1. Introduction
Dexibuprofen is the enantiomer of ibuprofen with a right-handed configuration. It serves as the primary contributor to the pharmacological effects of ibuprofen, exhibiting greater efficacy and superior safety and pharmacokinetic characteristics compared to ibuprofen. Nevertheless, the oral administration of dexibuprofen is hindered by its poor solubility degree, resulting in an unpleasant taste and a significant irritation to the gastrointestinal mucosa [1]. So the \( β \) -cyclodextrins were incorporated into dexibuprofen, inclusion compound can improve the solubility of dexibuprofen, promote its absorption and reduce stomach irritation, so as to expand its clinical application [2].
The inclusion of \( β- \) cyclodextrins to dexibuprofen can improve its solubility and absorption, cover up the bad odor, and reduce gastrointestinal irritation, which has made a contribution to the research in the medical field. Furthermore, the incorporation of the inclusion compound can be utilized to produce disperse tablets, so attaining the desirable attributes of prompt disintegration, dispersion, and convenient delivery. Dispersible tablets offer multiple administration options, including swallowing, rejection, sucking, or suspension in water. This makes them appropriate for a diverse range of patients, such as the elderly, young individuals, and those who struggle with swallowing conventional tablets or capsules. These versatile administration methods aim to optimize the clinical effectiveness of dexibuprofen.
This study examines the preparation and identification of dexibuprofen clathrate through a comprehensive analysis of relevant literature. The primary objective is to determine the most effective inclusion procedure for dexibuprofen based on yield and clathrate rate. Subsequently, an investigation was conducted on the methodology for the formulation of clathrate into tablets with dispersible properties. The preliminary pharmacokinetics of the dispersible pills were investigated in rats. At present, saturated aqueous solution method, ultrasonic method and grinding method are the most used for the inclusion of \( β \) -cyclodextrins on dexibuprofen. This paper will briefly describe these three methods, and then compare the inclusion effects of these three methods on dexibuprofen. In the study of preparing dexibuprofen \( β- \) cyclodextrin inclusion compound into dispersible tablets, the optimum formulation and preparation technology were selected, which achieved the characteristics of simple and reasonable prescription, stable process and good repeatability.
2. Overview
2.1. β-cyclodextrin
β-cyclodextrins are non-toxic macrocyclic oligosaccharides formed from 7 glucose molecules linked by \( α-1 \) , 4-glucoside bonds [3-5]. Cyclodextrins have annular or truncated conical shapes as a result of the absence of freely rotating links that connect the glucose-pyranose units. The distinctive characteristic of cyclodextrins enables guest molecules of suitable dimensions to be completely or partially enclosed within the hydrophobic cavity via non-covalent interactions, including hydrogen bonding and van der Waals forces [6-7]. \( β \) -cyclodextrin has moderate molecular holes, wide application range and low production cost, and is the most used cyclodextrin product in industry.
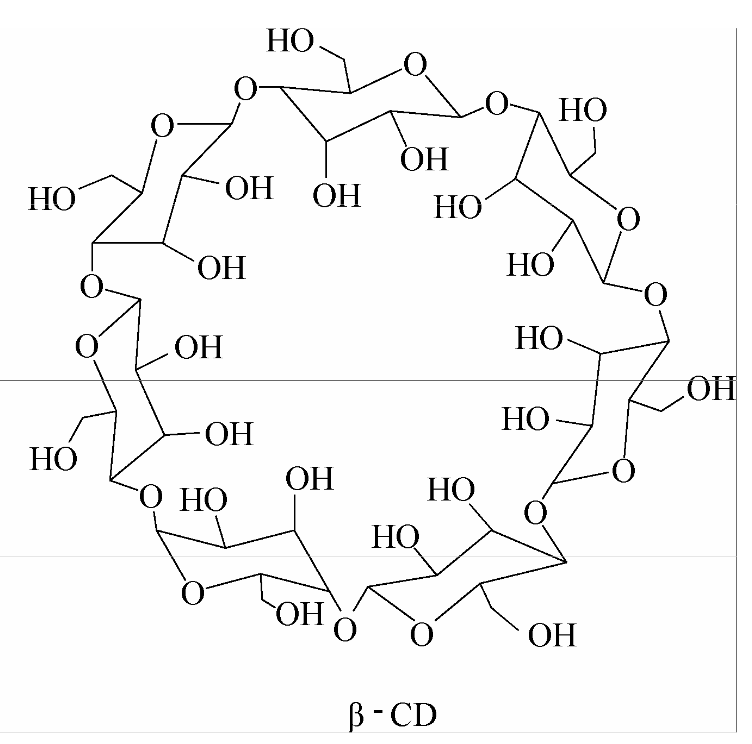
Figure1. Molecular structure of \( β \) -cyclodextrin [8].
