1. Introduction
Depressive disorder (also known as depression) is a common mental disorder with a depressed mood or loss of pleasure or interest in activities for long periods of time. It involves a depressed mood or loss of pleasure or interest in activities for long periods of time [1]. Depression is a disease which is a highly prevalent and debilitating mental disorder. It is recognized as one of the leading causes of global disease burden. According to the World Health Organization (WHO), major depressive disorder affects approximately 6% of the population annually, with a lifetime prevalence of up to 15-18% [2]. Although nowadays pharmacological and psychological therapies do exist, current treatments are not universally effective, and relapse rates remain high. This highlights our need to explore novel pathogenic mechanisms and therapeutic [3].
Growing evidence shows that inflammation in the brain, or neuroinflammation, plays a vital role in depression [4]. Diets high in fat or cholesterol are not only linked to metabolic problems but also to anxiety- and depression-like behaviours in both people and animal studies [5,6]. For example, mice fed a high-cholesterol diet (42% kcal fat + 1.25% cholesterol) developed anxiety- and depression-like behaviours accompanied by increased peripheral and central inflammation [7]. Similarly, obese rats on a high-fat diet exhibited elevated pro-inflammatory cytokines in the hippocampus and impaired serotonergic responses to escitalopram [8]. In ageing rats, even short-term high-fat intake led to hippocampal- and amygdala-dependent memory deficits, with increased microglia activation [9]. Notably, hypothalamic inflammation-including microgliosis, astrogliosis, and elevated TNF-α-was observed within 1-3 days of high-fat diet exposure. Recent preliminary analyses using lipidomic and transcriptomic approaches have begun to elucidate how saturated fat disrupts hypothalamic cAMP/PKA signalling via PDE4A activation, linking dietary intake with mood regulation pathways [10].
A high-fat diet (HFD) not only leads to obesity and metabolic syndrome but is also closely related to the occurrence of depression through multiple mechanisms. Long-term HFD can disrupt the intestinal barrier and homeostasis of intestinal flora, increase circulating levels of free fatty acids and inflammatory cytokines, thereby triggering low-grade chronic inflammation (Li., 2025). This peripheral inflammation can cross the blood-brain barrier and enter the central nervous system, activating microglia and astrocytes and inducing neuroinflammatory responses (Ma, 2025a). Meanwhile, abnormal lipid metabolism leads to mitochondrial dysfunction, oxidative stress and lipid droplet deposition, further altering the microglial phenotype and resulting in synaptic damage and impaired neural plasticity (Ma, 2025b). These dual imbalances in neuroimmunology and metabolism cause individuals to exhibit behavioural symptoms such as anxiety, anhedonia and cognitive impairment, forming an essential pathological bridge between obesity, metabolic disorders and depressive symptoms (Ma, 2025a and b). The relationship between obesity and depression is not one-way but rather an interactive and mutually reinforcing process. Under the state of obesity, pro-inflammatory factors (such as IL-6, TNF-α) secreted by excessive adipocytes and reactive oxygen species exacerbate peripheral inflammation through signalling pathways such as NF-κB, and induce excessive activation of M1-type microglia in the central nervous system, thereby damaging neurons and synaptic connections. Promoting the occurrence of depression [11]. Conversely, Depression can also lead to abnormal appetite and imbalance in energy metabolism through HPA axis disorder and elevated cortisol levels, thereby increasing the risk of obesity (Depression and obesity: evidence of shared)
Over time, such dietary patterns appear to induce inflammation in the hypothalamus, amygdala and hippocampus, which are directly implicated in regulating emotional responses within the brain. This also indicates that our diet directly influences brain health by altering immune pathways.
A study has revealed how diet-induced inflammation contributes to the development of depression [12]. Nevertheless, many questions remain unanswered. For instance, the precise molecular pathways linking high-fat diets to alterations in brain inflammation and depressive symptoms remain unclear.
Current treatments for depression also tend to overlook this diet-inflammation link, leaving a gap between what we know and how we treat patients. To deal with this challenge, this study focuses on the genomic and transcriptomic patterns of brain inflammation induced by a high-fat diet. By integrating multi-omics analysis and network pharmacology, we want to identify potential therapeutic targets and elucidate how everyday dietary habits may influence the onset and progression of depression. Ultimately, this work may open up new possibilities for more precise and personalised treatments for mood disorders influenced by HFD.
2. Materials and methods
2.1. Raw data source for RNAseq analysis
To identify differentially expressed genes associated with a high-fat diet (HFD), metabolic abnormalities, and depression, we retrieved relevant transcriptome sequencing (RNA-seq) data from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/). Finally, the GSE272983 dataset [13] was chosen. In the original study, mice were divided into the Control diet (CD) and the High-fat diet (HFD). Among them, the mice in the CD group received a standard diet throughout the experiment. In contrast, the mice in the HFD group were fed a high-fructose diet for an extended period to induce metabolic syndrome and related neuroinflammatory responses. Based on the experimental design from the original literature, the researchers further evaluated depressive and anxiety-like manifestations at the behavioural level (such as sucrose preference experiments and forced swimming experiments), and combined transcriptome sequencing to reveal the potential molecular mechanisms by which dietary factors influence neuroimmune responses and emotional disorders.
2.2. Differentially expressed gene and enrichment analysis of RNAseq raw data
The gene expression count matrix (GSE272983_countMatrix) was retrieved from the Gene Expression Omnibus (GEO) database (Accession: GSE272983). Differential expression analysis between the CD group and the HFD group under stress conditions was performed using the limma R package. Differentially expressed genes (DEGs) were identified based on the following criteria: adjusted P-value < 0.05 and absolute fold change (FC) ≥ 1.5. Volcano plots were generated using the ggplot2 R package to visualize the DEGs.
