1. Introduction
With the increasing prominence of health problems caused by excessive intake of added sugar, reducing and controlling sugar has become one of the most urgent dietary needs for people [1, 2]. Sugar substitutes are widely favored by consumers due to their high sweetness and low calorie properties [3]. These substitutes can be broadly categorized into three types: sugar alcohols, natural sweeteners, and artificial sweeteners. Erythritol, a representative sugar alcohol, has a sweetness of about 60%-80% of sucrose and a caloric value of only 0.4 kcal/g [4]. Steviol glycoside, another widely used natural sweetener, is approximately 250-350 times sweeter than sucrose and is metabolized into steviol and excreted in the urine [5, 6]. Common artificial sweeteners include aspartame and sucralose. Aspartame is approximately 180-200 times sweeter than sucrose[7], while sucralose is 600 times sweeter than sucrose [8]. Both aspartame and sucralose do not enter the human bloodstream [9]. The demand for sugar-substituted beverages has significantly increased in recent years, as consumers shift towards healthier dietary habits [10]. In China, the market for sugar substitutes has been steadily growing, reaching a size of approximately 22 billion dollars in 2021, with a compound annual growth rate (CAGR) of 35% [11]. However, along with the popularity of sugar substitutes, concerns about the long-term consumption of these products have emerged. Existing studies have shown a positive correlation between erythritol intake and the risk of cardiovascular disease (CVD) [12]. On July 14, 2023, the World Health Organization (WHO) classified aspartame as a Group 2B carcinogen, highlighting potential health risks [13]. Consequently, the safety of sugar substitutes has become an increasingly studied topic, as the existing health concerns are being addressed and further risks associated with these substitutes need clarification.
Learning and memory are crucial survival skills for human beings, with adolescence being a prime time for developing these functions as the nervous system undergoes significant developmental changes [5]. During this stage, continuous learning and exercise lead to the addition of new neurons in the granular cell layer of the dentate gyrus in the hippocampus, contributing to memory formation. When there is nerve damage, it can affect hippocampal function and result in symptoms of memory deficits in adolescents [14]. Several studies have indicated the potential neurotoxicity of sugar substitutes [15-17]. For instance, long-term consumption of sugar substitutes has been found to severely impair passive avoidance learning, affect cognitive and hippocampal integrity, and lead to difficulties in forming long-term memories in rats [15]. Lebda et al. [16]. conducted a two-month intervention experiment in which three groups of mice were daily fed water, aspartame, and cola [16]. The results showed significant brain damage in the mice that ingested aspartame and cola, with the mechanism involving circulatory system imbalance and neuronal apoptosis. Using adult zebrafish as a model, Li et al. investigated the behavioral and neurological effects of aspartame intake within the permissible daily intake range, revealing that it altered the behavioral characteristics of zebrafish and disrupted neurotransmitter homeostasis in the brain [17]. Although Finn and Lord’s study suggested that sucralose did not induce central nervous system lesions in mice and marmosets, there is still insufficient evidence to draw a definitive conclusion regarding the impact of sucralose on learning and memory functions in organisms [18].
In this study, Caenorhabditis elegans was employed as a model organism. C. elegans are multicellular invertebrates with transparent bodies, which facilitate experimental observation, making them commonly used for studying learning and memory functions. The nervous system of C. elegans is relatively simple, consisting of only 302 neurons, and the structure and connections of these neurons have been extensively described, enabling easier investigation of neural development and circuit formation. Due to their short life cycle and high reproductive capacity, C. elegans are highly suitable for large-scale behavioral experiments and genetic studies. Moreover, C. elegans share 60-80% genetic homology with humans, making them suitable for targeting conserved genes and molecular pathways [19]. Learning memory behavior in C. elegans involves both non-associative and associative learning, allowing them to respond effectively to a single stimulus and establish connections between multiple stimuli [20]. Rankin et al. developed a method to study the non-associative learning ability of nematodes using mechanical stimuli [21]. Additionally, the associative learning of C. elegans can be investigated through odor or chemotaxis experiments combined with starvation [22, 23]. Rashmi et al. confirmed the significance of sleep for learning and memory in C. elegans through a chemotaxis experiment involving the odor of butanone [24]. Furthermore, Vishnu et al. identified the crucial role of dopamine in the olfactory adaptive learning pathway of C. elegans [25]. Therefore, in this study, the tap stimulus assay and odor chemotaxis assay were employed to assess the non-associative learning ability of C. elegans, while chemotaxis experiments involving high concentrations of NaCl, combined with starvation, were utilized to examine their associative learning ability.