2.2. Dexibuprofen
Dexibuprofen (DIB) is classified as a phenylpropionic acid non-steroidal anti-inflammatory analgesic medication. It is considered the enantiomer of ibuprofen, exhibiting a more rapid and potent therapeutic effect compared to ibuprofen [2]. Nevertheless, dexibuprofen has limited solubility in water, a poor degree of absorption in the gastrointestinal system, irritating properties towards the gastric mucosa, and overall low bioavailability. These characteristics pose challenges to its extensive utilization. Furthermore, prolonged administration of dexibuprofen to patients can lead to the occurrence of gastrointestinal bleeding, ulcers, and perforations, along with other adverse gastrointestinal toxic effects. Additionally, variable degrees of liver, renal, and cardiovascular damage may be observed, which can also impact the drug's effectiveness [9-10].
2.3. The important role between dexibuprofen and \( β- \) cyclodextrin
Because the outer edge of \( β \) -cyclodextrins is hydrophilic and the inner cavity hydrophobic, it can provide a hydrophobic binding site like an enzyme, as the main body to enclose various suitable objects. Dexibuprofen can be used as a guest, completely or partially contained in the hydrophobic cavity of \( β- \) cyclodextrin by non-covalent interaction forces, resulting in the formation of dexibuprofen β-cyclodextrin inclusion complexes. After the formation of such clathrates, the dissolution rate of dexibuprofen can be effectively improved, which is convenient for the absorption of patients and can reduce the irritation to the stomach.
3. Review of preparation and analysis of inclusion compounds
3.1. Method
First, Saturated water solution method. β-cyclodextrin was added to water at 50°C to make \( β \) -cyclodextrin included aqueous solution. The fixed speed was 800r/min, and 5.0mL of dexibuprofen solution (containing about 0.55g dexibuprofen) was slowly dropped and dissolved with anhydrous ethanol. The solution was stirred at constant temperature for 1h. Remove, refrigerate for 12h, filter, wash the unencapsulated dexibuprofen with a small amount of anhydrous ethanol, and dry the filter residue at room temperature under reduced pressure to obtain the white powder clathrate, ground it, pass 100 mesh sieve, and then obtain [11].
Second, Ultrasonic method [11]. In this experiment, \( β \) -cyclodextrin was added to water at a temperature of 50℃ to create a saturated solution of \( β- \) cyclodextrin. The solution was then subjected to ultrasonic treatment using a cleaning machine. Subsequently, a solution of dexibuprofen dissolved in anhydrous ethanol (containing approximately 0.5g of dexibuprofen) was slowly added to the mixture. The resulting solution was subjected to ultrasonic treatment for a duration of 30 minutes before being removed. After refrigerating for a duration of 12 hours, proceed with the remaining procedures as previously described, and afterwards acquire the desired outcome.
Third, Grinding method [11]. In this experimental procedure, \( β \) -cyclodextrin in the amount of 3.0g is introduced into a mortar. Subsequently, 30 mL of water is added to the mixture. Following this, a solution of dexibuprofen dissolved in anhydrous ethanol, about weighing 0.55g and containing dexibuprofen, is gradually incorporated into the mixture. A volume of 5.0 mL was subjected to grinding for a duration of 1 hour. Subsequently, the sample was removed and refrigerated for a period of 12 hours. The other steps in the procedure remained consistent with the aforementioned protocol, leading to the final outcome.
3.2. The preparation technology of the inclusion compound was analyzed
The preparation technology of the inclusion compound was analyzed with the inclusion rate and yield rate as the index.
\( {R_{I}}=({W_{SD}}×\frac{{W_{S}}}{{W_{D}}})\ \ \ (1.1) \)
Where RI is the inclusion rate, WSD is the dexibuprofen content in sample, WS is the sample weight, \( {W_{D}} \) is the dexibuprofen input amount.
\( {R_{Y}}=(\frac{{W_{I}}}{{W_{F}}})×100\%\ \ \ (1.2) \)
Where \( {R_{Y}} \) is the yield rate, \( {W_{I}} \) is the weight of inclusion compound, \( {W_{F}} \) is the feeding amount.
It can be calculated by formulas (1.1) and (1.2).
Table 1. Comparison of three preparation methods of inclusion compounds [11].
Method | SWSM | UM | GM |
RI | 68.72 \( ± \) 4.46 | 65.63 \( ± \) 6.79 | 67.41 \( ± \) 7.98 |
RY | 73.67 \( ± \) 4.52 | 69.55 \( ± \) 7.71 | 61.38 \( ± \) 7.89 |
Where SWSM is the saturated water solution method, UM is the ultrasonic method, GM is the grinding method. It can be seen from Table 1 that saturated aqueous solution method is obviously the best method, which is easy to operate and convenient for industrial production.
3.3. Selection of optimum preparation technology for inclusion compound
Table 2. L9 (34) Orthogonal test factor level table [11].
