1. Introduction
Walter B. Cannon was noted for his research that coined the term “fight or flight responses” during the 20th century. According to Cannon’s research, there is an innate stress response that leads to the alteration of the homeostasis of the body and multiple physiological functions, such as the respiratory system, the endocrine system, and the circulatory system, to cope with emergency situations [1]. Later naturalistic, experimental, and mechanistic studies further refined this theory and discovered that not only life-threatening stressors would instigate this response but expected and chronic stimuli would also cause the body to initiate various stress responses [2]. Two fundamental stress pathways are the SNS and the HPA axis. Both pathways have been suggested as a coordinated hormone response system that regulates the release of modulating hormones [3, 4]. This study will later elaborate on the specific and collaborative effects of the SNS and HPA axis on the neuroendocrine system.
Some of the most common physiological responses to imminent stress include heart rate and blood pressure elevation, dilation of the pupil, and shortening of breath. Although there is a general understanding of the neuronal pathways that alter the autonomic function of these organs, little work has been done examining the intersections between the organs’ psychophysiology reactions and the neuroendocrinology of stress. This lack of studies may be partly due to the complexity of each individual system and the need to obtain adequate neuroscience tools. However, one of the many physiological responses, the respiratory system, has shown growing evidence of promising links between itself and the hypothalamus region of the brain for in vivo experiments with cats during the 20th century [5, 6]. These experiments provided the empirical foundation for further refined theories that described the possible connections between the HPA axis and the respiratory system [7, 8].
While these past researches have pointed out the HPA axis as a possible bridgeway that connects the respiratory system and the endocrine system of stress hormones, there are no evidence supporting its direct alteration on any aspects of the respiratory system, and the pathway only remains partially clear as merely certain stress hormones generated in the HPA axis would have an influence on breathing rhythm, without knowing the specific pathways and sites [7-9]. The pre-Bötzinger complex (pre-BötC) is a neuron network near the brainstem that functions as a modulator for the respiratory rhythm and, therefore, could serve as a potential interaction site for the stress hormones and the respiratory rhythm. To investigate this hypothesis, this paper will provide an overview of the main stress response systems and the pre-BötC. The article will then examine the neuroanatomical intersections between the endocrine stress response system and the respiratory system. Finally, based on these concepts, potential future research areas will be proposed.
2. Overview of the stress response system
The stress response system is often broadly perceived as an innate, sophisticated, evolutionary-conserved, and interlocking mechanism for organisms to cope with anticipated threats through alteration in the body’s physiology, predominately including the HPA axis and the autonomic nervous system (ANS).
The body’s unconscious actions are mainly regulated by the ANS. The system provides the most immediate response to stress stimuli by effecting physiological responses via the neural intervention of end organs (e.g. digestion, and respiration). The ANS is categorized into two separate systems: the sympathetic nervous system (SNS), and the parasympathetic nervous system (PSNS). Since the PSNS is the primary “rest and digest” response and does not affect the ANS stress response, we will primarily focus on the SNS stress response and the HPA axis.
2.1. Role of the sympathetic nervous system
SNS is often regarded as a fighting response system instigated by the stimulation of stressors to upcoming challenges or dangers. The sympathetic cells are located at the intermediolateral columns of the spine [10]. The neuro circuits expand to the paravertebral ganglia sites via the anterior rami of T1-L2 spinal nerves, and it is located near the ventral or lateral side of the spinal cord, via white rami communicants [10]. On varied regions of the paravertebral ganglion, the synapse of the SNS might extend to the prevertebral ganglions which are adjacent to the peripheral organs and tissues. Neurons within the paravertebral region extend without synapsing via axonal projections to initiate various physiological responses [11]. In contrast, neurons in the prevertebral site supply signals to the abdominal, pelvic, and perineal areas [11].
