug release for DOX-NB and it can deliver drug targeting at tumor under ultrasound. Meanwhile, DOX-NB could enhance the ultrasonic imaging significantly when applied to tumor area. Therefore, this strategy using nanobubbles has great potential in tumor therapy combined with ultrasound and offers a fire-new method for cancer treating and imaging.
Another research team employed the anti-GPC3 antibody as a method for tumor targeting. GPC3 is specifically expressed in most HCC cases, so it is seen as an important marker of liver cancer [12].In this study, Apatinib-loaded nanobubbles were prepared by a mechanical vibration method and anti-GPC3 antibody was coated onto the nanobubbles to target HepG2 cells (Figure 4) . Then the team evaluated the cells’ proliferation with different treatment. In contrast to non-treatment groups, group with GPC3-targeted and Apatinib-loaded nanobubbles (TALNBs) showed significantly higher rate, suggesting that under ultrasound this treatment effectively inhibited cell proliferation. Additionally, anti-GPC3 antibody conjugated with drug-loaded nanobubbles improved the attachment on HepG2 cells. Although this binding method performed well in in vitro tests, it may encounter some problems when applied in clinic. TALNBs might release GPC3 in the blood, resulting in an increase in the drug concentration in regions besides the tumor.
![Figure 4. Schematic diagram of fabrication of TALNBs [12]](https://file.ewadirect.com/press/media/markdown/document-image4_hPye4EJ.png)
2.2. Liposomes
Liposomes are phospholipids that can either be a monolayer bubble containing inert gas inside, or a bilayer bubble containing liquid.
There are several reasons why liposomes could be taken into consideration as the material of the bubbles used in ultrasound drug delivery.
Firstly, liposomes exist in our body as cell surface membranes or membranes of cell organelles. If the liposomes do not contain sugar chains on the surface of the cell that may be recognized as non-self-antigens, the chance of triggering an immune response will be much smaller than with materials like metals or ceramics [13].
In the comparison of bilayer liposomes (BL) and monolayer liposomes (ML), ML has shown to be a better material than bilayer liposomes. At the structural level, BL has a polar interior that holds liquid, while ML has a non-polar core filled with inert fluorous gas. The phospholipids in BL have their hydrophilic phosphate heads facing the inside of the bubble, so the bubbles are filled with polar liquid. MLs only have one layer of phospholipids, and the hydrophilic phosphate heads must face the outside, because the bubbles need to stay in blood and body fluids. Therefore, the interior must be hydrophobic as the non-polar hydrocarbon tails face inward. Both types of liposomes, however, contain regions for both hydrophilic and hydrophobic drugs due to the properties of phospholipids [14]. If there is one bubble of each kind of liposome, in comparison, ML has more space for inert fluorous gas, and as a result, if the gas leaves the bubble at a constant rate, the larger volume makes it harder for the bubble to run completely out of gas and collapse. A lower chance of collapsing enhances its stability, allowing it to complete circulation throughout the body. Also, if fluorous phospholipids are used, there will be fluorous-fluorous interactions between the membrane and the gas core, which makes the bubble more stable [15].
There is a kind of monolayer liposome microbubble that has already been clinically validated. The structure of lipid-coated microbubbles basically consists of two parts: an inert gas core and a lipid outer shell. The insoluble inert gas core makes the bubble more stable and the inert gas less likely to leak out, so the bubble is less likely to collapse [16].
Due to fluorophobic, there is strong interaction between fluoro-lipids and fluorous gas, which makes the bubble more gas-tight and increases its stability. Fluoro-lipids are made by chemically attaching fluorine atoms to lipids and are customized into different kinds of fluoro-lipids that provide different functions. The customized fluoro-lipids are mixed with DSPC and DSPE-PEG, two kinds of lipids, to form liposomes. Then the liposomes are placed into specific containers that contain the required fluorous gas, and sonication is used to form lipid-coated microbubbles, which are lipid-coated gas core microbubbles.