Factor Level | Charge ratio(mol) A ( \( β \) -CD : DIB) | Temperature(℃) B | Time (h) C |
1 | 2:1 | 40 | 3 |
2 | 1:1 | 50 | 4 |
3 | 1:2 | 60 | 5 |
Charge ratio, temperature and time are the three most significant factors affecting the inclusion compound. They are selected as the research objects, and the yield rate and inclusion rate are taken as indicators. \( {L_{9}}({3^{4}}) \) orthogonal test table is selected and factor level table is designed. The primary, secondary and optimal influence levels of factors are determined by the range R of each factor.
The comprehensive score was determined by allocating a weight of 0.6 to the inclusion rate and a weight of 0.4 to the yield rate of the inclusion compound. The upper limit for the inclusion rate was established at 100, with the highest recorded values being 87.97 and 86.78.
\( y=({y_{1}}+100-87.97)×0.6+({y_{2}}+100-86.78)×0.4\ \ \ (1.3) \)
Where y is the comprehensive score, \( {y_{1}} \) is the inclusion rate, \( {y_{2}} \) is the yield rate.
Table 3. Orthogonal test result [11].
No | 1(A) | 2(B) | 3(C) | 4(D) | y1(%) | y2(%) | y |
1 | 1 | 1 | 1 | 1 | 70.64 | 63.36 | 80.23 |
2 | 1 | 2 | 2 | 2 | 78.57 | 72.83 | 88.78 |
3 | 1 | 3 | 3 | 3 | 75.85 | 69.74 | 85.91 |
4 | 2 | 1 | 2 | 3 | 79.45 | 76.53 | 90.79 |
5 | 2 | 2 | 3 | 1 | 87.61 | 86.78 | 99.78 |
6 | 2 | 3 | 1 | 2 | 87.97 | 79.18 | 96.96 |
7 | 3 | 1 | 3 | 2 | 61.46 | 55.60 | 71.62 |
8 | 3 | 2 | 1 | 3 | 68.76 | 54.64 | 75.62 |
9 | 3 | 3 | 2 | 1 | 69.96 | 63.31 | 79.81 |
K1 | 254.92 | 242.64 | 252.81 | ||||
K2 | 287.53 | 264.18 | 259.38 | ||||
K3 | 227.05 | 262.68 | 257.31 | ||||
R | 20.16 | 7.18 | 2.19 |
Table 4. Anova result [11].
Source of variance | SS | FS | MS | F | Significance |
A | 610.89 | 2 | 305.44 | 62.69 | * |
B | 96.42 | 2 | 48.21 | 9.90 | |
C | 7.52 | 2 | 3.76 | 0.77 | |
Error | 9.74 | 2 | 4.87 |
Variance analysis was employed to assess the impact of each element on the preparation process. The findings indicate that component A exerts the greatest influence on the comprehensive score, with factors B and C following in significance. The charge ratio exerts a substantial impact on the outcomes of inclusion, with a 1:1 feed ratio being optimal. The experimental outcomes were not significantly influenced by variations in temperature and duration. The temperature of inclusion was recorded at 50℃, while the duration of the process was 4 hours. The aforementioned findings represent the optimal procedure for the construction of an inclusion complex.
4. Preparation of inclusion complex dispersion tablets
4.1. Prescription single factor
First, Choice of filler [12-14]. The DIB- \( β \) -cyclodextrin inclusion complex was mixed with lactose, mannitol, pre-gelatinized starch and MCC at the ratio of 1:1 after 100 mesh sieve, and the tablet was pressed by wet method. The tensile strength can be calculated according to formula 1.4. The compressive formability of the excipients was evaluated by tensile strength. The results showed that MCC had the highest tensile strength.
\( T=\frac{2F}{πDL}\ \ \ (1.4) \)
Where T is the tensile strength, F is the radial destructive force, D is the tablet diameter, L is the tablet height.
Table 5. Tensile strength of several adjuvants.
Adjuvants | Lactose | Mannitol | Pre-gelatinized | MCC |
T/MPa | 0.35 \( ± \) 0.033 | 0.25 \( ± \) 0.028 | 0.30 \( ± \) 0.065 | 0.65 \( ± \) 0.026 |
Second, Choice of disintegrating agent. Swelling test [15]: The disintegrant is dried to constant weight, and 1g is taken into the scale test tube respectively, and the height is gently tapped until the height no longer changes. At this time, the height of the powder is determined as \( {h_{1}} \) . After shaking with water, the powder height was determined to be \( {h_{2}} \) . The swelling volume ratio ( \( {h_{2}}/{h_{1}} \) ) of the powder was calculated. The results showed that CMS-Na was always unsaturated, and the swelling of the excipients with unsaturated water absorption was greater than that with saturated water absorption, so the swelling degree of CMS-Na was the largest.
Table 6. Swelling data of several disintegrants and microcrystalline cellulose.