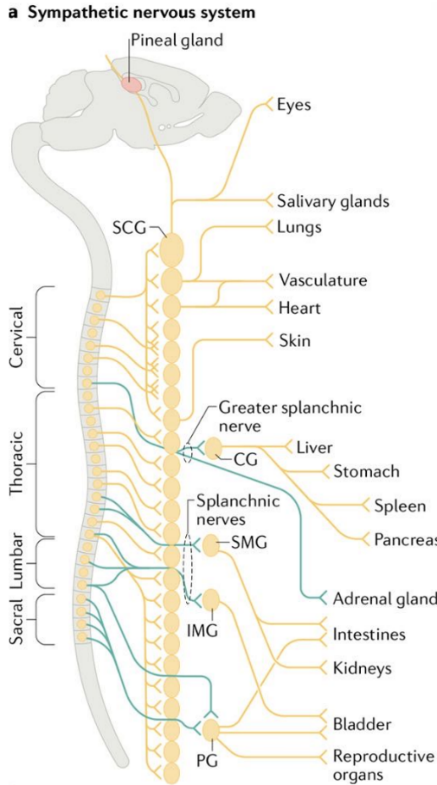
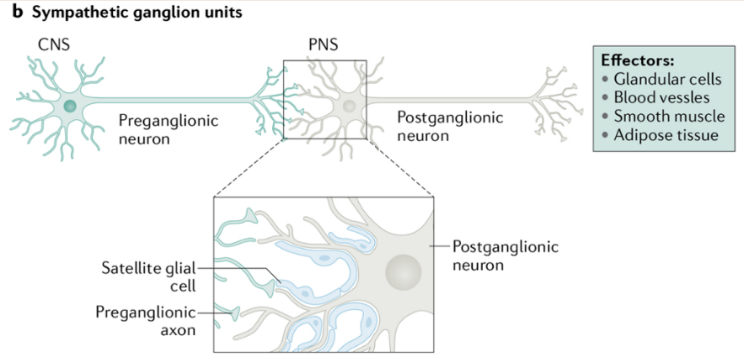
The SNS is mainly composed of two populations of neurons: preganglionic and post-ganglionic neurons which are connected by synapses. The preganglionic neurons are the trigger neurons that are linked to transmit signals to post-ganglionic neurons which are found at the paravertebral ganglia in bilateral chains and the prevertebral ganglia in clusters [11]. The preganglionic neurons release acetylcholine (Ach), which binds to the nicotinic acetylcholine receptors resided on the postganglionic neurons. The post-ganglionic neurons then release their main neurotransmitter (norepinephrine, epinephrine) that binds to the adrenergic receptors, classified as alpha and beta, in the target tissue [10], as shown in figure 1.
|
|
Figure 1. Organization of the mouse sympathetic nervous system and sympathetic ganglion units. Remodified from [11]. (a) This is an illustration of the neuronal circuits of the SNS which is assembled mainly by paravertebral or prevertebral sympathetic ganglia, sending signals through diverse networks to innervate different body organs. (b) The preganglionic neuron and postganglionic neuron form synaptic connections at either the paravertebral or prevertebral ganglia regions. The postganglionic neurons contain satellite glial cells that cover their synapses, dendrites, and cell bodies.
As the first stress response system to emergent dangers, SNS does this mainly through its ability to regulate the constriction (vasoconstriction) of the blood vessels. This effect is mediated via the binding of alpha-1 adrenergic receptors with the predominant neurotransmitter, norepinephrine, released from the post-ganglionic sympathetic neurons [12]. Activation of the a1- adrenergic receptor leads to the contraction of smooth muscles around the blood vessels and hence decreases blood flow and thus the heart rate increases [12].
2.2. Role of the HPA axis
In response to stress, corticotrophin-releasing hormone (CRH) and arginine vasopressin (AVP) are released out of the paraventricular nucleus (PVN) [7]. These peptides then travel down via the hypophyseal portal to reach the APLG and bind to the cognate receptors on corticotropes, which activates the release of adrenocorticotropic hormone (ACTH) [11]. Although CRH is a principal mediator for the release of ACTH, AVP could potentially assume control to mediate the acute reactivity under chronic stress conditions where CRHs are decentralized [7, 13]. Besides the primary ACTH mediators, there are also various other sub-neuro peptides and transmitters, including catecholamines, angiotensin II, serotonins, and vasoactive intestinal peptides [7, 13], that induce the release of ACTH, as can some cytokines involved with regulation of inflammatory activities such as interleukin -1 and interleukin-6 [14, 15]. ACTH has a relatively shorter life span and after being released, it travels down quickly through the body’s circulation system and reaches the adrenal cortex, and regulates glucocorticoid cortisol concentration, via binding of melanocortin type 2 receptor (MC2-R) [7, 13], as shown in figure 2.