Functional enrichment analysis of the DEGs was conducted online platform (https://www.bioinformatics.com.cn/, accessed on 9 September 2025), including Gene Ontology (GO) analysis across three domains-biological process (BP), molecular function (MF), and cellular component (CC)-as well as KEGG pathway analysis.
2.3. Network pharmacology analysis
The Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) (https://www.tcmsp-e.com/) and the HERB database (http://herb.ac.cn/) were employed to predict the potential targets of these six substances. Screening criteria included an oral bioavailability (OB) value >30% and a drug-likeness (DL) index ≥0.18. Differentially expressed genes (DEGs) were used as depression-related targets. To identify the potential therapeutic targets of Medicine-Food Homology (MFH) in depression, an intersection analysis was conducted between MFH-related targets and DEGs targets. A Venn diagram was generated using the online platform SRplot (https://www.bioinformatics.com.cn/) to visualize the overlapping targets. The STRING online database (https://cn.string-db.org/) was utilized to construct and analyze the protein-protein interaction (PPI) network of the overlapping targets between the herbal compounds and depression-related genes. Hub genes were identified from the PPI network and subsequently subjected to Gene Ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis to explore their biological functions and underlying mechanisms.
3. Results
3.1. Analysis result of differential expression genes
In the original research, the animals in the HFD showed major depressive disorder (MDD) like-phenotype through the forced swim test and tail suspension test following the chronic unpredictable mild stress (CUMS) treatment. And in the Novel Object Recognition Test we found CUMS induced cognitive impairment-like phenotype in mice on HFD [13]. To investigate the role of HFD-related neuroinflammation in the development of depression, this study primarily focused on the differentially expressed genes in the CD group and the HFD group under stress, using these as the basis for subsequent bioinformatics analysis.