Adjuvants | \( {h_{1}} \) /cm | \( {h_{2}} \) /cm | Swelling volume ratio |
CMS-Na | 1.93 \( ± \) 0.11 | / | / |
PVPP | 2.66 \( ± \) 0.19 | 4.34 \( ± \) 0.26 | 1.63 \( ± \) 0.12 |
L-HPC | 3.14 \( ± \) 0.21 | 4.41 \( ± \) 0.29 | 1.40 \( ± \) 0.09 |
L-HPC(Xinyue) | 1.56 \( ± \) 0.21 | 5.81 \( ± \) 0.33 | 3.72 \( ± \) 0.36 |
MCC101 | 2.41 \( ± \) 0.19 | 3.55 \( ± \) 0.44 | 1.47 \( ± \) 0.09 |
MCC301 | 2.20 \( ± \) 0.20 | 3.16 \( ± \) 0.27 | 1.44 \( ± \) 0.23 |
Water absorption test [16-17]: An absorbent cotton layer was applied onto the surface of the petri dish. Subsequently, a small basket constructed from metal wire was positioned within the aforementioned container, and filter paper was carefully inserted into the small basket. Following the soaking process, the disintegrants were compressed into tablet form and positioned at the center of the basket. Subsequently, the weight of the little basket was measured at regular intervals. The findings indicated that L-HPC exhibited the highest water absorption capacity, while CMS-Na demonstrated a progressive increase in water absorption. On the other hand, although MCC did not exhibit significant water absorption, it had a faster rate of water absorption.
4.2. Disintegration time comparison
The average disintegration time of DIB- \( β \) -cyclodextrin inclusion complex was 45min. Disintegration is mainly done in the form of surface dissolution, and some even fail to disintegrate [18].
First, the disintegration time of excipient blanks is examined. In order to compare the disintegration times, various disintegrators and prescription dosages were used, with lactose serving as the filler and a 2% PVP solution as the binder. The findings of the study indicate that there is an inverse relationship between the disintegrant content and the disintegration time. Specifically, as the content of microcrystalline cellulose (MCC) increases, the disintegration time decreases. Notably, when the MCC content exceeds 20%, there is a considerable reduction in the disintegration time.
Second, disintegration time of tablet after addition of main drug. Lactose was used as filler, 2% PVP solution as binder, magnesium stearate as lubricant and DIB- \( β \) -cyclodextrin inclusion complex as main drug. The disintegrating time of different formulations was determined by wet granulation of different kinds of disintegrators according to different dosage. The results show that the disintegration time decreases with the increase of the disintegrating agent, but when the amount of disintegrant reaches 20%, the disintegrant time limit still cannot be reached.
Third, the combination of disintegrating agent. Given the inability of a singular disintegrating agent to achieve the specified disintegration criterion within a 3-minute timeframe, it is prudent to incorporate 20% microcrystalline cellulose (MCC) into the prescription, as tablets containing this percentage of MCC exhibit superior disintegration performance. Polyvinylpyrrolidone-polyvinyl acetate copolymer (L-HPC) in a 1:1 ratio was introduced into the mixture at various tablet weight proportions, while lactose was added to compensate for any deficiency in tablet weight. The determination of disintegration time was conducted using the wet granulation method. The findings of the study indicated that a notable reduction in disintegrant time was observed when the combined disintegrant constituted 10% of the prescribed quantity. This reduction in disintegrant time led to the attainment of the disintegrant time limit for the dispersible tablets.
4.3. Formulation optimization
The content of DIB in DIB- \( β \) -cyclodextrin inclusion complex dispersion tablets and the dissolution of DIB- \( β \) -cyclodextrin inclusion complex dispersion tablets were determined to optimize the prescription. Test according to the following table, 50% DIB- \( β \) -cyclodextrin inclusion complex, tablet weight 500mg, tablet quality is insufficient to supplement with lactose, Table 7 to Table 9 to determine the best prescription. Visual analysis and analysis of variance showed that the amount of disintegrating agent had significant influence on the disintegrating and dissolution of dispersible tablets, and the excessive amount of disintegrating agent affected the dissolution of drugs. Therefore, the optimal prescription regimen is \( {A_{2}}{B_{2}}{C_{2}} \) , that is, L-HPC(Xinyue): PVPP is 1:2, accounting for 15% of the total prescriptions, and \( MC{C_{301}} \) is 22.5%.
Table 7. L9(34) factors and levels of orthogonal experiment [18].
Level | A:L-HPC:PVPP | B:MCC/% | C: Disintegration dose/% |
1 | 1:1 | 20 | 10 |
2 | 1:2 | 22.5 | 15 |
3 | 2:1 | 25 | 20 |
Table 8. Results of L9(34) orthogonal experiment [18].