For example, the product Definity, which is called perflubron lipid microspheres, is a kind of fluoro-lipid-coated microbubble. The gas core is mainly composed of perfluoro propane (C₃F₈) [17]. C₃F₈ is an inert gas, and because the bond between fluorine and carbon atoms is very strong, it will not react under human body conditions. It also has low water solubility and is highly hydrophobic. The lipid shell contains three kinds of lipids that provide different functions: primary phospholipids, helper lipids, and PEGylated lipids. Primary lipids such as dipalmitoyl phosphatidylcholine (DPPC) contain two saturated carbon chains, each with 16 carbon atoms. The saturated carbon chains make the lipids more stable, so they will not react easily under body temperature, and they also provide mechanical support. Helper lipids such as cholesterol are interspersed between primary lipids; they help maintain the fluidity of the whole lipid coat under different temperatures. PEGylated lipids such as 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000) are made when the lipid DSPE and the compound PEG are connected by a chemical bond. PEG then forms a hydrophilic layer on the surface of the lipid-coated microbubbles in order to prevent them from collapsing into each other. This product uses the features of PEG and the fluorophobic between fluorine compounds to improve the load capacity and the stability of lipid-coated microbubbles.
2.3. Nanoparticle
To begin with, nanoparticles are a kind of engineered particles which have a size within the 1-100 nm size range. However, functionally extended to submicron scales (from about 250-450nm) demonstrate their biomedical applications [18]. Through their unique chemical properties, these tiny particles have basically changed the way medical diagnosis and treatment are conducted. In the field of diagnostic imaging, specialized nanoparticles greatly improve detection capabilities as contrast agents: superparamagnetic iron oxide nanoparticles (SPIONs) improve magnetic resonance imaging (MRI) sensitivity for hepatocellular carcinoma. Furthermore, gold nanoparticles (AuNPs) have provided superior contrast results in computed tomography (CT) imaging instead of traditional iodine-based contrast media. In addition, hyaluronic acid nanoparticles with echogenic properties were able to extend the time for ultrasound visualization of liver tissue, which can maintain signal integrity for more than 180 minutes, far exceeding the performance of traditional micro vesicles.
2.3.1. ADM-NMC
The nanoparticles successfully synthesized ADM-NMC by covalently connecting drug-loaded PLGA nanoparticles to microbubble shells (Figure 5) . Moreover, it hugely enhances drug loading capabilities while retaining the critical ultrasound contrast capabilities of image-guided delivery, and in vivo imaging confirmed effective tumor contrast enhancement.
![Figure 5. A) Size distribution of ADM-NMCs by dynamic light scattering (DLS) measurement; B) optical microscope image of ADM-NMCS; C) laser confocal microscope image of NMCs (DiI-labeled NPs, FITC labeled MBs). REF _Ref4538 \r \h \* MERGEFORMAT [19]](https://file.ewadirect.com/press/media/markdown/document-image5_9F3JN4j.png)
One of the most crucial points is that LIFU provides superior spatial control for localized microbubble disruption as well as efficient drug release compared to off-focus ultrasound, allowing for precise targeting. In the rabbit VX2 liver tumor model, the "LIFU + ADM-NMCs" combination produced extraordinary therapeutic influences: it reached the highest tumor volume suppression rate (VIR = 56.73%). And induced the lowest proliferation index and the highest apoptosis index demonstrated the most effective tumor suppression (Figure 6) . Moreover, it largely extended median survival to 71 days compared to 31 days in the control group. It encapsulation within ADM-NMC significantly reduces cardiotoxicity associated with free alkathromycin, thereby improving the safety of the treatment.
![Figure 6. Kaplan–Meier curve of each group. The survival rate of LIFU + ADM-NMCs group was obviously higher than that of other groups REF _Ref4538 \r \h \* MERGEFORMAT [19]](https://file.ewadirect.com/press/media/markdown/document-image6_WTUE4Vr.png)
This approach combining ADM-NMC with LIFU-mediated delivery offers a higher efficient, targeted, and safer strategy for liver tumor chemotherapy. And also offering huge potential for future clinical translation [19].