First, we found that a HFD caused clear changes in brain gene expression, including 22 genes upregulated, 416 genes downregulated and 55,049 genes showing no significant change (Fig. 1). Next, we performed enrichment analysis on the up- and down-regulated genes among the 438 DEGs, including GO and KEGG pathway analysis.
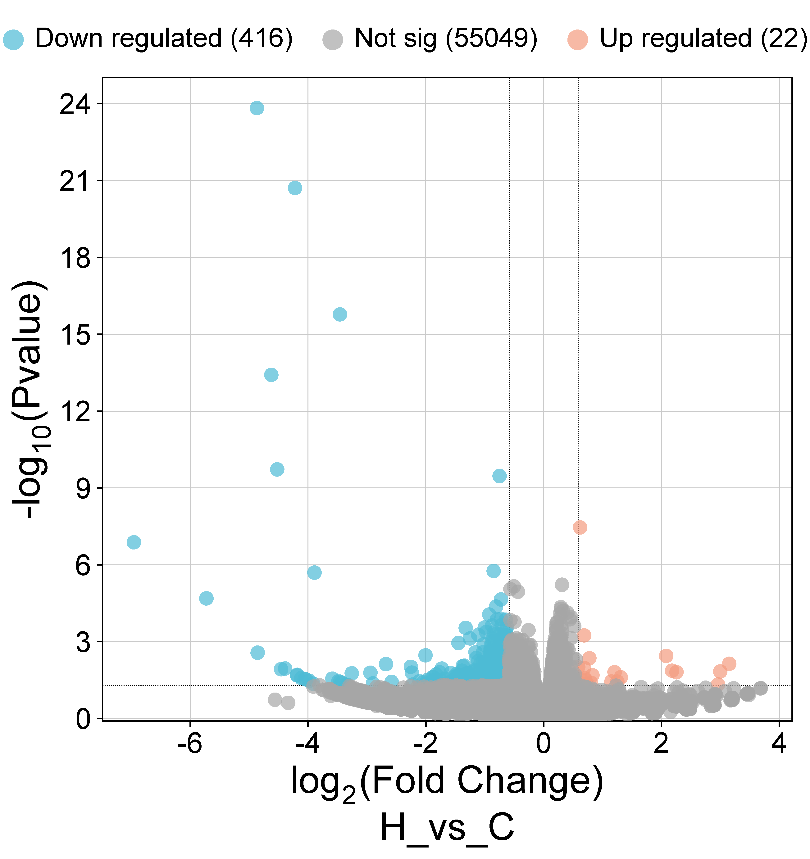
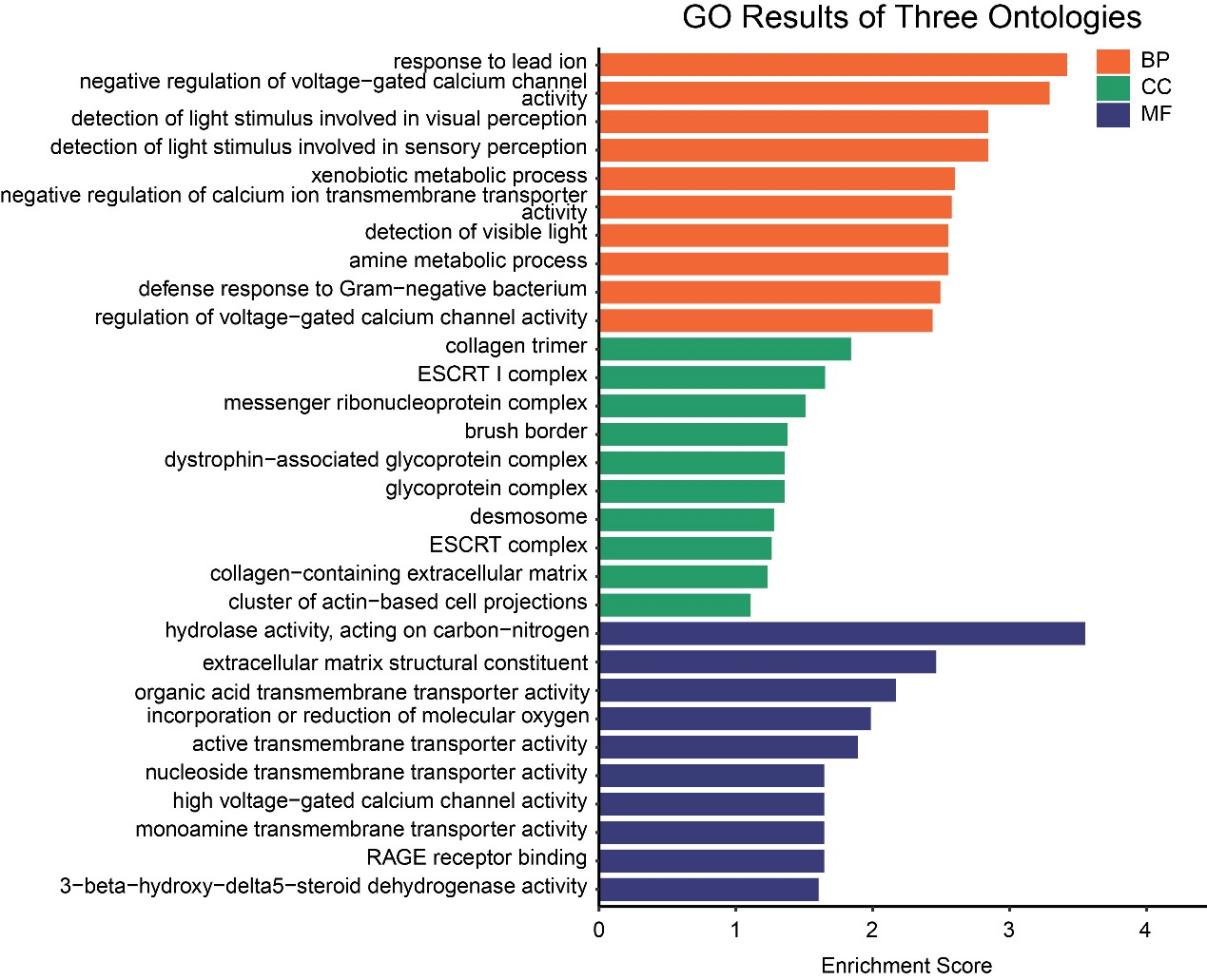
3.2. HFD downregulated the genes involved in neurotransmitter transport and regulation of calcium ion channel activity
GO enrichment analysis of down-regulated genes revealed significant enrichment (p < 0.05) in biological processes (BPs), cellular components (CCs), and molecular functions (MFs) (Fig. 2). In terms of biological processes, these genes were primarily enriched in response to lead ion, negative regulation of voltage-gated calcium channel activity, negative regulation of calcium ion transmembrane transporter activity, amine metabolic process, defense response to Gram-negative bacterium, and regulation of voltage-gated calcium channel activity-processes involved in neuroendocrine function, neuroplasticity, neurotransmission, and the immune-inflammatory system, all of which are closely associated with the mechanisms of depression. Moreover, light perception and xenobiotic metabolism were observed in processes, suggesting that high-fat diets may impact environmental adaptability and metabolic detoxification functions. At the cellular component level, significant enrichment was noted for the ESCRT I complex, messenger ribonucleoprotein complex, atrophin-related glycoprotein complex, and glycoprotein complex. In the molecular functions, monoamine transmembrane transport activity was markedly enriched, supporting the hypothesis that 'a high-fat diet may induce depression by inhibiting monoamine neurotransmitter transport and disrupting associated signalling pathways’. Furthermore, we found oxidoreductase activity and organic acid transmembrane transport activity, further linking these processes to the pathological mechanisms of depression. Overall, A high-fat diet may impair neural function and homeostasis regulation by suppressing the expression of genes associated with calcium ion and monoamine neurotransmitter transport, thereby ultimately promoting the development of depression.