NO. | 1(A) | 2(B) | 3(C) | 4(D) | Disintegration time /s | Dissolution degree /% |
1 | 1 | 1 | 1 | 1 | 139.2 | 50.76 |
2 | 1 | 2 | 2 | 2 | 81.7 | 70.88 |
3 | 1 | 3 | 3 | 3 | 79.3 | 54.36 |
4 | 2 | 1 | 2 | 3 | 58.7 | 76.55 |
5 | 2 | 2 | 3 | 1 | 56.6 | 62.95 |
6 | 2 | 3 | 1 | 2 | 100.5 | 67.39 |
7 | 3 | 1 | 3 | 2 | 58.6 | 54.98 |
8 | 3 | 2 | 1 | 3 | 97.7 | 68.36 |
9 | 3 | 3 | 2 | 1 | 68.2 | 74.25 |
K1 | 300.20 | 256.50 | 337.40 | 264.00 | ||
K2 | 215.8 | 236.00 | 208.6 | 240.80 | ||
K3 | 224.5 | 248.00 | 194.5 | 235.70 | ||
R1 | 28.13 | 6.83 | 47.63 | 9.43 | ||
K1` | 176.00 | 182.29 | 186.51 | 187.96 | ||
K2` | 206.89 | 202.19 | 221.68 | 193.25 | ||
K3` | 197.59 | 196.00 | 172.29 | 199.27 | ||
R2 | 10.30 | 6.63 | 16.46 | 3.77 |
Where K1,K2,K3,R1 are the disintegration, K1`,K2`,K3`,R2 are the dissolution.
Table 9. Variance analysis of crosscut data [18].
Source of variance | Sum of squares of deviation | Degree of freedom | Mean square | Value of F | Significance |
A | 1436.62 | 2 | 718.31 | 9.47 | |
B | 70.72 | 2 | 35.36 | 0.47 | |
C | 4134.3 | 2 | 2067.15 | 27.26 | * |
Error | 151.68 | 2 | 75.84 | ||
A` | 167.42 | 2 | 83.71 | 7.84 | |
B` | 69.14 | 2 | 34.57 | 3.24 | |
C` | 430.95 | 2 | 215.47 | 20.19 | * |
Error | 21.35 | 2 | 10.67 |
Where A,B,C are the disintegration, A`,B`,C` are the dissolution.
4.4. Preparation process optimization
First, Comparison between wet pellet pressing and full powder direct pressing.The tablet produced by the direct compression method using powdered materials has favorable disintegration characteristics and a polished tablet surface. However, due to the requirement of applying substantial pressure during the compression process, it results in increased wear on the die. Furthermore, the inadequate fluidity of the inclusion complex results in a significant disparity in sheet weight, hence leading to an increased generation of dust during the tablet compression procedure. The tablets produced using the wet granulation method exhibit rapid disintegration, improved flow of the inclusion complex, minimal pressure requirements, ease of tablet formation, and negligible weight variation. These characteristics are advantageous for large-scale industrial manufacturing processes. In conclusion, moist granulation is considered the most optimal method.
Second, comparison of adding methods of disintegrators. The disintegration time was measured by internal addition, external addition and internal and external addition respectively, and then the tablet was pressed by wet method. It can be seen from Table 10 that there is little difference in disintegration time, but the internal addition is selected from the overall consideration of appearance.
Table 10. Selection of adding methods for disintegrants [18].
Prescription number | Adding method | Disintegration time/s | Tablet appearance |
1 | Internal addition | 64 | Smooth surface, good finish. |
2 | External addition | 59 | The surface has more fine powder, not smooth, poor finish. |
3 | Internal and external addition | 62 | The surface has a small amount of fine powder, smooth, good finish. |
Third, Selection of grain size. Since particle size is an important factor affecting drug dissolution, DIB- \( β \) -cyclodextrin inclusion complex and excipients are prepared through different drug sieve numbers. The results showed that the larger the number of sieve sizes, the rounder the particles, the more uniform the particle size, the better the fluidity, the better the tablet finish and the smaller the difference in tablet weight. Therefore, the selection of 40 mesh sieve granulation is the best.
Fourth, Determination of tablet force. By measuring the disintegration time of the tablet, checking the appearance and hardness of the tablet, respectively pressing the tablet under different pressing forces to determine the pressing force. The results showed that the optimum pressing force was 5kg· \( {cm^{-2}} \) .
Fifth, Determination of prescription [18]. DIB-β-cyclodextrin inclusion complex (50g as DIB) 250g, MCC112.5g, PVPP50g, L-HPC25g, lactose 52.5g, erythritol 5g, magnesium stearate 5g, passed 100 mesh sieve respectively, set aside. The DIB- \( β \) -cyclodextrin inclusion complex was evenly mixed with MCC, L-HPC and PVPP, then granulated with 2%PVP aqueous solution as binder through 40 mesh sieve, dried at 45℃, whole granule, mixed with magnesium stearate and erythritol, pressed 1000 tablets, each containing 50mg DIB, and obtained.