2.3.2. Echo-NPS
However, nanoparticle can also be used for present ultrasound imaging. In the contemporary medicine, it presents an increasingly trend for instant real-time ultrasound imaging of specific organ such as liver, target-specific and long -circulating ultrasound contrast agents are of special interest. Ultrasound has been the one of the most significant clinical imaging modalities for several decades but conventional (UCAs) still exists many drawbacks such as it poor-imaging contrast and short time windows (especially without the help with ultrasound contrast agent). A highly qualified UCA can provide more informative images to clinicians. Nonetheless, the insuperable conflicting problem of traditionally UCAs is intravascular imaging, especially for micro bubbles that are from 1 to 8 micrometers. Because conventional UCAs is too unstable during blood circulation and the imaging will be unclear and also traditional UCAs have a very large size and so it is hard for it to diffuse out the microvasculature into surrounding tissues after injection.
Conventionally, there has a huge hindrance to the exploitation for ideal UCAs, because the size of particles decreases, the high echo ability be offset, so image cannot be presented perfectly. Moreover, the optimum particles size for effective extravasation should be smaller than 750 nm and particle size should be sub-micrometer to obtain effective ultrasound contrast. However, there is a new species of echogenic hyaluronic acid nanoparticles is presented as an ultra-long-acting, liver-specific UCA which is totally different from traditional UCAs. A novel ultrasound contrast agent was developed through an oil-in-water (O/W) emulsion process. In this work, biologically inert perfluoro pentane (PFP), a hydrophobic ultrasound-responsive gas, becomes encapsulated within hyaluronic acid nanoparticles (HANPs) via hydrophobic interactions. The HANPs form spontaneously through self-assembly of amphiphilic hyaluronic acid-5β-cholinic acid (HA-CA) conjugates in aqueous solution. There resulting PFP-loaded HANPs (Echo-NPs) possess distinct solid nano-structures, which are different from conventional gas-core microbubbles [20]. In this graph it shows clearly that the unique morphology and size distribution (Figure 7) .
![Figure 7. a) Physical properties in terms of hydrodynamic size and zeta potential, b) observation of monodispersed and Echo-HANPs in contrast to extremely heterogeneous and large-sized Sonovue and Echo-HA. c) observation of the inner structure and PFP localization inside Echo-NPs contrasted to HANPs without PFP and HA and PFP simple mixtures (Echo-HA). REF _Ref5061 \r \h \* MERGEFORMAT [20]](https://file.ewadirect.com/press/media/markdown/document-image7_PJG3cS0.png)
Echo-NPs mainly have three significant advantages that are better than traditional ones. Firstly, it can enhance structural stability with optimal hydrodynamic properties for in vivo use. Secondly, gradual PFP vaporization leading to controlled expansion in physiological conditions. Moreover, it can generate strong, sustained ultrasound signals at target sites after multiple circulatory passes.
3. Conclusion and outlook
The carriers mentioned above content can all achieve targeted drug delivery through ultrasound-mediated means, but each has its own advantages and disadvantages in practical application. The circulation time of particles is relatively long (20 hours), whereas bubbles typically persist only for a few minutes. However, compared with bubbles and liposomes, the production process of particles is more complex and high-cost. In term of stability, both particles and bubbles have relatively excellent stability, while liposomes are comparatively less stable. Depending on the different application scenarios, different types of carriers with corresponding properties can be selected.
Currently, clinical applications of ultrasound-targeted drug delivery for cancer treatment are very limited, indicating that this technology largely remains at the laboratory research stage. Therefore, the main direction of the next research is to conduct clinical application attempts while ensuring safety. Based on the current results obtained from in vivo and vitro cultures, the targeted strategy using ultrasound shows outstanding potential. With continuous exploration and optimization, there is a possibility that they could replace conventional radiotherapy and chemotherapy, ultimately becoming a novel approach for cancer treatment.
References
[1]. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries [J]. CA: a cancer journal for clinicians, 2024, 74(3): 229-263.
[2]. Sagnelli, E., Macera, M., Russo, A., Coppola, N., & Sagnelli, C. (2020). Epidemiological and etiological variations in hepatocellular carcinoma. Infection, 48(1), 7-17.
[3]. Balogh, J., Victor III, D., Asham, E. H., Burroughs, S. G., Boktour, M., Saharia, A., ... & Monsour Jr, H. P. (2016). Hepatocellular carcinoma: a review. Journal of hepatocellular carcinoma, 41-53.