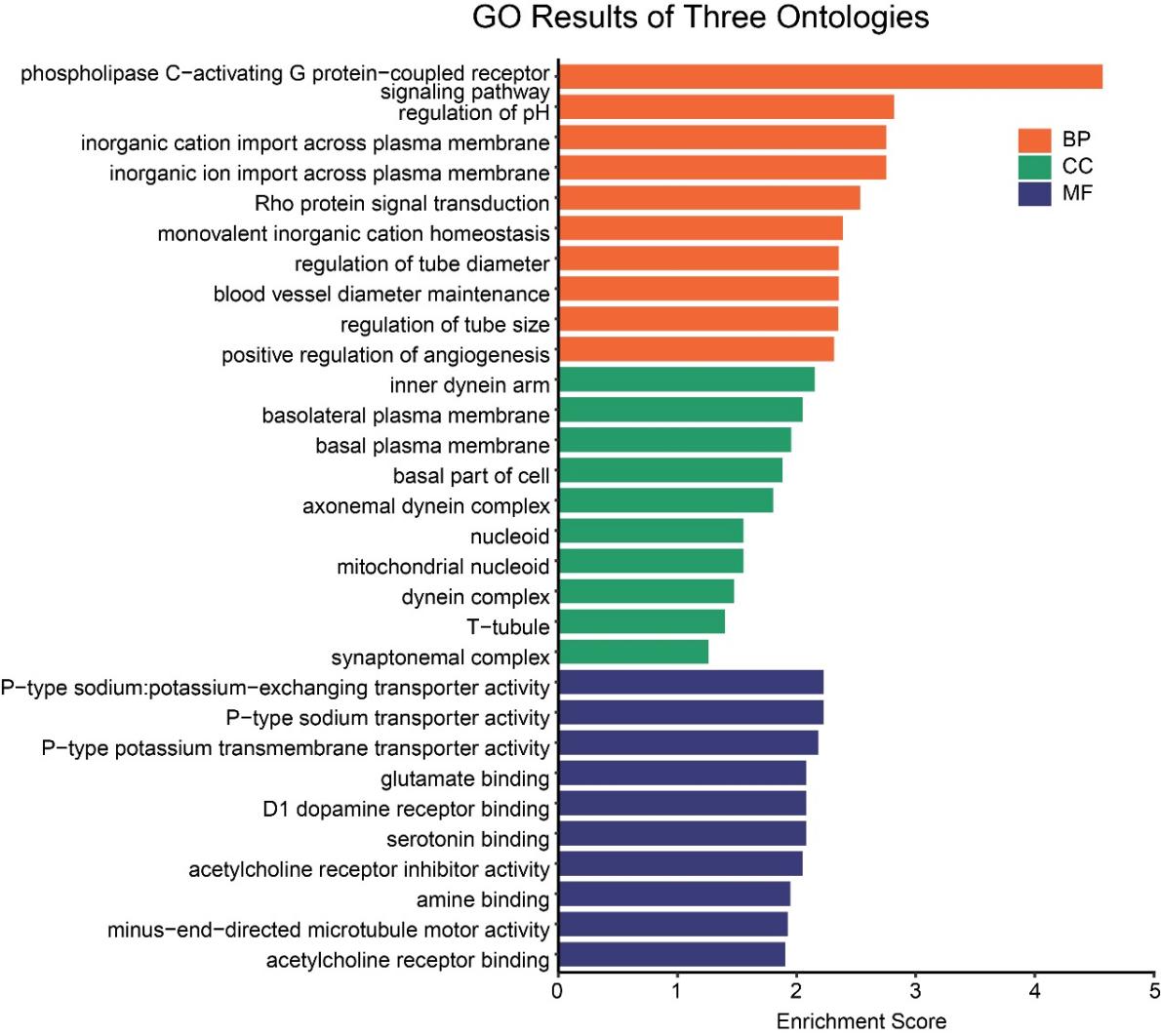
3.3. HFD mainly upregulated the genes involved in neurotransmitter receptor binding and neuronal signaling pathways
GO enrichment analysis of upregulated genes revealed significant enrichment (Fig. 3). In the biological process (BP) category, the phospholipase C-activating G protein-coupled receptor signaling pathway was significantly enriched and stood out. Meanwhile, regulation of pH, inorganic cation import / inorganic ion import / monovalent cation homeostasis, rho protein signal transduction, and positive regulation of angiogenesis were also significantly enriched. Cellular components were enriched in mitochondrial nucleoid, dynein complex, basal plasma membrane/basolateral plasma membrane, indicating associations with membrane-related functions. Molecular functions were significantly enriched in binding activities related to glutamate, D1 dopamine receptors, serotonin, acetylcholine, and other amine compounds, suggesting that HFD substantially affects multiple neurotransmitter systems.
Combined with the observed inhibition of monoamine transporter functions in downregulated genes, we hypothesize that HFD may induce a low-clearance, high-sensitivity neural state: the downregulation of monoamine transporters might represent a compensatory mechanism to reduce neurotransmitter reuptake, while the upregulation of multiple neurotransmitter receptors may reflect enhanced neuronal sensitivity in response to diminished signaling. However, prolonged overactivation of glutamate and acetylcholine systems could disrupt neural circuit balance, potentially triggering or exacerbating depression-related symptoms such as anxiety, anhedonia, and cognitive impairment, ultimately leading to compensatory failure.