5. Preliminary pharmacokinetics study of inclusion complex dispersion tablets in rats
5.1. Administration and Blood sample collection and plasma sample pretreatment
The tested preparation was self-made DIB- \( β \) -cyclodextrin inclusion complex dispersion tablets, and the reference preparation was commercially available DIB tablets. The dosage was referred to adults, and the equivalent dose was converted according to the ratio of animal surface area. The dosage of both preparations was equivalent to DIB 50mg/kg [19-20]. A total of twelve SD rats, deemed to be in good health, were subjected to random assignment into two groups: the test preparation group and the reference preparation group. Each group consisted of six rats. The experimental group underwent a 12-hour fasting period before to intragastric administration. In this group, the test preparation consisted of intragastric administration using dispersible tablets containing DIB- \( β \) -cyclodextrin inclusion complex. The control group, on the other hand, received intragastric administration using commercially available DIB tablets.
Blood samples were collected from the orbital region of mice and transferred into anticoagulant tubes. The tubes were kept at ambient temperature for a duration of 1 hour, following which the plasma was separated using centrifugation. The resulting plasma was subsequently stored in a refrigerator set at a temperature of -20℃ for future utilization. The plasma samples were subjected to pretreatment, where 0.1mL of plasma was absorbed using a precision suction method. Subsequently, 0.4 mL of methanol was added, followed by vortex mixing and centrifugation. The resulting supernatant was then used for sample injection, as described in reference [21].
5.2. Methodological investigation and Relative bioavailability of disperse tablets
The peak area of the plasma sample solution was assessed by introducing control solutions with varying concentrations. This allowed for the establishment of a standard curve, which facilitated the determination of the linear correlation between drug concentration and peak area. The findings indicated that medication DIB had a strong linear correlation within the concentration range of 0.625-20.000 \( μ \) g/mL. The instrument's accuracy and stability were assessed by conducting many measurements of plasma samples at various concentrations. Intraday and daytime precision were calculated to evaluate the device's performance. The obtained results indicate that the instrument demonstrates favorable precision. Plasma samples of known concentration were obtained and subjected to varying circumstances, including freezing, ice melting, and ambient temperature, for different durations. The findings indicate that plasma samples obtained via DIB may be maintained in a stable manner for a duration of 21 days when subjected to freezing conditions, and for a period of 12 hours when exposed to ice melting and ambient temperature settings. This finding suggests that plasma samples obtained from DIB demonstrate favorable stability when subjected to specific temperature settings. A volume of 0.4 mL of blank plasma was combined with reference solutions of high, medium, and low concentrations of DIB. This resulted in the acquisition of plasma samples with varying quantities. The recovery rate demonstrated a satisfactory outcome as determined through mathematical computation. The pharmacokinetic parameters were calculated by 3p97 pharmacokinetic software, and the relative bioavailability of DIB \( -β \) -cyclodextrin inclusion complex dispersive tablets was calculated to be 160.88% [21].
6. Conclusion
Inclusion of dexibuprofen with β-cyclodextrin can make up for the deficiency of dexibuprofen. The best preparation method of the inclusion complex is saturated aqueous solution method. The best inclusion process is that the molar ratio of dexibuprofen to \( β \) -cyclodextrin is 1:1, the inclusion temperature is 50℃, and the inclusion time is 4h. The inclusion process is simple, reasonable and feasible. When the inclusion complex was made into dispersible tablets, the optimal prescription composition was DIB \( -β \) -cyclodextrin inclusion complex 50%, MCC22.5%, lactose 10.5%, PVPP10%, L-HPC5%. The design of this prescription was simple and reasonable, and the process was stable. The relative bioavailability of the tablets was 160.88%, which significantly improved the pharmacokinetic behavior and bioavailability of the drugs.
The research prospects of \( β \) -cyclodextrin and dexibuprofen include the study of inclusion complex properties, drug delivery systems, drug therapeutic effects, structural optimization, and the development of new drugs based on inclusion complex. These studies are expected to further promote the development and application of drugs and provide new solutions for drug treatment and disease management.
References
[1]. Du X L, Ren S Q, & Hu Y L.(2001). Gastrointestinal adverse reactions of non-steroidal anti-inflammatory drugs and their prevention and treatment. Chinese Journal of Hospital Pharmacy, 21(4), 235.
[2]. Xie J T, Wang C Y, Li J, & Zhang T T. (2009). Determination of D-ibuprofen β-cyclodextrin inclusion complex by HPLC. Journal of Anhui Medicine, 13(2), 157-158.
[3]. Prabu, S., Swaminathan, M., Sivakumar, K., & Rajamohan, R. (2015). Preparation, characterization and molecular modeling studies of the inclusion complex of Caffeine with Beta-cyclodextrin. Journal of Molecular Structure, 1099, 616-624.
[4]. Kfoury, M., Auezova, L., Greige-Gerges, H., Ruellan, S., & Fourmentin, S. (2014). Cyclodextrin, an efficient tool for trans-anethole encapsulation: Chromatographic, spectroscopic, thermal and structural studies. Food chemistry, 164, 454-461.
[5]. Jin G Y. (2017). Analysis of 7 heavy metal elements in β-cyclodextrin. Journal of Medicine Today, 27(4), 228-231.
