1. Introduction
The Flaviviridae family consists of four genera: Flavivirus, Pestivirus, Hepacivirus, and Pegivirus. Among them, the mosquito-borne yellow fever virus mainly belongs to the genus Flavivirus. It can cause diseases and death in both human and animal hosts. Fever, malaise, hemorrhagic fever, and fatal encephalitis are some of the symptoms of infection, among others [1].
Mosquito-borne yellow fever virus is primarily transmitted by mosquitoes of the Aedes and Haemagogus genera and can cause severe diseases in humans. Over the past 70 years, mosquito-borne yellow fever virus has rapidly and extensively spread worldwide. Dengue virus infects approximately 400 million people each year, with over a quarter of the world’s population living in areas where dengue virus is prevalent. The outbreak of West Nile virus in 2000 resulted in 5 million infections. Despite the availability of commercial vaccines for yellow fever, the ongoing transmission of yellow fever virus remains a serious public health challenge.
Gene editing technology is a technique used to modify the genome of organisms. It allows for precise modifications of DNA sequences in cells or organisms to alter or repair specific gene functions. The most commonly used gene editing technology is the CRISPR-Cas9 system, which is a natural defense mechanism found in bacteria to fend off viral attacks. The system utilizes the Cas9 protein and RNA guide sequences to lead the Cas9 protein to the final DNA sequence, enabling DNA editing through cutting and repair processes.
Gene editing technology has broad prospects for applications in various fields, including basic scientific research, agricultural production, and medical treatments. This review focuses on the application of gene editing technique in the detection and control of mosquito-borne diseases. It reviews the advantages and disadvantages of the technology and proposes integration strategies with other cutting-edge technologies.
2. The current status of major mosquito-borne disease
2.1. Dengue virus
Dengue fever is mainly transmitted through The Mosquito vector Aedes aegypti and Is endemic to the majority of tropical and subtropical areas worldwide. The global incidence of dengue infections has increased by 300% in the past 50 years, posing a continuous threat to human health in areas where Aedes aegypti is prevalent. According to literature reports, the incidence of urban dengue fever in the Western Pacific region has significantly increased [2]. In addition to historically affected regions, the dengue virus has successfully spread to newer ecological environments [3], possibly due to the expanding range of Aedes aegypti. Another concerning reality is the increasing transmission rate and scale of dengue viruses carried by Aedes albopictus mosquitoes. Historically, Aedes albopictus was considered a minor vector of dengue fever and rarely caused large-scale outbreaks. However, in recent years, an epidemic caused by serotype 1 dengue virus (DENV1) transmitted by Aedes albopictus has resulted in one of the largest public health crises in history. In 2014, There are over 37,000 cases that have been confirmed by the laboratory in Guangzhou [4], China, indicating the potential for Aedes albopictus to trigger large-scale dengue fever outbreaks in urban environments.
2.2. Japanese encephalitis virus
The virus is the primary cause of viral encephalitis in the Asian region. Currently, it is estimated that approximately 3 billion people live in 24 countries with the risk of Japanese encephalitis infection. The Culex tritaeniorhynchus mosquito is the most important vector for Japanese encephalitis, primarily breeding in water pools and flooded rice fields, and biting humans and animals during the night. Japanese encephalitis results in around 67,900 cases annually. The epidemiology of Japanese encephalitis virus is also undergoing constant changes, with cases in multiple countries showing the co-circulation of various genotypes. Strains from genotype I are present in East Asia, specifically the GⅠ-b lineage, Since the 1990s, they have gradually taken over genotype III (GIII) and become the dominant genotype. Currently, little is recognized about the molecular mechanisms behind the appearance of GⅠ-b and GⅤ, and their substitution of GⅢ. Laboratory studies have shown that GⅠ-b and GⅤ can also be effectively transmitted by the vector mosquito (Culex spp.) [5]. Furthermore, the pathogenicity is not significantly different between GⅠ-b and GⅢ [6-8].
3. The development of CRISPR-Cas system
Since Escherichia coli discovered sequences of short palindromic repeats (CRISPR) regularly spaced in clusters in 1987, researchers have successively revealed the presence of CRISPR clusters in different bacteria and identified cas genes and the classification of CRISPR systems. Subsequent studies have revealed the adaptive immune function of CRISPR systems and their targeted effects on DNA and RNA. In 2013, scientists successfully applied the CRISPR-Cas9 gene editing technology in mammalian cells, bringing new possibilities for gene therapy and disease treatment. In the following years, CRISPR technology also made significant progress in clinical trials, including trials targeting HIV-1 and cataracts. These milestone events mark the rapid development of CRISPR technology and lay the foundation for its wide-ranging applications in gene editing and therapy (figure1).