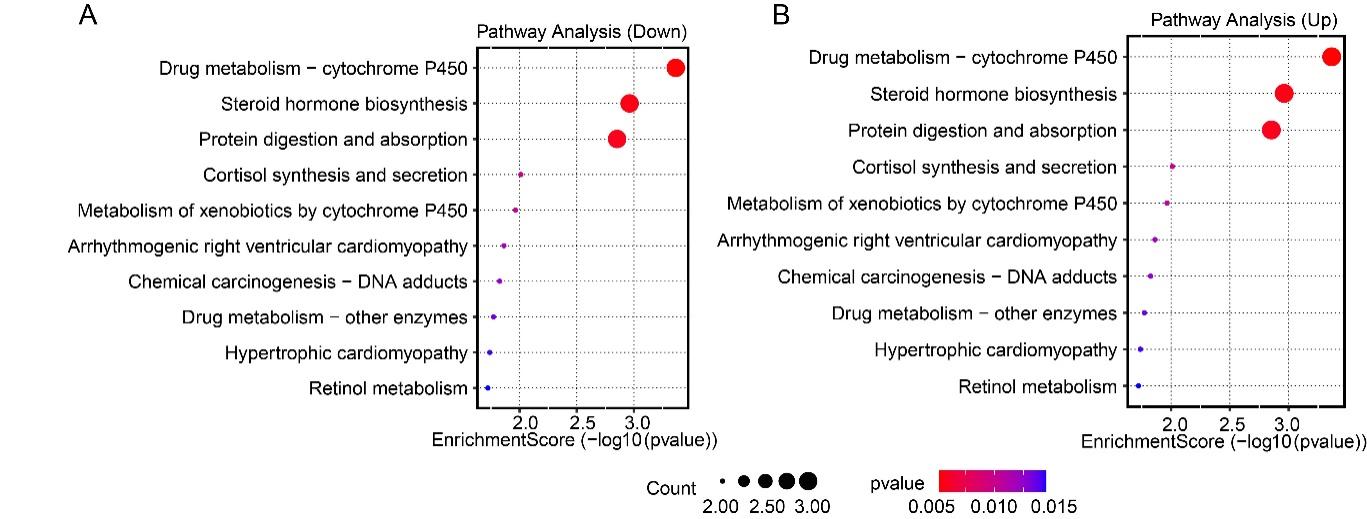
3.4. The different expression genes are involved in neurotransmitter signaling, metabolic regulation, and neuroendocrine pathways
KEGG enrichment analysis revealed that down-regulated genes were significantly enriched in drug metabolism (CYP450), steroid hormone biosynthesis, protein digestion and absorption, as well as cortisol synthesis and secretion pathways (p < 0.05) (Fig. 4A); up-regulated genes were enriched in neuroactive ligand-receptor interaction, calcium signaling pathway, hormone signaling, alanine, aspartate and glutamate metabolism, renin-angiotensin system, and arginine biosynthesis, all of which are closely associated with depression (Fig. 4B). These results are highly consistent with the GO analysis, indicating that HFD may disrupt brain homeostasis through multi-system coordination (neurotransmitters, calcium signaling, metabolism, endocrine, etc.). The core mechanism might involve impaired neurotransmitter clearance, compensatory receptor hypersensitivity, and reduced metabolic and stress regulatory capacity, collectively promoting the development of depression.
3.5. Drug combination schemes targeting HFD-related depression selected using MFH
Dietary habits do indeed influence depression. In traditional Chinese medicine theory, the concept of “Medicine and Food Homology (MFH)” posits that many substances possess dual functions as both food and medicine, capable of providing nutrition while also treating illness.
Based on clinical guidelines for the diagnosis and treatment of depression in both traditional Chinese medicine and Western medicine, some formulations can shorten the course of antidepressant medication and reduce side effects. We summarized the composition of these Chinese patent medicines as follows:
Bupleurum, White Peony Root, Angelica Root, Poria, Atractylodes, Fried Licorice Root (or Licorice Root), Mint, Ginger, Cyperus Rhizome, Tangerine Peel, Ligusticum Root, Bitter Orange Peel, Moutan Bark, Gardenia Fruit, Curcuma Rhizome.According to China's latest list of food and drug substances (totalling 106 items), the following drugs are classified as MFH: Angelicae Sinensis Radix, Poria cocos, Glycyrrhiza uralensis, Mentha haplocalyx, Gardenia jasminoides, Zingiber officinale.In summary, we have identified six MFH substances with potential antidepressant effects: Angelicae Sinensis Radix, Poria cocos, Glycyrrhiza uralensis, Mentha haplocalyx, Gardenia jasminoides, and Zingiber officinale.
3.6. Network pharmacology analysis
3.6.1. Screening of potential targets for the anti-depression effects of MFH
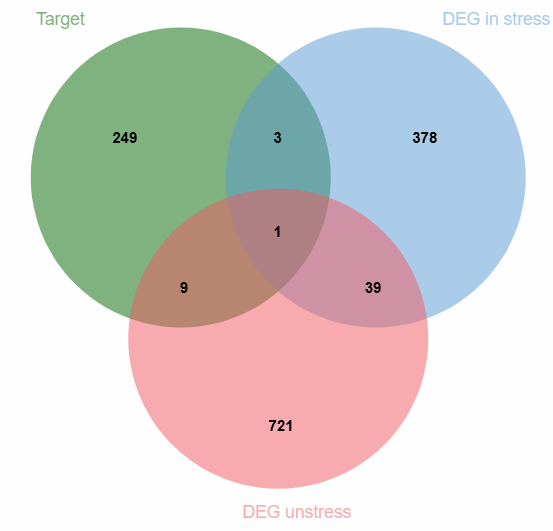
Using the TCMSP and HERB databases, we predicted potential target sites for six food-medicine dual-use substances in MFH. After removing duplicate targets, we identified 262 MFH-specific potential targets and obtained Depression-related targets under stress (421 genes) and non-stress (770 genes) conditions from the DEG database. We found the same targets from MFH-predicted and depression-associated through Venn diagram analysis. The 13 common targets are potential mediators of catechin's antidepressant effects (Figure 5).