[4]. Liu, Y., Yang, S., Zhou, Q., Zhou, J., Li, J., Ma, Y., ... & Zhao, Y. (2022). Nanobubble-based anti-hepatocellular carcinoma therapy combining immune check inhibitors and sonodynamic therapy. Nanoscale Advances, 4(22), 4847-4862.
[5]. Motola-Kuba, D., Zamora-Valdés, D., Uribe, M., & Méndez-Sánchez, N. (2006). Hepatocellular carcinoma. An overview. Annals of hepatology, 5(1), 16-24.
[6]. Befeler, A. S., & Di Bisceglie, A. M. (2002). Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology, 122(6), 1609-1619.
[7]. Wu, J., & Nyborg, W. L. (2008). Ultrasound, cavitation bubbles and their interaction with cells. Advanced drug delivery reviews, 60(10), 1103-1116.
[8]. Wu, P., Wang, X., Lin, W., & Bai, L. (2022). Acoustic characterization of cavitation intensity: A review. Ultrasonics Sonochemistry, 82, 105878.
[9]. Zhou Q, Wang Y, Cheng Q, et al. Graphene Oxide Modified Microbubble for the Ultrasonic Imaging and Treatment of Hepatocellular Carcinoma [J]. ACS Applied Nano Materials, 2025, 8(15): 7825-7839.
[10]. Rinaldi L, Folliero V, Palomba L, et al. Sonoporation by microbubbles as gene therapy approach against liver cancer [J]. Oncotarget, 2018, 9(63): 32182.
[11]. Meng M, Gao J, Wu C, et al. Doxorubicin nanobubble for combining ultrasonography and targeted chemotherapy of rabbit with VX2 liver tumor [J]. Tumor Biology, 2016, 37(7): 8673-8680.
[12]. Tian Y, Liu Z, Zhang L, et al. Apatinib-loaded lipid nanobubbles combined with ultrasound-targeted nanobubble destruction for synergistic treatment of HepG2 cells in vitro [J]. OncoTargets and therapy, 2018: 4785-4795.
[13]. Schroeder, Avi, Joseph Kost, and Yechezkel Barenholz. "Ultrasound, liposomes, and drug delivery: principles for using ultrasound to control the release of drugs from liposomes." Chemistry and physics of lipids 162.1-2 (2009): 1-16.
[14]. Blume, Alfred. "A comparative study of the phase transitions of phospholipid bilayers and monolayers." Biochimica et Biophysica Acta (BBA)-Biomembranes 557.1 (1979): 32-44.
[15]. Mukherjee, Biswajit, et al. "Size dependent variations of phospholipid based vesicular drug carriers in systemic drug activity." Current Pharmaceutical Biotechnology 16.4 (2015): 380-391.
[16]. Tikhonova, Elena G., et al. "Study of Physico-Chemical Properties and Morphology of Phospholipid Composition of Indomethacin." Nanomaterials 12.15 (2022): 2553.
[17]. Oda Y, Suzuki R, Mori T, et al. Development of fluorous lipid-based nanobubbles for efficiently containing perfluoropropane [J]. International Journal of Pharmaceutics, 2015, 487(1-2): 64-71.
[18]. Moghadam F F. Using nanoparticles in medicine for liver cancer imaging [J]. Oman medical journal, 2017, 32(4): 269.
[19]. Gong Y, Wang Z, Dong G, et al. Low-intensity focused ultrasound mediated localized drug delivery for liver tumors in rabbits [J]. Drug delivery, 2016, 23(7): 2280-2289.
[20]. Min H S, Son S, Lee T W, et al. Liver‐specific and echogenic hyaluronic acid nanoparticles facilitating liver cancer discrimination [J]. Advanced Functional Materials, 2013, 23(44): 5518-5529.
Cite this article
Feng,Y.;Chen,X.;Shen,Z. (2025). Different Carriers for Ultrasound-Enhanced Drug Delivery of Hepatocellular Carcinoma. Theoretical and Natural Science,139,28-39.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: AI for Healthcare: Advanced Medical Data Analytics and Smart Rehabilitation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries [J]. CA: a cancer journal for clinicians, 2024, 74(3): 229-263.