[6]. Charoenchaitrakool, M., Dehghani, F., & Foster, N. R. (2002). Utilization of supercritical carbon dioxide for complex formation of ibuprofen and methyl-β-cyclodextrin. International journal of pharmaceutics, 239(1-2), 103-112.
[7]. Riekes, M. K., Tagliari, M. P., Granada, A., Kuminek, G., Silva, M. A. S., & Stulzer, H. K. (2010). Enhanced solubility and dissolution rate of amiodarone by complexation with β-cyclodextrin through different methods. Materials Science and Engineering: C, 30(7), 1008-1013.
[8]. Zhang S Y, Zou Y, Wei T Y, Mou C X, Liu X J, & Tong Z F. (2016). Preparation of β-cyclodextrin/polyether coacetamide filled membrane and pervaporization of trace phenol in water. Journal of Chemical Engineering, 67(11), 4662-4670.
[9]. Caunedo-Alvarez, A., Gómez-Rodríguez, B. J., Romero-Vázquez, J., Argüelles-Arias, F., Romero-Castro, R., García-Montes, J. M., ... & Herrerías-Gutiérrez, J. M. (2010). Macroscopic small bowel mucosal injury caused by chronic nonsteroidal anti-inflammatory drugs (NSAID) use as assessed by capsule endoscopy. Revista Espanola De Enfermedades Digestivas, 102(2), 80.
[10]. Lanas, A., Tornero, J., & Zamorano, J. L. (2010). Assessment of gastrointestinal and cardiovascular risk in patients with osteoarthritis who require NSAIDs: the LOGICA study. Annals of the rheumatic diseases, 69(8), 1453-1458.
[11]. Zhong J C, & Xie J T. (2014). Preparation of D-ibuprofen clathrate. Journal of Mudanjiang Medical College, 35(6), 32-36.
[12]. Gao Yan, Cui Fude, Liu Peili, Zhang Yulong, Li Qian, & Zhan Xiaoliang. (2006). Optimization of ketoprofen enteric orally disintegrating tablets. Journal of Shenyang Pharmaceutical University, 23(10), 625-629.
[13]. Kamboj, M., Goyal, S., Rakha, P., Arora, G., Dureja, H., & Nagpal, M. (2011). Formulation and evaluation of metformin oro-dispersible tablets. Acta Pol Pharm, 68(5), 717-723.
[14]. Zhang Y. (2013). Preparation and quality control of disperse tablets of total flavones of Populus flavones. Journal of Practical Medicine and Clinic, 16(10), 939-941.
[15]. Gu Wangwen, Yu Lifeng, Yang Haosong, & Pei Yuan-ying. (2001). Comparison of properties of domestic microcrystalline cellulose and Avicel. Chinese Journal of Pharmacology, 36(8), 532-534.
[16]. Lin Wenhui, Cui Fude, Lei Yong, He Jidong, & Pu Hongze. (2001). Study on rapid calcium collapse tablets. Journal of Shenyang Pharmaceutical University, 18(3), 177-180.
[17]. Setty, C. M., Prasad, D. V. K., Gupta, V. R. M., & Sa, B. (2008). Development of fast dispersible aceclofenac tablets: effect of functionality of superdisintegrants. Indian journal of pharmaceutical sciences, 70(2), 180.
[18]. hong J C, & Xie J T. (2015). Preparation of D-ibuprofen β-cyclodextrin inclusion complex dispersion tablets. Chinese Journal of Hospital Pharmacy, 35(18), 1671-1675.
[19]. Ha N, Du Z M, & Guo M H. (2007). Chronopharmacokinetics of ibuprofen granules in rats. Chinese Journal of Pharmacology, 23(11), 1539-1540.
[20]. Xie Bin, Fang Jianguo, Wang Wenqing, Rao Zichao, Zhang Guangxun, Ren Xia, & Xiao Jieyu. (2012). Pharmacokinetics and bioavailability of domestic D-ibuprofen sustained release capsules in rats. Medical Review, 31(10), 1291-1294.
[21]. Zhong JC, & Xie JTAO. (2019). Preliminary pharmacokinetic study of D-ibuprofen β-cyclodextrin inclusion compound dispersion tablets in rats. Collection, 3.
Cite this article
Li,Z. (2023). Research and prospect of inclusion complexes of dexibuprofen β-cyclodextrin. Theoretical and Natural Science,8,53-62.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Du X L, Ren S Q, & Hu Y L.(2001). Gastrointestinal adverse reactions of non-steroidal anti-inflammatory drugs and their prevention and treatment. Chinese Journal of Hospital Pharmacy, 21(4), 235.
[2]. Xie J T, Wang C Y, Li J, & Zhang T T. (2009). Determination of D-ibuprofen β-cyclodextrin inclusion complex by HPLC. Journal of Anhui Medicine, 13(2), 157-158.