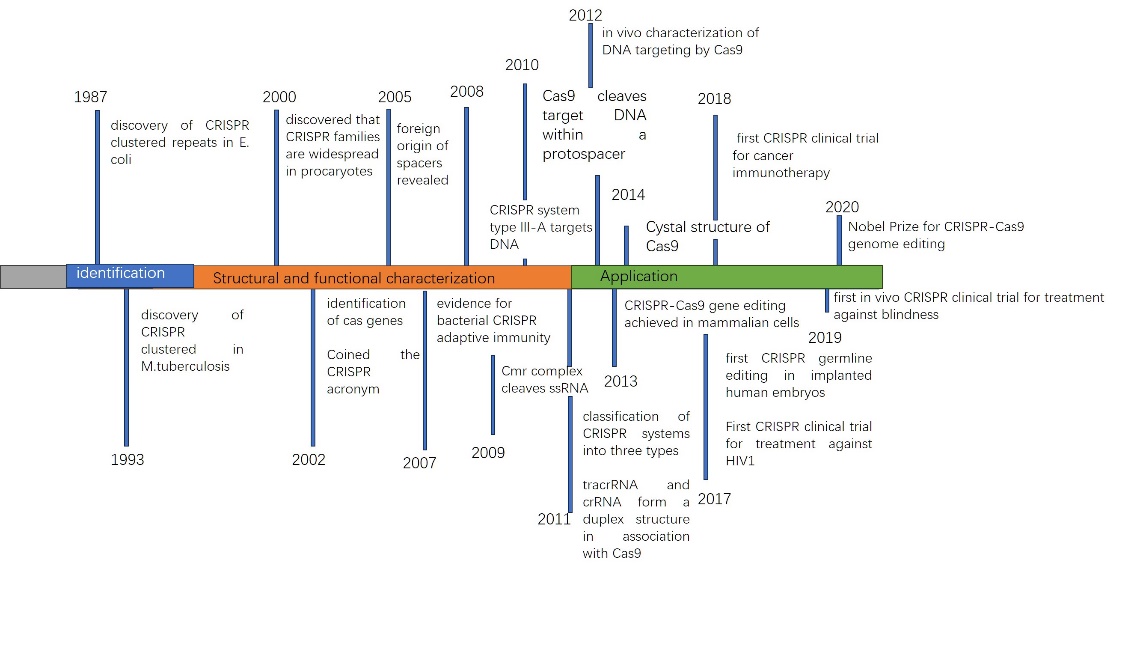
Figure 1. Timeline of CRISPR Technology Development.
The CRISPR-Cas system is a naturally occurring immune system found in bacteria and archaea, used to defend against viral and foreign DNA invasions. The system consists of the sequences and Cas proteins. CRISPR sequences are composed of repetitive sequences and spacer sequences, with the spacer sequences containing fragments that interact with viruses or other foreign DNA. These spacer sequences serve to store the genetic information of previous interactions between the bacteria or archaea and viruses or foreign DNA.
The CRISPR-Cas system is made up of Cas proteins that can recognize and cleave foreign DNA. Cas proteins interact with the spacer sequences in the CRISPR sequence, identifying and binding to foreign DNA that matches these spacers. Once bound to the foreign DNA, Cas proteins can cleave it using their intrinsic nuclease activity, thereby defending against viral invasions.
Cas9 is the major commonly used Cas protein in the CRISPR-Cas system. It is coupled with a guide RNA (gRNA) which has a similarity to the target DNA sequence. This allows Cas9 to precisely target the desired DNA and cleave it using its nuclease activity. Once the DNA is cut, the cell’s repair mechanisms can mediate the addition, deletion, or modification of DNA sequences, enabling genome editing.
In addition to Cas9, there are other types of Cas proteins, such as Cas12 and Cas13, which have similar functions but possess distinct characteristics.
In figure2, RNA-guided DNA editing tools include Cas9 nucleases and Cas12 nucleases, while RNA-guided RNA cleavage activity exists for CRISPR-Cas13 nucleases. Non-target DNA/RNA templates can also be cut by Cas12/13 nucleases with collateral cutting capability. SpCas9 can recognize the 3’NGG PAM and function best with a 20-nt spacer, Cas12a orthologs typically take a 5’T-rich PAM by contrast. The PAM prototype is adjacent to the spacer element, while the PFS prototype is located on the flanking side.