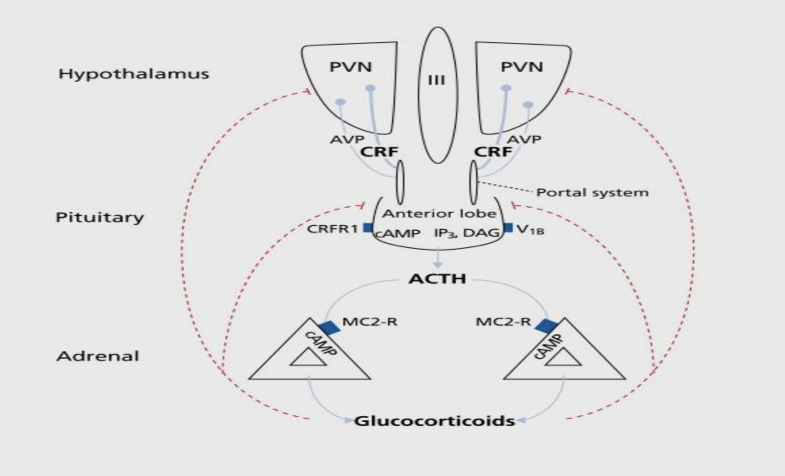
Figure 2. Demonstration of the hypothalamic-pituitary-adrenal axis. Remodified from [13]. The neurons in the PVN synthesize CRH and AVP which access the anterior pituitary through hypophysial portal. The activation of the CRH-1 receptor releases corticotropes and cyclic adenosine monophosphate (cAMP) pathway that increase ACTH concentration. In the decentralized presence of CRH, AVP elicits synergistic effects on ACTH via vasopressin V1b receptor binding. The ACTH then travels down and binds to MC2-R in the adrenal cortex, initiates the release of glucocorticoid.
Unlike the SNS stress response, the HPA axis does not provide a rapid physiology adaption when facing emergent dangers [16]. The pathway is often considered as the sluggish secondary phase of stress responses that allows the body to continuously remain alert through the regulation of glucocorticoid levels [16]. Glucocorticoids can affect every organ system and play a primary physiological effect on the body through alteration of energy mobilization (stimulation of glucose level), suppression of the immune system, and release of catecholamine [10, 17]. The principal function behind glucocorticoids’ wide effects might be related to the restoration of homeostasis and shaping longer adaptation to reduce the impacts of future similar challenges [7, 18]. If overused, the restorative process has deleterious effects on the body and produces organismic “wear and tear” [7]. Therefore, a mechanism to regulate it is needed. Glucocorticoids are meditated by negative feedback inhibition via rapid shut-off of the CRH neurons [4]. In the PVN, glucocorticoids bind to its receptor (GR receptor) which causes rapid release and synthesis of endocannabinoids (Ecs) [4, 13]. The Ecs then bind to Cannabinaoid-1 receptors, inhibiting the glutamate release thereby terminating the drive toward CRH neurons [4].
3. Overview of the pre-Bötzinger Complex
Understandings behind the mechanism of breathing remain opaque and vague. This surprisingly complex behavior is affected by various distinct key natural elements that alter its pattern generation. Being able to identify these key natural elements has therefore become a primary target that would lead to the prominent discoveries of molecular, cellular, and synaptic networks that give rise to the mechanism. One essential key element of the respiratory system is the pre-BötC.
3.1. Anatomy of the pre-BötC
The pre-BötC is a neuronal network that is part of the ventral respiratory group which forms a rostro caudal column at the medulla oblongata that connects the brain stem and spinal cord [19]. The column is divided into four distinct sections: the pre-Bötzinger complex, the rostral Bötzinger complex, the rostral ventral respiratory group, and the caudal ventral respiratory group [20], as shown in figure 3.