In this study, it was found that sucrose treatment reduced the non-associative learning ability and associative learning ability of C. elegans to a certain extent. The study investigated the impact of sucrose treatment on the learning memory function of C. elegans. Different concentrations of sucrose were examined to evaluate their effects on non-associative and associative learning behaviors of C. elegans. Additionally, the study analyzed the expression of 11 well-known genes associated with learning memory using RT-qPCR. The findings revealed that sucrose modulates learning memory pathways by extensively regulating pathways related to learning and memory. Notably, the glutamate signaling pathway was identified as one of the pathways affected by sucrose treatment. This study lays the foundation for further in-depth research on the regulation of learning memory pathways by sugar substitutes in mammals, and suggests the potential harm of sugar substitutes consumption on learning memory ability in developing adolescents.
2. Materials and Methods
2.1. Experimental Materials
Sucralose (BR, HPLC ≥ 98%) was obtained from Shanghai Yuanye Biotechnology Co. Ltd. Benzaldehyde (AR, GC > 98.5%) was purchased from Shanghai Macklin Biochemical Technology Co., Ltd. Peptone, yeast dipping powder, and tryptone were acquired from Beijing AOBOX Biotechnology Co., Ltd. The remaining reagents were purchased from Beijing Solarbio Science & Technology Co., Ltd.
Wild-type Caenorhabditis elegans N2 and Escherichia coli OP50 strains were obtained from the Chinese Institute of Science and Biophysics.
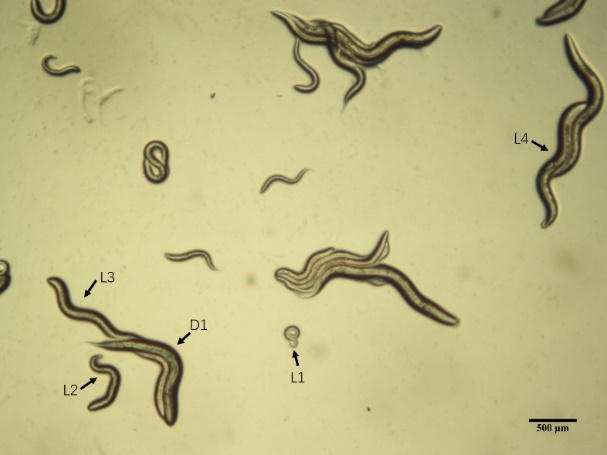
Figure 1. Caenorhabditis elegans observed under a stereomicroscope, with different growth stages indicated by arrows. The larval stages 1 to 4 (L1-L4) and the first day of adulthood (D1) are specifically highlighted. Scale bar = 250 μm.
2.2. Experimental Methods
2.2.1. Design of market research forms and implementation. Through field observation of supermarkets in Beijing, China, we identified the mainstream sugar substitute beverages on the market and recorded data such as manufacturer, beverage name, sugar substitute category, and name of sugar substitute.
2.2.2. Culture and synchronization of C. elegans. The nematode growth medium (NGM) was prepared by first weighing 0.75 g of NaCl, 0.625 g of tryptone, and 5.0 g of agar powder. The weighed ingredients were then dissolved and the volume was adjusted to 250 mL using deionized water. The medium was sterilized at 115 °C for 30 minutes and subsequently cooled to approximately 65 °C at room temperature. To the cooled medium, 0.25 mL of 1 M CaCl2, 0.25 mL of a 5 mg/mL cholesterol ethanol solution, 0.25 mL of 1 M MgSO4, and 6.25 mL of 1 M K3PO4 were added, and the mixture was thoroughly mixed. NGM plates were made using the prepared medium, which were then allowed to cool and coated with 100 μL of E. coli OP50 as the food source.