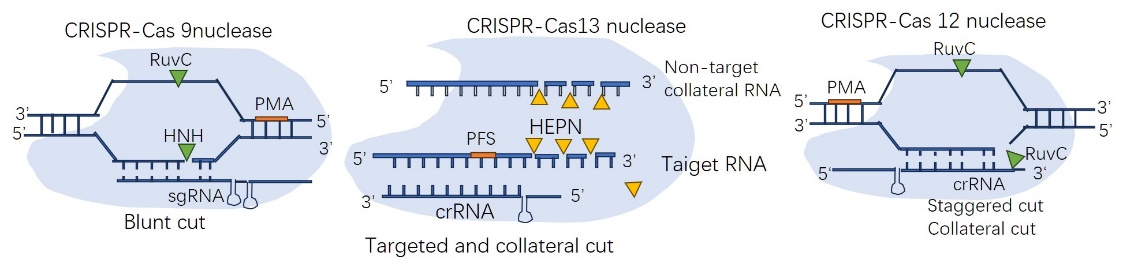
Figure 2. The diagram of CRISPR.
4. Specific examples of CRISPR in mosquito-borne disease control
4.1. CASE1
Dengue fever, caused by the dengue virus, is widely spread in Latin America and Asia. The CRISPR-Cas9-based detection system NASBA combines programmable RNA sensors Using a platform that uses freeze-dried paper to express cell-free proteins, which can differentiate between three different serotypes of dengue virus RNA. The CRISPR Cas13-based SHERLOCK v2 system developed by Jonathan et al. achieves a sensitivity as low as 2 aM and enables instrument-free detection of dengue virus within 90 minutes. It can also detect mutations in dengue virus RNA through lateral flow. While the SHERLOCK system performs well in nucleic acid testing, technologies that eliminate the extraction step are more suitable for on-site detection.
To further simplify the detection process, Cameron Myhrvold et al. take in the HUDSON (To obliterate nucleases, heating unextracted diagnostic samples is necessary) technique, Viral particles can be reduced and high levels of RNases in bodily fluids can be inactivated by using scorching and chemical reduction. When integrated with the SHERLOCK system, the HUDSON method allows direct detection of dengue virus from whole blood, serum, saliva, and other samples within 2 hours. Additionally, it can differentiate between four serotypes of dengue virus. CRISPR-Cas12a, widely used in genome editing, has shown promise in rapid detection of dengue virus by binding to dengue virus ssDNA reporter genes, as indicated by recent studies [9].
4.2. CASE2
Sherlock technology can also be applied to the detection of the COVID-19 virus to achieve rapid and highly sensitive results. In this instance, scientists designed specific CRISPR primers and Cas13 enzyme to target the RNA of the COVID-19 virus for detection. Primers are DNA molecules with specific sequences that can specifically bind to the RNA sequence of the COVID-19 virus.
Firstly, the RNA in the sample is extracted and reacted with the primers and Cas13 enzyme. If there is COVID-19 virus RNA present in the sample, the primers will bind to it and activate the Cas13 enzyme. The activated Cas13 enzyme will then start cleaving the co-existing RNA molecules. This cleavage process releases a large quantity of signal RNA fragments.
Next, fluorescent probes or other signal probes are used to detect these released signal RNA fragments. If there is COVID-19 virus present in the sample, a large quantity of signal RNA fragments will be generated, resulting in a significant signal amplification effect.
Finally, by detecting the intensity of the fluorescence or other signals, it can be determined whether the COVID-19 virus is present in the sample. If the fluorescence or signal intensity exceeds the predefined threshold, the presence of the virus can be confirmed. The application of Sherlock technology in COVID-19 virus detection can provide rapid and highly sensitive results, helping to identify infected individuals early and take appropriate measures for isolation and treatment.