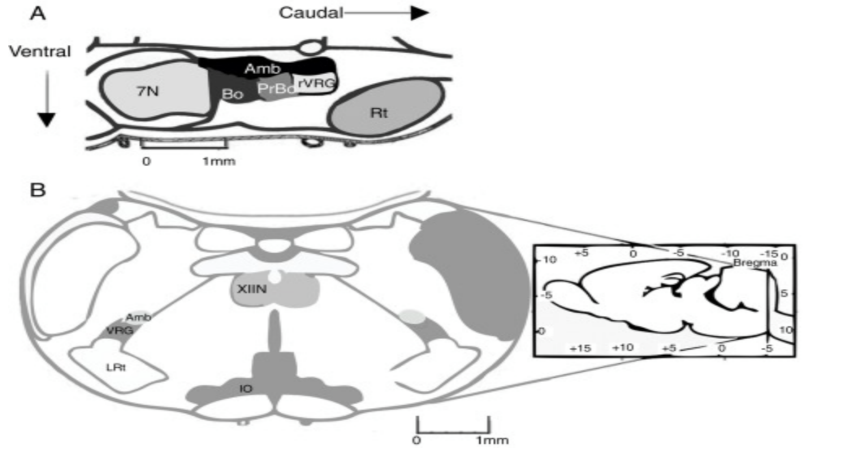
Figure 3. Illustration of the location of the pre-BötC. Remodified from [21]. (a) Demonstration of the location of the pre-BötC in the rodent brain. The pre-BötC is located at the same level as the obex, rostral to the lateral reticular nucleus, caudal to the level of the facial nucleus, and ventral to nucleus ambiguous. (b) Coronal illustration of pyramidal decussation to show the location for the pre-BötC via recognizable landmarks. IO: inferior olivary nucleus; VRG: ventral respiratory group; XIIN: hypoglossal nucleus [19].
Although there is a comprehensive understanding of the location of experimental mammals, information regarding the respiratory control complexes in humans is limited. There has only been one recent published research have claimed to successfully identify the precise location of the human pre-BötC [20].
The pre-BötC consists mainly of interneurons. 600 out of 1000 neurons in the pre-BötC of rodents possess the neurokinin 1 receptor (NK1R), producing substance P and somatostatin [19]. These neurons are often considered the biomarker for the pre-BötC. Brain autopsy conducted on patients with Lewy body dementia and multiple system atrophy have shown a great reduction in the number of NK1R- immunoreactive neurons of the pre-BötC, suggesting a high likelihood that respiratory diseases are caused by loss of neurons in the pre-BötC [19].
The pre-BötC also contains other populations of neurons, such as glycinergic, glutamatergic, and GABAergic neurons [21]. Most of the neurons in the pre-BötC develop inspiratory drive potentials which top off at 10-20 mV synchronic with a depolarization lasting 0.3-0.8 s [19]. Another major subpopulation of neurons in the pre-BötC is silent neurons, which do not fire any action potential despite they are located in the same network [19].
3.2. Pre-BötC inspiratory rhythm
In many models, the bursts generated by pre-BötC correlate with the body’s inspiratory activity and are believed the be an essential part of rhythmogenesis. The underlying mechanism of rhythmogenesis, however, remains unclear. When pre-BötC was first discovered, there was an initial study that found the presence of pacemaker neurons and proposed it as a potential drive for rhythmogenesis [22]. While the theory does sound convincing, there were no in vitro experiments that showed pacemaker neurons are necessary for rhythmogenesis [23]. Another popular theory for the resulting rhythm generated is synaptic inhibition. This, however, also fails critical tests for its presence [24]. Therefore, there is reason to doubt both mechanisms of synaptic inhibition and packer neurons as the primary control for inspiratory rhythm in the pre-BötC. Recent data, however, have provided another relatively reliable theory as an essential factor that constitutes the inspiratory pattern generation – the burstlet theory.