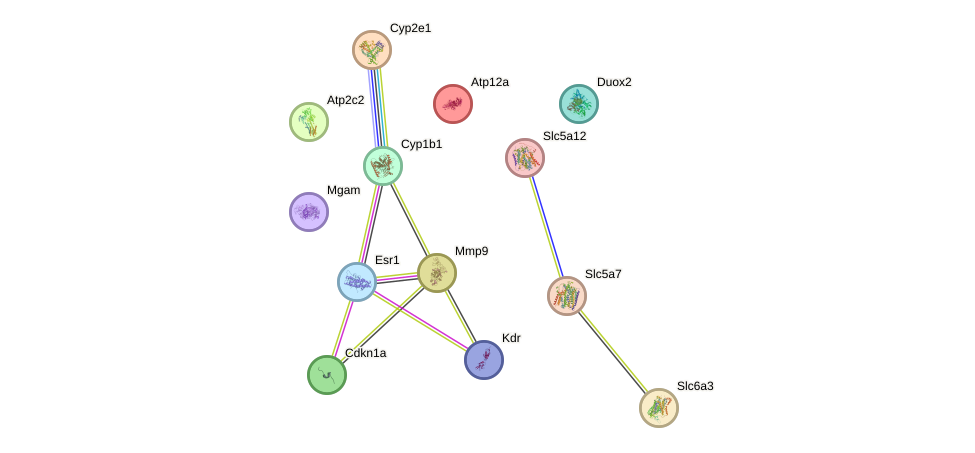
We set up a protein-protein interaction (PPI) network for the 13 overlapping targets using the STRING database (Figure 6). There are 13 nodes and 10 connecting lines in the net. MMP9, ERα1, and Cyp1b1 exhibit the highest connectivity (4, 4, and 3 connections, respectively), indicating that they may function as hub genes and key regulatory factors within this network.
3.6.2. GO enrichment analysis of the potential targets
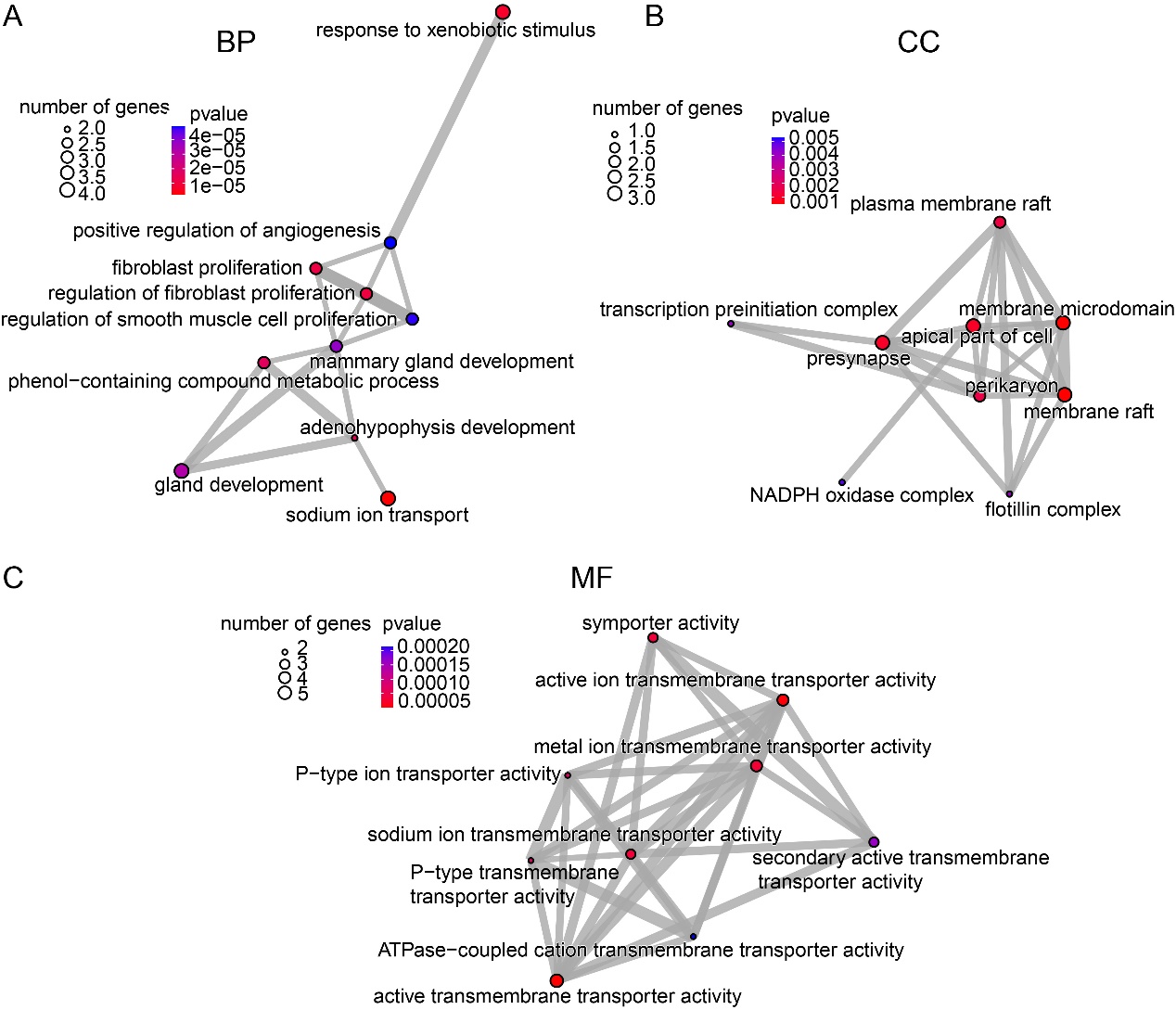
GO enrichment analysis of the 13 identified genes revealed significant enrichment (p < 0.05) (Figs. 7). Among biological processes, sodium ion transport emerged as the top enriched term, highlighting its prominent role (Fig. 7A). Enriched cellular components were predominantly associated with membrane microdomains, including membrane rafts, the apical part of the cell, and presynaptic regions, suggesting involvement in membrane-related organization and neural signal transduction (Fig. 7B). Molecular function analysis showed significant enrichment in transporter activities, particularly active transmembrane transporter activity and active ion transmembrane transporter activity (Fig. 7C). These findings indicate that MFH may exert its effects on depression primarily by modulating neural signaling through the regulation of transmembrane ion transport and maintenance of ion homeostasis.