C. elegans were inoculated on NGM medium and cultured at 20 °C until egg-conceiving adults were obtained. Next, the C. elegans were treated with 1% sodium hypochlorite and 0.5 M potassium hydroxide. Embryos were collected during incubation. The embryos were then washed three times using M9 buffer and transferred onto a restriction medium that was devoid of tryptone and E. coli OP50. Following incubation, the C. elegans experienced stagnation under conditions of food deprivation. They were subsequently collected and inoculated with NGM medium containing food to initiate normal growth and development, effectively achieving synchronization of the growth cycle of C. elegans.
2.2.3. Effect of sucralose on tap stimulus in the C. elegans. According to the method described by Rankin et al [26], C. elegans synchronized to the L1 stage were transferred to different groups of NGM medium. These groups included a control group without any substituted sugar (0 mg/mL), as well as groups with sucralose concentrations of 0.3 mg/mL, 1 mg/mL, and 10 mg/mL. Additionally, there was a non-substituted sugar control with 10 mg/mL of glucos. The C. elegans were then incubated for 24 hours.
After incubation, individual C. elegans were carefully transferred to new petri plates containing the respective groups of NGM medium (35 mm in diameter). The plates were incubated overnight. On the following day, a needle was used to tap the edge of the petri plate corresponding to the head of the C. elegans, and their evasive response to the tap stimulus was observed. The time it took for the C. elegans to recover their movement was recorded. This process was repeated several times until the C. elegans no longer evasively responded to the tap. If the C. elegans still exhibited an evasive response after 10 taps, the time from the start of the evasive response to the recovery of movement on the tenth tap was recorded as the final state.
2.2.4. Effect of sucralose on odor chemotaxis in the C. elegans. According to the method described by Wang et al [27]. , C. elegans synchronized to the L1 stage were transferred to NGM medium in each group and incubated at 20 °C for 48 hours. Adult C. elegans that did not harbor eggs were selected and placed onto NGM medium plates (90 mm in diameter) that did not contain E. coli OP50. These C. elegans were positioned at the center of the medium.
Once the C. elegans had slightly crawled away from the edge of the medium, 1 μL of elicitor (0.1% benzaldehyde ethanol solution) was added at a distance of 1 cm from the edge of the plate. As a control, 1 μL of anhydrous ethanol was added to the edge of the medium opposite to the elicitor. Afterwards, 1 μL of 1 M NaN3 was added to both the elicitor and control points to paralyze any C. elegans that approached these areas. After the C. elegans had crawled for 2 hours at 20 °C, the number of paralyzed C. elegans within a radius of 1 cm around the elicitor and the control was recorded.
\( Convergence Index (CI)=\frac{number of attracted C. elegans-number of control C. elegans}{total number of C. elegans×100\%} \)
2.2.5. Effect of sucralose on NaCl chemotaxis in the C. elegans. According to the method described by Bargamann et al. [28], a petri plate with a diameter of 90 mm was prepared. On this plate, two points labeled as A and B were selected, positioned 4 cm apart from each other. The NGM medium used at these points did not contain NaCl and E. Coli OP50. Point A served as the control point, while at point B, a piece of agar containing 100 mM NaCl was placed. The plate was then left overnight at 4 °C. Afterward, the agar at point B was carefully removed, resulting in the formation of a continuous NaCl concentration gradient extending from point B towards point A. Point C was selected on the center vertical line between points A and B. The distance between point C and both points A and B was 3 cm. Point C served as the starting point for subsequent procedures.
C. elegans synchronized to the L1 stage were transferred to the NGM medium of each group and incubated at 20 °C for 48 hours (Table 1). After that, the groups were washed with NaCl-free M9 buffer.