There has always been a long-standing view which believed periodic movements of large amplitude bursts are essential for the generation of rhythmogenesis in the pre-BötC. In vitro, when the neuron excitability is high, action potentials can be measured in every pre-BötC cycle driving motor outputs via the hypoglossal nerve (XII). When the excitability is low, however, there is no expected pattern occurring in the motor outputs. Instead, within the pre-BötC, there is observed synchronous low-level activities, called the burstlets [25]. Studies conducted while recording both cranial nerve (XII) and specialized rhythm-generating pre-BötC have illustrated burstlets to be the preceding factors for the high amplitude inspiratory factors [25]. Although there is yet a comprehensive understanding or study demonstrating how dose the burstlets affect the XII motor neuron output, studies have pointed out that when there is sufficient excitability, there could be potential burstlets leaks from the pre-BötC that cause high amplitude bursts in the motor output [25].
4. Intersections between the endocrine stress response system and the respiratory system
Although as mentioned earlier there are limited studies that directly investigate the relationship between the respiratory system and the endocrine stress response system, there is extensive research conducted on the relationship between the dysregulation of the HPA axis with panic disorders and SNS with respiratory pathologies such as acute and chronic intermittent hypoxia
4.1. The HPA axis and the respiratory system
Respiration changes are often considered as one of the most common physiological symptoms caused by panic disorders (PD) and panic attacks (PA). Different abnormalities in respiration, such as shortness of breath and enhanced CO2 activities have been detected and are well-documented in PA patients. The effects of PD on neuroendocrine and respiratory systems are extensively studied but the linkage between these two systems is rarely examined. Recent research by James L. Abelson that attempted to elucidate the neurobiological connection between PA and the HPA axis provided a potential explanation for this linkage [26]. The experiment was conducted on PD patients in the context of stressful simulation and has shown a rise in HPA activity, measured via both ACTH and cortisol levels, compared to the controlled resting state [26]. Based on these findings and speculations that respiratory dysregulation is associated with subjective dyspnea, James L. Abelson’s team proposed and verified that elevated HPA activity (rise of ACTH level) is parallel to tidal volume abnormalities, measured based on comparisons between PD patients and normal patient via injection of doxapram [27].
Besides the correlation between PD-induced respiratory change and the HPA axis, past studies that focused on the anatomy of the brain have suggested activity changes in the hypothalamus are associated with respiratory controls, and since the hypothalamus is an essential part of the HPA axis, this adds another potential cue to the relationship between the HPA axis and the respiratory system [28].
4.2. The SNS and the respiratory system
In contrast to the HPA axis, which does not directly innervate organs in the body but rather affects the body upon release of glucocorticoid, the SNS axons ramify from paravertebral and prevertebral ganglions and regulate various physiology functions of the organs mainly via release of norepinephrine and epinephrine. Since the release, diffusion, and buildup of sufficient glucocorticoids inhibit more time and is the main mechanism of the HPA axis to regulate body endocrine and organ physiology functions, the SNS is relatively more direct and rapid. However, like the HPA axis, the SNS also lacks studies for its underlying regulation of rhythmogenesis. There were merely examinations conducted between respiratory-related conditions that cause irregular control of breathing patterns and norepinephrine released by SNS.
Acute intermittent hypoxia (AIH) is a recurrent episode of low oxygen breaths that is used as a therapy to assist walking recovery after spinal cord injury. The late study has discovered AIH leads to fundamental changes in the pre-BötC both in vitro and in vivo, by increasing synaptic inhibition activity when norepinephrine is increased, and since norepinephrine is an essential neurotransmitter that contributes to the innervation of SNS towards various organs, one can conclude a potential interaction between the respiratory patterns generated in the pre-BötC and the SNS [29]. Like the AIH study, observations on chronic intermittent hypoxia (episodes of low oxygen breaths that have a longer period spang than AIH) also show changes in the strength or pattern of respiratory couples with changes in the SNS activities.