4. Discussion
Under stress conditions, HFD primarily affects the process or biological function of monoamine neurotransmitter transport and imbalance in glutamate and acetylcholine system activities, suggesting that HFD may promote the development of depression through potential targets such as monoamine transporters and multiple neurotransmitter receptors. Through network pharmacology analysis, we identified six food-medicine homologous substances with potential therapeutic effects against depression, whose mechanism may involve modulating the activity of membrane transporters, thereby influencing neural signalling and maintenance of ion homeostasis.
This study innovatively utilised GEO database analysis to examine the effects of a high-fat diet (HFD) on mouse brain transcriptional profiles under both stressed and unstressed conditions. Through differential gene expression and enrichment analysis, it was discovered that the HFD disrupts brain homeostasis via multi-system synergistic actions (involving neurotransmitters, calcium signalling, metabolism, endocrine systems, etc.). HFD may disrupt brain homeostasis through multi-system coordination (neurotransmitters, calcium signalling, metabolism, endocrine, etc.). The core mechanism might involve impaired neurotransmitter clearance, compensatory receptor hypersensitivity, and reduced metabolic and stress regulatory capacity, collectively promoting the development of depression.
Subsequently, another innovative approach was taken by focusing on substances with MFH using network pharmacology. Based on integrated Chinese and Western medicine guidelines for depression diagnosis and treatment, practical components against high-fat diet (HFD)-induced depression were screened. We use the TCMSP and HERB databases to predict the action targets of six active herbal constituents in MFH, revealing potential multi-target mechanisms that contribute to its therapeutic effects on depression. MFH may be incorporated into food products, and also enhances therapeutic efficacy and reduces adverse reactions during pharmaceutical treatments. They may also serve as adjunctive therapies to calm the mind, replenish vital energy, and enhance cognitive function.
Of course, there are limitations. Our analysis is based on RNA-seq data from animal models, which cannot fully capture the complexity of human depression. Gene expression changes also do not necessarily prove causality; further experiments are needed to test these mechanisms directly. Moreover, diet-induced depression is shaped by many factors—including genetics, gut–brain communication, and lifestyle—which were not addressed in this study. Future research should integrate multiple “omics” approaches and, ideally, human data to give us a more precise and more complete picture.
In short, this work supports the idea that what we eat can profoundly shape brain health. A high-fat diet not only affects weight and metabolism-it also changes inflammatory and metabolic pathways in the brain that regulate mood. By uncovering these molecular patterns, we lay the groundwork for new strategies that integrate nutritional, metabolic, and psychiatric approaches to more effectively prevent and treat depression.
Acknowledgements
If any, should be placed before the references section without numbering.
References
[1]. WHO, (2025, August 29) Depressive disorder (depression). https: //www.who.int/news-room/fact-sheets/detail/depression
[2]. Major depressive disorder. In Wikipedia.Retrieved from https: //en.wikipedia.org/wiki/Major_depressive_disorder
[3]. Gin S. Malhi, J. John Mann.Depression. Lancet. 2018 Nov 24; 392(10161): 2299-2312. doi: 10.1016/S0140-6736(18)31948-2. Epub 2018 Nov 2. PMID: 30396512.
[4]. Beurel, E., Toups, M., Nemeroff, C.B.(2020)The Bidirectional Relationship of Depression and Inflammation: Double Trouble , Neuron, 107( 2), 234-256,https: //doi.org/10.1016/j.neuron.2020.06.002
[5]. Barrett CE, Jiang M, O’Flaherty BG, Dias BG, Rainnie DG, Young LJ et al (2023) Early life exposure to high fructose diet induces metabolic dysregulation associated with sex-specific cognitive impairment in adolescent rats. J Nutriochem 114: 109220. https: //doi.org/10.1016/j.jnutbio.2022.109220
[6]. Spagnuolo MS, Iossa S, Cigliano L (2020) Sweet but bitter: focus on fructose impact on brain function in rodent models. Nutrients 13(1). https: //doi.org/10.3390/nu13010001
[7]. Wang, H., Chen, X., Li, S., Zhao, H., & Li, J. (2023). High-cholesterol diet induces anxiety/depression-like behavior and neuroinflammation in mice. Brain, Behavior, and Immunity, 108, 122–134. https: //pubmed.ncbi.nlm.nih.gov/36738999
[8]. Rivera, P., Romero-Zerbo, Y., Pavón, F. J., Serrano, A., Sánchez, S., & Rodríguez de Fonseca, F. (2021). Obesity induced by high-fat diet dysregulates the serotonergic system and impairs escitalopram response in rats. Neuropharmacology, 190, 108560. https: //pmc.ncbi.nlm.nih.gov/articles/PMC8319113
[9]. Sobesky, J. L., Barrientos, R. M., De May, H. S., Thompson, B. M., & Weber, M. D. (2017). High-fat diet consumption disrupts memory and primes microglia in aged rats. Neurobiology of Aging, 58, 88–99. https: //pubmed.ncbi.nlm.nih.gov/287198555
[10]. Thaler, J. P., Yi, C. X., Schur, E. A., Guyenet, S. J., Hwang, B. H., Dietrich, M. O., … & Schwartz, M. W. (2012). Obesity is associated with hypothalamic injury in rodents and humans. The Journal of Clinical Investigation, 122(1), 153–162. https: //pmc.ncbi.nlm.nih.gov/articles/PMC6893649
[11]. Stephanie Fulton, Léa Décarie-Spain, Xavier Fioramonti, Bruno Guiard, Shingo Nakajima.The menace of obesity to depression and anxiety prevalence.Trends in Endocrinology and Metabolism, 2022 Jan, 18-35.https: //doi.org/10.1016/j.tem.2021.10.005
[12]. Cheng Xinglei. Evaluation of a depression model established by a high-fat, high-sugar diet and exploration of related mechanisms in the mesolimbic system [D]. Chengdu Medical College, 2024.