4.3. CASE3
The Zika virus is a pathogen transmitted by mosquitoes that can cause infection and disease in humans. In order to detect and prevent the spread of the Zika virus earlier, scientists have developed a genetic editing biosensor using gene editing technology. In this case, scientists chose a specific mosquito species, such as Aedes aegypti, as the target. They used gene editing technology to modify the mosquitoes, enabling them to produce specific biomarkers when infected with the Zika virus. Specifically, scientists combined genes associated with Zika virus infection with fluorescent genes or other marker genes using gene editing technology. When the mosquitoes are infected with the Zika virus, these genes are activated and produce visual markers, such as a fluorescence signal. Subsequently, scientists use optical sensor devices to detect the fluorescence signal in the mosquito’s bodily fluids. When the fluorescence signal in the mosquito’s bodily fluids reaches a specific threshold, the sensor generates a corresponding signal indicating whether the mosquito is carrying the Zika virus.
This genetic editing biosensor can significantly improve the speed and accuracy of Zika virus detection. Compared to traditional detection methods, it can identify infected mosquitoes earlier and take appropriate control and preventive measures, effectively reducing the risk of Zika virus transmission.
5. Limitations and future development of CRISPR technology in mosquito-borne disease
The main focus of CRISPR-based diagnostics in infectious diseases is the detection of pathogens. CRISPR-based methods have been used to detect RNA viruses, including parvovirus B19, members of the Flavivirus family such as yellow fever virus, Japanese encephalitis virus, Ebola virus, and coronaviruses [10-12].
A biggest drawback of most CRISPR-based diagnostic methods currently is their reliance on pre-amplification to detect targets below the femtomolar extent. While caps used for pre-amplification add an additional layer of specificity, this process increases the complexity of detection, adds to the cost, and prolongs the reaction time.
To solve such problem, this review proposes the idea of applying digital twin technology in gene editing experiments.
Digital twin refers to the real-time interconnection, interaction, and synchronization between a digital model and a physical entity in the real world. In the field of CRISPR gene editing, digital twin can simulate and predict the effects of CRISPR editing processes to guide actual gene editing experiments.
Digital twin will use computational models to simulate the working mechanisms of the Cas9 system, including the interactions between Cas9 protein and RNA guide sequences, among others. The model will then calculate and predict the effects of the CRISPR-Cas9 system editing based on the characteristics of the target gene sequence. Digital twin can also interact with actual experimental data to continuously improve and optimize the model by comparing the predicted editing effects with the experimental results.
By utilizing digital twin technology, researchers can assess the editing effects and potential side effects before conducting actual CRISPR gene editing, thereby enhancing editing efficiency and accuracy. Digital twin technology can simulate and test various prevention and control strategies by establishing digital twin models to simulate the spread of vector-borne diseases and mosquito population changes under different control strategies. These simulations can evaluate the effectiveness of different strategies, guiding decision-making and optimizing the implementation of prevention and control measures.
Although the application of digital twin in CRISPR is still developing, it provides researchers with a more efficient, cost-effective, and reliable method to predict and optimize the CRISPR gene editing process. This technology accelerates the progress of gene editing research and supports the development of fields like gene therapy.
6. Conclusion
Currently, the detection of malaria parasites, dengue virus, and Zika virus has been made possible through the use of CRISPR-Cas-based detection techniques. However, there are many mosquito-borne diseases, such as yellow fever virus, chikungunya virus, Rift Valley fever virus, and West Nile virus, which have been found in China but have not been studied for detection using the CRISPR-Cas system. This is also a future direction for further research on the system detection. In coming future, detection techniques based on the CRISPR-Cas system can be combined with automation, artificial intelligence, and other technologies to improve detection efficiency while ensuring accuracy. It is hoped that they will play a greater role in responding to newly emerging mosquito-borne diseases, as well as in disease detection and large-scale screening.
Authors contribution
All the authors contributed equally and their names were listed in alphabetical order.
References
[1]. Simmonds P, Becher P, Bukh J, et al. ICTV virus taxonomy profile: Flaviviridae. The Journal of General Virology, 98(1):2-3 (2017).
[2]. Guo C C, Zhou Z X, wen Z H, et al. Global epidemiology of dengue outbreaks in 1990—2015: a systematic review and meta-analysis[J]. Frontiers in Cellular and Infection Microbiology, 7:317 (2017).
[3]. Dhimal M, Gautam I, Joshi H D, et al. Risk factors for the presence of chikungunya and dengue vectors (Aedes aegypti and Aedes albopictus), their altitudinal distribution and climatic determinants of their abundance in central Nepal. PLoS Neglected Tropical Diseases, 9(3): e0003545 (2015).
[4]. Yang L, Chen Y, Yan H C, et al. A survey of the 2014 dengue fever epidemic in Guangzhou, China. Emerging Microbes & Infections, 4(9): e57 (2015).