5. Areas of Future Research
The two main stress response systems (HPA axis and SNS) regulate various physiological responses of the body mainly via the control and release of stress-related hormones such as ACTH, epinephrine, and norepinephrine. Despite there being hardly any research conducted on the correlations between respiratory rhythmogenesis and endocrine stress response, different concentrations of these hormones, in the cases mentioned above, show an underlying relationship with the irregular activity of various respiratory-related diseases and conditions. Noting that (a) respiratory conditions (AIH and CIH) have illustrated cases that when there is high level of epinephrine, there is frequency irregularities in the ventilatory motor output; (b) PD and PA patients exhibit elevated levels of ACTH; (c) HPA axis and SNS activities can be measured via concentration levels of ACTH, epinephrine, and norepinephrine; (d) pre-BötC is the main neuro network site for breathing pattern generation and regulation. Based on these cases, one can make a reasonable hypothesis that there exists a correlation between the activities of the stress response system, recorded via concentration levels of the listed stress hormones (ACTH and norepinephrine), and pre-BötC breathing rhythmogenesis. In order to verify this hypothesis, future research records stress-respiratory coupling stress hormones concentration and the pre-BötC population activities upon various concentrations of the stress-respiratory coupling hormones. In 2014, there were already preexisting records from Sébastien Zanella’s team’s research to measure the effects of norepinephrine on the breathing irregularities from the pre-BötC [29]. The experiment’s recording, however, mainly focused on the activity of the overall neuron population, cranial nerve XII, as the purpose was to record the pattern of the breathing activity. As previously noted, burstlets that are generated within neurons of pre-BötC are the main modulators for respiratory rhythmogenesis. Thus, future research could provide additional recording of the effects of stress-respiratory coupling hormones on the pre-BötC population, instead of solely on the overall motor neuron output.
6. Conclusion
Much remained to learn about the complex interactions between the stress neuroendocrinology and psychophysiology of the respiratory system. There is abundant information on each individual discipline, but there is a need for studies on the system’s linkage instead of a deepened specific understanding of the systems separately. This linkage is of great relevance towards the buildup of a comprehensive understanding of the interaction between the nervous system and many other physiological responses, such as the cardiovascular system, and the digestive system, besides just the respiratory system. Many of the outcomes of these future researches could in turn lead to the development of useful clinical drugs that target stress-triggered physiological diseases.
References
[1]. Cannon WB. Bodily changes in pain, hunger, fear, and rage. An account of recent researches into the function of emotional excitement (2nd ed.). Boston: Charles T. Branford Company, 1953.
[2]. Pulopulos MM, Baeken C, De Raedt R. Cortisol response to stress: The role of expectancy and anticipatory stress regulation. Horm Behav. 2020 Jan;117:104587.
[3]. Zygmunt A, Stanczyk J. Methods of evaluation of autonomic nervous system function. Arch Med Sci. 2010 Mar 1;6(1):11-8.
[4]. Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, Scheimann J, Myers B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr Physiol. 2016 Mar 15;6(2):603-21.
[5]. Kristensen MP, Poe GR, Rector DM, Harper RM. Activity changes of the cat paraventricular hypothalamus during phasic respiratory events. Neuroscience. 1997 Oct;80(3):811-9.
[6]. DASGUPTA SR. Effect of frontal section through the hypothalamus on respiration in diencephalic cats. Am J Physiol. 1956 Jul;186(1):139-41.
[7]. Abelson JL, Khan S, Giardino N. HPA axis, respiration and the airways in stress--a review in search of intersections. Biol Psychol. 2010 Apr;84(1):57-65.
[8]. Abelson JL, Weg JG, Nesse RM, Curtis GC. Persistent respiratory irregularity in patients with panic disorder. Biol Psychiatry. 2001 Apr 1;49(7):588-95.
[9]. Kristensen MP, Poe GR, Rector DM, Harper RM. Activity changes of the cat paraventricular hypothalamus during phasic respiratory events. Neuroscience. 1997 Oct;80(3):811-9.
[10]. Waxenbaum JA, Reddy V, Varacallo M. Anatomy, Autonomic Nervous System. [Updated 2023 Jul 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-.
[11]. Scott-Solomon E, Boehm E, Kuruvilla R. The sympathetic nervous system in development and disease. Nat Rev Neurosci. 2021 Nov;22(11):685-702.
[12]. Sheng Y, Zhu L. The crosstalk between autonomic nervous system and blood vessels. Int J Physiol Pathophysiol Pharmacol. 2018 Mar 10;10(1):17-28.