Table 1. Treatment of Different Groups
Group | Treatment | Sample size |
Standard NGM | No hunger at 20 °C | 50 |
NGM without NaCl and OP50 | Starvation at 20 °C for 4 h | 50 |
NGM of 100 mM NaCl without OP50 | Starvation at 20 °C for 4 h | 50 |
At the end of the treatment period, the C. elegans were dropped onto the starting point C on the petri plate. Any excess solution was carefully removed using paper. Once the C. elegans had crawled away from the starting point C, 1 μL of 1 M NaN3 was added to both points A and B on the petri plate. The C. elegans were then allowed to freely move for 2 hours at a temperature of 20 °C. At the end of this period, the number of C. elegans present within a radius of 1 cm around points A and B were counted separately.
\( Convergence Index (CI)=\frac{number of C. elegans in point B area-number of C. elegans in point A area}{total number of C. elegans×100\%} \)
2.2.6. Effect of sucralose on the related gene expression in the C. elegans. RNA Extraction. C. elegans eggs, with a minimum of 50 eggs per group, were cultured using NGM medium supplemented with different concentrations of sucralose. The control group was cultured using standard NGM medium (in 60 mm plates). For each group, 2000 clean D1 C. elegans were carefully picked and transferred to separate 1.5 mL EP tubes. The C. elegans were then washed three times using H2O. Next, RNase-free H2O was added to the tubes, and the C. elegans were transferred to RNase-free centrifugation tubes. The tubes were centrifuged to remove the supernatant. Subsequently, the TRUEscript RT MasterMix kit was used to prepare for PCR. 50 μL of lysate was added to each centrifugation tube containing the C. elegans. 20 μL of the sample was then transferred to RNase-free PCR tubes. The PCR tubes were then incubated at 65°C for 10 minutes in a PCR machine. After incubation, the samples were inactivated at 85 °C for 1 minute and cooled on ice. The RNA concentration and quality were determined to ensure the suitability of the extracted RNA for further analysis.
cDNA reverse transcription. 2 mg of total RNA was taken and first-strand cDNA synthesis was performed using the TransScript First-Strand cDNA Synthesis kit.
RT-qPCR. The expression levels of age-1, add-1, flp-34, dop-1, tph-1, eat-4, glr-1, nmr-1, nmr-2, casy-1 and unc-43 in each group of samples were detected by using SYBR Green PCR Master Mix with gapdh as an internal reference (IR). The sequences of PCR primers used in the experiment are shown in Table 2. The relative expression of target genes was analyzed by the 2^ (-ΔΔCt) method.
Table 2. RT-qPCR primers sequences.
No. | Primer | Sequence (5’ to 3’ direction) |
IR | gapdh-F | GCTGACGGACCAATGAAG |
gapdh-R | TGACGAAGTGTGGGTTGA | |
1 | age-1-F | CGTTCGGAACTGGAAAGCTATCG |
age-1-R | GAGTACTGCAGATGGTGGCATATC | |
2 | add-1-F | GTTCATGACGTCAACGTTCCATCC |
add-1-R | GGATTCGGCGCATAGATTTGGTG | |
3 | flp-34-F | GCAGACATTTCCACATTTGCATCAG |
flp-34-R | GTACTGATCTTCCGATGATGGAATGATG | |
4 | dop-1-F | GCTATTTGCTGCAGTCAACGATATC |
dop-1-R | CTTCCAATTGCATACGGAAGCGG | |
5 | tph-1-F | CATGGCTCTATTCGCTGATCCAG |
tph-1-R | GACATTCTTGCTCAACAACACGATCC | |
6 | eat-4-F | GCAAGAAGAAGGAAACGAAAACCCG |
eat-4-R | GCCCTTGAGTAATTTGAATGAAAGCC | |
7 | glr-1-F | GGTGGAGATGATGTTAGTGTTGAGG |
glr-1-R | CACCTTGTCGCCACGCTAATAC | |
8 | nmr-1-F | GTTCAACGTTACATTGAGGTAGAGCTG |
nmr-1-R | GAAGGGAATTCCATTCAGCATCTACAC | |
9 | nmr-2-F | GTTCCCAAATCTACAGTATCCCGATTG |
nmr-2-R | GTCAAGCACCACAGCGTCATAG | |
10 | casy-1-F | CATTCTGGAAATGGACCTCCCG |
casy-1-R | CCGATGACGAGCAACACTAACAG | |
11 | unc-43-F | CAGGATATTGTACGGGTGACTCAGAC |
unc-43-R | GCCTTCGATAAGGTTACCAAGTGC |
2.2.7. Homology analysis. The NCBI Homologene tool was utilized to determine whether the genes associated with learning and memory in C. elegans, which are regulated by sucralose, have homologous counterparts in humans. Homology comparisons were conducted, and the results were summarized for documentation purposes.