[13]. Sachin Singh, Nitesh Kumar Singh, Kottapalli Srividya1, Unis Ahmad hat, Divya Tej Sowpati1, Sumana Chakravarty, Arvind Kumar.Elucidating neural molecular mechanisms underlying metabolic disorders-induced neuropsychiatric disorders in mice on prolonged high fructose diet.Metabolic Brain Disease, (2025) 40: 226 https: //doi.org/10.1007/s11011-025-01648-0.
Cite this article
Hu,K. (2025). Exploring the Correlation Between High-Fat Diets and Depression Based on Transcriptomic Features and Network Pharmacology Analysis. Theoretical and Natural Science,152,166-176.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICMMGH 2026 Symposium: Biomedical Imaging and AI Applications in Neurorehabilitation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. WHO, (2025, August 29) Depressive disorder (depression). https: //www.who.int/news-room/fact-sheets/detail/depression
[2]. Major depressive disorder. In Wikipedia.Retrieved from https: //en.wikipedia.org/wiki/Major_depressive_disorder
[3]. Gin S. Malhi, J. John Mann.Depression. Lancet. 2018 Nov 24; 392(10161): 2299-2312. doi: 10.1016/S0140-6736(18)31948-2. Epub 2018 Nov 2. PMID: 30396512.
[4]. Beurel, E., Toups, M., Nemeroff, C.B.(2020)The Bidirectional Relationship of Depression and Inflammation: Double Trouble , Neuron, 107( 2), 234-256,https: //doi.org/10.1016/j.neuron.2020.06.002
[5]. Barrett CE, Jiang M, O’Flaherty BG, Dias BG, Rainnie DG, Young LJ et al (2023) Early life exposure to high fructose diet induces metabolic dysregulation associated with sex-specific cognitive impairment in adolescent rats. J Nutriochem 114: 109220. https: //doi.org/10.1016/j.jnutbio.2022.109220
[6]. Spagnuolo MS, Iossa S, Cigliano L (2020) Sweet but bitter: focus on fructose impact on brain function in rodent models. Nutrients 13(1). https: //doi.org/10.3390/nu13010001
[7]. Wang, H., Chen, X., Li, S., Zhao, H., & Li, J. (2023). High-cholesterol diet induces anxiety/depression-like behavior and neuroinflammation in mice. Brain, Behavior, and Immunity, 108, 122–134. https: //pubmed.ncbi.nlm.nih.gov/36738999
[8]. Rivera, P., Romero-Zerbo, Y., Pavón, F. J., Serrano, A., Sánchez, S., & Rodríguez de Fonseca, F. (2021). Obesity induced by high-fat diet dysregulates the serotonergic system and impairs escitalopram response in rats. Neuropharmacology, 190, 108560. https: //pmc.ncbi.nlm.nih.gov/articles/PMC8319113
[9]. Sobesky, J. L., Barrientos, R. M., De May, H. S., Thompson, B. M., & Weber, M. D. (2017). High-fat diet consumption disrupts memory and primes microglia in aged rats. Neurobiology of Aging, 58, 88–99. https: //pubmed.ncbi.nlm.nih.gov/287198555
[10]. Thaler, J. P., Yi, C. X., Schur, E. A., Guyenet, S. J., Hwang, B. H., Dietrich, M. O., … & Schwartz, M. W. (2012). Obesity is associated with hypothalamic injury in rodents and humans. The Journal of Clinical Investigation, 122(1), 153–162. https: //pmc.ncbi.nlm.nih.gov/articles/PMC6893649
[11]. Stephanie Fulton, Léa Décarie-Spain, Xavier Fioramonti, Bruno Guiard, Shingo Nakajima.The menace of obesity to depression and anxiety prevalence.Trends in Endocrinology and Metabolism, 2022 Jan, 18-35.https: //doi.org/10.1016/j.tem.2021.10.005
[12]. Cheng Xinglei. Evaluation of a depression model established by a high-fat, high-sugar diet and exploration of related mechanisms in the mesolimbic system [D]. Chengdu Medical College, 2024.
[13]. Sachin Singh, Nitesh Kumar Singh, Kottapalli Srividya1, Unis Ahmad hat, Divya Tej Sowpati1, Sumana Chakravarty, Arvind Kumar.Elucidating neural molecular mechanisms underlying metabolic disorders-induced neuropsychiatric disorders in mice on prolonged high fructose diet.Metabolic Brain Disease, (2025) 40: 226 https: //doi.org/10.1007/s11011-025-01648-0.