[13]. Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8(4):383-95.
[14]. Venihaki M, Dikkes P, Carrigan A, Karalis KP. Corticotropin-releasing hormone regulates IL-6 expression during inflammation. J Clin Invest. 2001 Oct;108(8):1159-66.
[15]. Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987 Oct 23;238(4826):522-4.
[16]. Godoy LD, Rossignoli MT, Delfino-Pereira P, Garcia-Cairasco N, de Lima Umeoka EH. A Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front Behav Neurosci. 2018 Jul 3;12:127.
[17]. Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000 Feb;21(1):55-89.
[18]. Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003 Jul;24(3):151-80.
[19]. Muñoz-Ortiz J, Muñoz-Ortiz E, López-Meraz ML, Beltran-Parrazal L, Morgado-Valle C. Pre-Bötzinger complex: Generation and modulation of respiratory rhythm. Neurologia (Engl Ed). 2019 Sep;34(7):461-468. English, Spanish.
[20]. Schwarzacher SW, Rüb U, Deller T. Neuroanatomical characteristics of the human pre-Bötzinger complex and its involvement in neurodegenerative brainstem diseases. Brain. 2011 Jan;134(Pt 1):24-35.
[21]. Liu YY, Ju G, Wong-Riley MT. Distribution and colocalization of neurotransmitters and receptors in the pre-Bötzinger complex of rats. J Appl Physiol (1985). 2001 Sep;91(3):1387-95.
[22]. Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991 Nov 1;254(5032):726-9.
[23]. Del Negro CA, Funk GD, Feldman JL. Breathing matters. Nat Rev Neurosci. 2018 Jun;19(6):351-367.
[24]. Janczewski WA, Tashima A, Hsu P, Cui Y, Feldman JL. Role of inhibition in respiratory pattern generation. J Neurosci. 2013 Mar 27;33(13):5454-65.]
[25]. Kam K, Worrell JW, Janczewski WA, Cui Y, Feldman JL. Distinct inspiratory rhythm and pattern generating mechanisms in the preBötzinger complex. J Neurosci. 2013 May 29;33(22):9235-45.
[26]. Abelson JL, Khan S, Liberzon I, Young EA. HPA axis activity in patients with panic disorder: review and synthesis of four studies. Depress Anxiety. 2007;24(1):66-76.]
[27]. Abelson JL, Weg JG, Nesse RM, Curtis GC. Persistent respiratory irregularity in patients with panic disorder. Biol Psychiatry. 2001 Apr 1;49(7):588-95.
[28]. REDGATE ES. Inspiratory area excitability and the hypothalamus. Am J Physiol. 1960 Jun;198:1299-303.
[29]. Zanella S, Doi A, Garcia AJ 3rd, Elsen F, Kirsch S, Wei AD, Ramirez JM. When norepinephrine becomes a driver of breathing irregularities: how intermittent hypoxia fundamentally alters the modulatory response of the respiratory network. J Neurosci. 2014 Jan 1;34(1):36-50.
Cite this article
Wan,C. (2023). An overview of the correlation between the stress response system and the respiratory system. Theoretical and Natural Science,16,1-9.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Cannon WB. Bodily changes in pain, hunger, fear, and rage. An account of recent researches into the function of emotional excitement (2nd ed.). Boston: Charles T. Branford Company, 1953.
[2]. Pulopulos MM, Baeken C, De Raedt R. Cortisol response to stress: The role of expectancy and anticipatory stress regulation. Horm Behav. 2020 Jan;117:104587.
[3]. Zygmunt A, Stanczyk J. Methods of evaluation of autonomic nervous system function. Arch Med Sci. 2010 Mar 1;6(1):11-8.
[4]. Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, Scheimann J, Myers B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr Physiol. 2016 Mar 15;6(2):603-21.
[5]. Kristensen MP, Poe GR, Rector DM, Harper RM. Activity changes of the cat paraventricular hypothalamus during phasic respiratory events. Neuroscience. 1997 Oct;80(3):811-9.
[6]. DASGUPTA SR. Effect of frontal section through the hypothalamus on respiration in diencephalic cats. Am J Physiol. 1956 Jul;186(1):139-41.