2.2.8. Data processing. All experiments pertaining to learning and memory were conducted three times to ensure reliability, and the data obtained were expressed as Mean ± SEM (standard error of the mean). Statistical analyses were performed to assess the differences between two groups using Student’s t-test. For comparisons involving multiple groups, the data were analyzed using the One-way ANOVA test. The statistical analysis was carried out using GraphPad Prism 9.
3. Results
3.1. Market research results
A comprehensive field observation was conducted in six small, medium, and large supermarkets to record the presence of various mainstream sugar-substituted beverages. The study focused on beverages from three distinct beverage companies. The observations revealed that all six beverages examined in the study contained sucralose as a sweetener. The details of these findings are summarized in Table 3.
Table 3. Results of market research on sugar-substituted beverages.
Manufacturer | Name | Sugar substitutes type | Sugar substitute name | Ingredient list | Contained sucralose |
Chi Forest | White Peach Flavored Sparkling Water | Sugar alcohols, synthetic | Erythritol, Sucralose | Water, Erythritol, Carbon dioxide, Sodium bicarbonate, Citric acid, Sucralose, Potassium sorbate, Food flavor. | Yes |
Chi Forest | Alien Electrolyte Water | Sugar alcohols, synthetic | Erythritol, Sucralose | Water, Erythritol, Vitamin E, Vitamin B6, Salt, Calcium Lactate, Potassium Chloride, Zinc gluconazole, Citric Acid, Sodium Citrate, Sucralose, Food Flavor | Yes |
Coca-Cola | Coca-Cola Zero | Synthetic | Aspartame, Acesulfame, Sucralose | Carbonated Water, Caramel Color, Phosphoric Acid, Aspartame, Potassium Benzoate (To Protect Taste), Natural Flavors, Potassium Citrate, Acesulfame Potassium, Caffeine | Yes |
Master Kong | Iced Lemon Black Tea | Sugar alcohols, synthetic | Erythritol, Sucralose, Acesulfame | Water, Polydextrose, Instant Black Tea, Grape Juice Concentrate, Edible Salt, Black Tea Concentrate, Erythritol, Citric Acid, Sodium Citrate, DL-Malic Acid, Sucralose, Sodium D-Iscorbylate, Vitamin C, Caramel Color, Acesulfame, Edible Flavors | Yes |
Coca-Cola | Sprite Zero | Synthetic | Aspartame, Acesulfame, Sucralose | Carbonated Water, Citric Acid, Potassium Citrate, Natural Flavors, Potassium Benzoate (To Protect Taste), Aspartame, Acesulfame Potassium | Yes |
Coca-Cola | Fanta Zero | Synthetic | Aspartame, Acesulfame, Sucralose | Carbonated Water, Citric Acid, Potassium Citrate, Aspartame, Natural Flavors, Modified Food Starch, Potassium Benzoate, Acesulfame Potassium, Glycerol Ester Of Rosin, Yellow 6, Medium Chain Triglycerides, Sucrose Acetate Isobutyrate, Red 40 | Yes |
3.2. Effect of sucralose on tap stimulus in the C. elegans
The comparison of experimental data on the first evasive response time of C. elegans revealed the following results. There was no statistically significant difference in the first evasive response time between the blank control group and the groups treated with 0.3 mg/mL sucralose, 1 mg/mL sucralose, and 10 mg/mL glucose. However, the group treated with 10 mg/mL sucralose exhibited a significant prolongation of the evasive response time compared to the blank control group (**** P < 0.0001), and this difference was statistically significant (Fig. 2A). These findings indicate that a high concentration of sucralose significantly increased the evasive response time, reduced the sensitivity of the nematodes, and affected their non-associative learning ability.