[2]. Sagnelli, E., Macera, M., Russo, A., Coppola, N., & Sagnelli, C. (2020). Epidemiological and etiological variations in hepatocellular carcinoma. Infection, 48(1), 7-17.
[3]. Balogh, J., Victor III, D., Asham, E. H., Burroughs, S. G., Boktour, M., Saharia, A., ... & Monsour Jr, H. P. (2016). Hepatocellular carcinoma: a review. Journal of hepatocellular carcinoma, 41-53.
[4]. Liu, Y., Yang, S., Zhou, Q., Zhou, J., Li, J., Ma, Y., ... & Zhao, Y. (2022). Nanobubble-based anti-hepatocellular carcinoma therapy combining immune check inhibitors and sonodynamic therapy. Nanoscale Advances, 4(22), 4847-4862.
[5]. Motola-Kuba, D., Zamora-Valdés, D., Uribe, M., & Méndez-Sánchez, N. (2006). Hepatocellular carcinoma. An overview. Annals of hepatology, 5(1), 16-24.
[6]. Befeler, A. S., & Di Bisceglie, A. M. (2002). Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology, 122(6), 1609-1619.
[7]. Wu, J., & Nyborg, W. L. (2008). Ultrasound, cavitation bubbles and their interaction with cells. Advanced drug delivery reviews, 60(10), 1103-1116.
[8]. Wu, P., Wang, X., Lin, W., & Bai, L. (2022). Acoustic characterization of cavitation intensity: A review. Ultrasonics Sonochemistry, 82, 105878.
[9]. Zhou Q, Wang Y, Cheng Q, et al. Graphene Oxide Modified Microbubble for the Ultrasonic Imaging and Treatment of Hepatocellular Carcinoma [J]. ACS Applied Nano Materials, 2025, 8(15): 7825-7839.
[10]. Rinaldi L, Folliero V, Palomba L, et al. Sonoporation by microbubbles as gene therapy approach against liver cancer [J]. Oncotarget, 2018, 9(63): 32182.
[11]. Meng M, Gao J, Wu C, et al. Doxorubicin nanobubble for combining ultrasonography and targeted chemotherapy of rabbit with VX2 liver tumor [J]. Tumor Biology, 2016, 37(7): 8673-8680.
[12]. Tian Y, Liu Z, Zhang L, et al. Apatinib-loaded lipid nanobubbles combined with ultrasound-targeted nanobubble destruction for synergistic treatment of HepG2 cells in vitro [J]. OncoTargets and therapy, 2018: 4785-4795.
[13]. Schroeder, Avi, Joseph Kost, and Yechezkel Barenholz. "Ultrasound, liposomes, and drug delivery: principles for using ultrasound to control the release of drugs from liposomes." Chemistry and physics of lipids 162.1-2 (2009): 1-16.
[14]. Blume, Alfred. "A comparative study of the phase transitions of phospholipid bilayers and monolayers." Biochimica et Biophysica Acta (BBA)-Biomembranes 557.1 (1979): 32-44.
[15]. Mukherjee, Biswajit, et al. "Size dependent variations of phospholipid based vesicular drug carriers in systemic drug activity." Current Pharmaceutical Biotechnology 16.4 (2015): 380-391.
[16]. Tikhonova, Elena G., et al. "Study of Physico-Chemical Properties and Morphology of Phospholipid Composition of Indomethacin." Nanomaterials 12.15 (2022): 2553.
[17]. Oda Y, Suzuki R, Mori T, et al. Development of fluorous lipid-based nanobubbles for efficiently containing perfluoropropane [J]. International Journal of Pharmaceutics, 2015, 487(1-2): 64-71.
[18]. Moghadam F F. Using nanoparticles in medicine for liver cancer imaging [J]. Oman medical journal, 2017, 32(4): 269.
[19]. Gong Y, Wang Z, Dong G, et al. Low-intensity focused ultrasound mediated localized drug delivery for liver tumors in rabbits [J]. Drug delivery, 2016, 23(7): 2280-2289.
[20]. Min H S, Son S, Lee T W, et al. Liver‐specific and echogenic hyaluronic acid nanoparticles facilitating liver cancer discrimination [J]. Advanced Functional Materials, 2013, 23(44): 5518-5529.