[7]. Abelson JL, Khan S, Giardino N. HPA axis, respiration and the airways in stress--a review in search of intersections. Biol Psychol. 2010 Apr;84(1):57-65.
[8]. Abelson JL, Weg JG, Nesse RM, Curtis GC. Persistent respiratory irregularity in patients with panic disorder. Biol Psychiatry. 2001 Apr 1;49(7):588-95.
[9]. Kristensen MP, Poe GR, Rector DM, Harper RM. Activity changes of the cat paraventricular hypothalamus during phasic respiratory events. Neuroscience. 1997 Oct;80(3):811-9.
[10]. Waxenbaum JA, Reddy V, Varacallo M. Anatomy, Autonomic Nervous System. [Updated 2023 Jul 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-.
[11]. Scott-Solomon E, Boehm E, Kuruvilla R. The sympathetic nervous system in development and disease. Nat Rev Neurosci. 2021 Nov;22(11):685-702.
[12]. Sheng Y, Zhu L. The crosstalk between autonomic nervous system and blood vessels. Int J Physiol Pathophysiol Pharmacol. 2018 Mar 10;10(1):17-28.
[13]. Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8(4):383-95.
[14]. Venihaki M, Dikkes P, Carrigan A, Karalis KP. Corticotropin-releasing hormone regulates IL-6 expression during inflammation. J Clin Invest. 2001 Oct;108(8):1159-66.
[15]. Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987 Oct 23;238(4826):522-4.
[16]. Godoy LD, Rossignoli MT, Delfino-Pereira P, Garcia-Cairasco N, de Lima Umeoka EH. A Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front Behav Neurosci. 2018 Jul 3;12:127.
[17]. Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000 Feb;21(1):55-89.
[18]. Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003 Jul;24(3):151-80.
[19]. Muñoz-Ortiz J, Muñoz-Ortiz E, López-Meraz ML, Beltran-Parrazal L, Morgado-Valle C. Pre-Bötzinger complex: Generation and modulation of respiratory rhythm. Neurologia (Engl Ed). 2019 Sep;34(7):461-468. English, Spanish.
[20]. Schwarzacher SW, Rüb U, Deller T. Neuroanatomical characteristics of the human pre-Bötzinger complex and its involvement in neurodegenerative brainstem diseases. Brain. 2011 Jan;134(Pt 1):24-35.
[21]. Liu YY, Ju G, Wong-Riley MT. Distribution and colocalization of neurotransmitters and receptors in the pre-Bötzinger complex of rats. J Appl Physiol (1985). 2001 Sep;91(3):1387-95.
[22]. Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991 Nov 1;254(5032):726-9.
[23]. Del Negro CA, Funk GD, Feldman JL. Breathing matters. Nat Rev Neurosci. 2018 Jun;19(6):351-367.
[24]. Janczewski WA, Tashima A, Hsu P, Cui Y, Feldman JL. Role of inhibition in respiratory pattern generation. J Neurosci. 2013 Mar 27;33(13):5454-65.]
[25]. Kam K, Worrell JW, Janczewski WA, Cui Y, Feldman JL. Distinct inspiratory rhythm and pattern generating mechanisms in the preBötzinger complex. J Neurosci. 2013 May 29;33(22):9235-45.
[26]. Abelson JL, Khan S, Liberzon I, Young EA. HPA axis activity in patients with panic disorder: review and synthesis of four studies. Depress Anxiety. 2007;24(1):66-76.]
[27]. Abelson JL, Weg JG, Nesse RM, Curtis GC. Persistent respiratory irregularity in patients with panic disorder. Biol Psychiatry. 2001 Apr 1;49(7):588-95.
[28]. REDGATE ES. Inspiratory area excitability and the hypothalamus. Am J Physiol. 1960 Jun;198:1299-303.
[29]. Zanella S, Doi A, Garcia AJ 3rd, Elsen F, Kirsch S, Wei AD, Ramirez JM. When norepinephrine becomes a driver of breathing irregularities: how intermittent hypoxia fundamentally alters the modulatory response of the respiratory network. J Neurosci. 2014 Jan 1;34(1):36-50.