[5]. De wispelaere M, Desprès P, Choumet V. European Aedes albopictus and culex pipiens are competent vectors for Japanese encephalitis virus. PLoS Neglected Tropical Diseases, 11(1): e0005294 (2017).
[6]. Fan Y C, Lin J W, Liao S Y, et al. Virulence of Japanese encephalitis virus genotypes Ⅰand Ⅲ, Taiwan[J/OL]. Emerging Infectious Diseases, 23(11):1883-1886 (2017).
[7]. Park S L, Huang Y J S, Lyons A C, et al. North American domestic pigs are susceptible to experimental infection with Japanese encephalitis virus. Scientific Reports, 8:7951 (2018).
[8]. Ricklin M E, Garcìa-nicolàs O, Brechbühl D, et al. Japanese encephalitis virus tropism in experimentally infected pigs. Veterinary Research, 47:34 (2016).
[9]. Kellner, M.J., Koob, J.G., Gootenberg, J.S.et al.SHERLOCK: nucleic acid detection with CRISPR nucleases. Nat Protoc 14, 2986–3012 (2019).
[10]. Dai, Y. et al. Exploring the trans-cleavage activity of CRISPR–Cas12a (cpf1) for the development of a universal electrochemical biosensor. Angew. Chem. Int. Ed. 58, 17399–17405 (2019).
[11]. Pardee, K. et al. Rapid, low-cost detection of Zika virus using programmable biomolecular components. Cell 165, 1255–1266 (2016).
[12]. Li, S.-Y. et al. CRISPR–Cas12a-assisted nucleic acid detection. Cell Discov. 4, 20 (2018).
Cite this article
Luo,L.;Wang,J.;Wang,L. (2023). Application and prospects of gene editing in mosquito-borne disease control. Theoretical and Natural Science,8,40-45.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Simmonds P, Becher P, Bukh J, et al. ICTV virus taxonomy profile: Flaviviridae. The Journal of General Virology, 98(1):2-3 (2017).
[2]. Guo C C, Zhou Z X, wen Z H, et al. Global epidemiology of dengue outbreaks in 1990—2015: a systematic review and meta-analysis[J]. Frontiers in Cellular and Infection Microbiology, 7:317 (2017).
[3]. Dhimal M, Gautam I, Joshi H D, et al. Risk factors for the presence of chikungunya and dengue vectors (Aedes aegypti and Aedes albopictus), their altitudinal distribution and climatic determinants of their abundance in central Nepal. PLoS Neglected Tropical Diseases, 9(3): e0003545 (2015).
[4]. Yang L, Chen Y, Yan H C, et al. A survey of the 2014 dengue fever epidemic in Guangzhou, China. Emerging Microbes & Infections, 4(9): e57 (2015).
[5]. De wispelaere M, Desprès P, Choumet V. European Aedes albopictus and culex pipiens are competent vectors for Japanese encephalitis virus. PLoS Neglected Tropical Diseases, 11(1): e0005294 (2017).
[6]. Fan Y C, Lin J W, Liao S Y, et al. Virulence of Japanese encephalitis virus genotypes Ⅰand Ⅲ, Taiwan[J/OL]. Emerging Infectious Diseases, 23(11):1883-1886 (2017).
[7]. Park S L, Huang Y J S, Lyons A C, et al. North American domestic pigs are susceptible to experimental infection with Japanese encephalitis virus. Scientific Reports, 8:7951 (2018).
[8]. Ricklin M E, Garcìa-nicolàs O, Brechbühl D, et al. Japanese encephalitis virus tropism in experimentally infected pigs. Veterinary Research, 47:34 (2016).
[9]. Kellner, M.J., Koob, J.G., Gootenberg, J.S.et al.SHERLOCK: nucleic acid detection with CRISPR nucleases. Nat Protoc 14, 2986–3012 (2019).
[10]. Dai, Y. et al. Exploring the trans-cleavage activity of CRISPR–Cas12a (cpf1) for the development of a universal electrochemical biosensor. Angew. Chem. Int. Ed. 58, 17399–17405 (2019).
[11]. Pardee, K. et al. Rapid, low-cost detection of Zika virus using programmable biomolecular components. Cell 165, 1255–1266 (2016).
[12]. Li, S.-Y. et al. CRISPR–Cas12a-assisted nucleic acid detection. Cell Discov. 4, 20 (2018).