[3]. Prabu, S., Swaminathan, M., Sivakumar, K., & Rajamohan, R. (2015). Preparation, characterization and molecular modeling studies of the inclusion complex of Caffeine with Beta-cyclodextrin. Journal of Molecular Structure, 1099, 616-624.
[4]. Kfoury, M., Auezova, L., Greige-Gerges, H., Ruellan, S., & Fourmentin, S. (2014). Cyclodextrin, an efficient tool for trans-anethole encapsulation: Chromatographic, spectroscopic, thermal and structural studies. Food chemistry, 164, 454-461.
[5]. Jin G Y. (2017). Analysis of 7 heavy metal elements in β-cyclodextrin. Journal of Medicine Today, 27(4), 228-231.
[6]. Charoenchaitrakool, M., Dehghani, F., & Foster, N. R. (2002). Utilization of supercritical carbon dioxide for complex formation of ibuprofen and methyl-β-cyclodextrin. International journal of pharmaceutics, 239(1-2), 103-112.
[7]. Riekes, M. K., Tagliari, M. P., Granada, A., Kuminek, G., Silva, M. A. S., & Stulzer, H. K. (2010). Enhanced solubility and dissolution rate of amiodarone by complexation with β-cyclodextrin through different methods. Materials Science and Engineering: C, 30(7), 1008-1013.
[8]. Zhang S Y, Zou Y, Wei T Y, Mou C X, Liu X J, & Tong Z F. (2016). Preparation of β-cyclodextrin/polyether coacetamide filled membrane and pervaporization of trace phenol in water. Journal of Chemical Engineering, 67(11), 4662-4670.
[9]. Caunedo-Alvarez, A., Gómez-Rodríguez, B. J., Romero-Vázquez, J., Argüelles-Arias, F., Romero-Castro, R., García-Montes, J. M., ... & Herrerías-Gutiérrez, J. M. (2010). Macroscopic small bowel mucosal injury caused by chronic nonsteroidal anti-inflammatory drugs (NSAID) use as assessed by capsule endoscopy. Revista Espanola De Enfermedades Digestivas, 102(2), 80.
[10]. Lanas, A., Tornero, J., & Zamorano, J. L. (2010). Assessment of gastrointestinal and cardiovascular risk in patients with osteoarthritis who require NSAIDs: the LOGICA study. Annals of the rheumatic diseases, 69(8), 1453-1458.
[11]. Zhong J C, & Xie J T. (2014). Preparation of D-ibuprofen clathrate. Journal of Mudanjiang Medical College, 35(6), 32-36.
[12]. Gao Yan, Cui Fude, Liu Peili, Zhang Yulong, Li Qian, & Zhan Xiaoliang. (2006). Optimization of ketoprofen enteric orally disintegrating tablets. Journal of Shenyang Pharmaceutical University, 23(10), 625-629.
[13]. Kamboj, M., Goyal, S., Rakha, P., Arora, G., Dureja, H., & Nagpal, M. (2011). Formulation and evaluation of metformin oro-dispersible tablets. Acta Pol Pharm, 68(5), 717-723.
[14]. Zhang Y. (2013). Preparation and quality control of disperse tablets of total flavones of Populus flavones. Journal of Practical Medicine and Clinic, 16(10), 939-941.
[15]. Gu Wangwen, Yu Lifeng, Yang Haosong, & Pei Yuan-ying. (2001). Comparison of properties of domestic microcrystalline cellulose and Avicel. Chinese Journal of Pharmacology, 36(8), 532-534.
[16]. Lin Wenhui, Cui Fude, Lei Yong, He Jidong, & Pu Hongze. (2001). Study on rapid calcium collapse tablets. Journal of Shenyang Pharmaceutical University, 18(3), 177-180.
[17]. Setty, C. M., Prasad, D. V. K., Gupta, V. R. M., & Sa, B. (2008). Development of fast dispersible aceclofenac tablets: effect of functionality of superdisintegrants. Indian journal of pharmaceutical sciences, 70(2), 180.
[18]. hong J C, & Xie J T. (2015). Preparation of D-ibuprofen β-cyclodextrin inclusion complex dispersion tablets. Chinese Journal of Hospital Pharmacy, 35(18), 1671-1675.
[19]. Ha N, Du Z M, & Guo M H. (2007). Chronopharmacokinetics of ibuprofen granules in rats. Chinese Journal of Pharmacology, 23(11), 1539-1540.
[20]. Xie Bin, Fang Jianguo, Wang Wenqing, Rao Zichao, Zhang Guangxun, Ren Xia, & Xiao Jieyu. (2012). Pharmacokinetics and bioavailability of domestic D-ibuprofen sustained release capsules in rats. Medical Review, 31(10), 1291-1294.
[21]. Zhong JC, & Xie JTAO. (2019). Preliminary pharmacokinetic study of D-ibuprofen β-cyclodextrin inclusion compound dispersion tablets in rats. Collection, 3.