Regarding the number of evasive response times of C. elegans (Fig. 2B), the experimental data showed no statistically significant difference between the blank control group and all the experimental groups. Therefore, sucralose did not have an impact on the number of evasive responses in C. elegans.
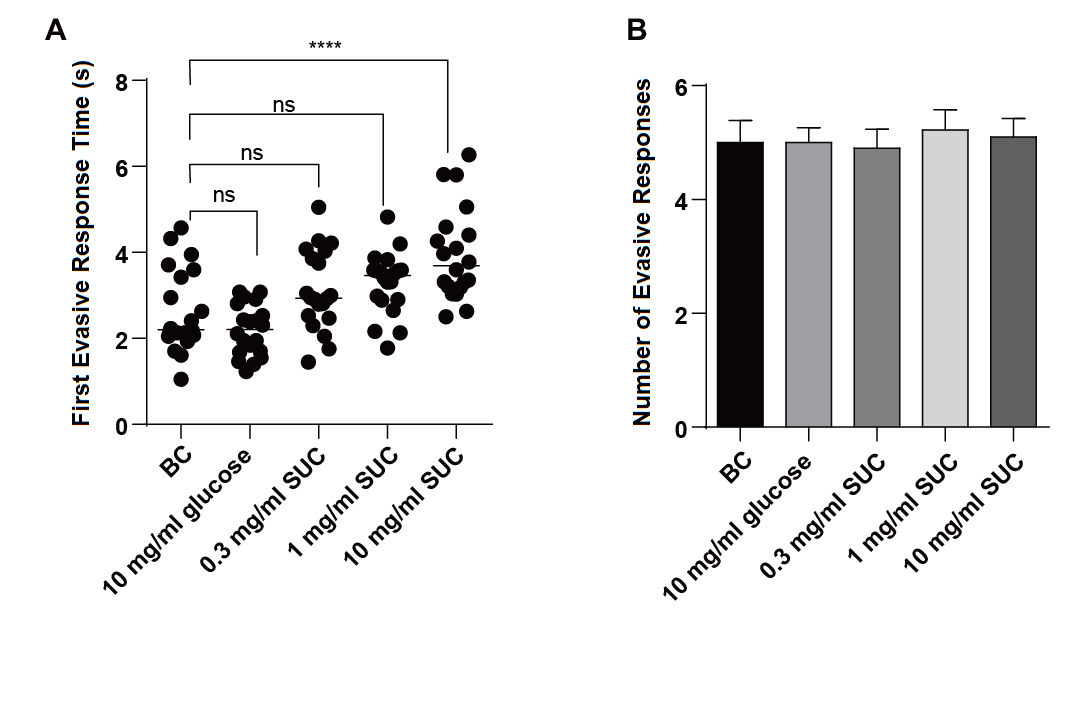
Figure 2. Effect of different concentrations of sucralose on tap stimulus of C. elegans.
3.3. Effect of sucralose on odor chemotaxis in the C. elegans
The comparison of experimental data on the percentage of benzaldehyde in C. elegans odor chemotaxis (Fig. 3A, ** P < 0.01, ns for no statistical difference) revealed the following findings. There was no statistically significant difference in odor chemotaxis between the blank control group and the groups treated with 1 mg/mL sucralose, 10 mg/mL sucralose, and 10 mg/mL glucose. However, the odor chemotaxis of C. elegans in the 0.3 mg/mL sucralose group was significantly lower compared to that of the blank control group, and this difference was statistically significant. These results indicate that the low concentration of sucralose had an impact on the odor chemotaxis of C. elegans towards the odor of benzaldehyde.
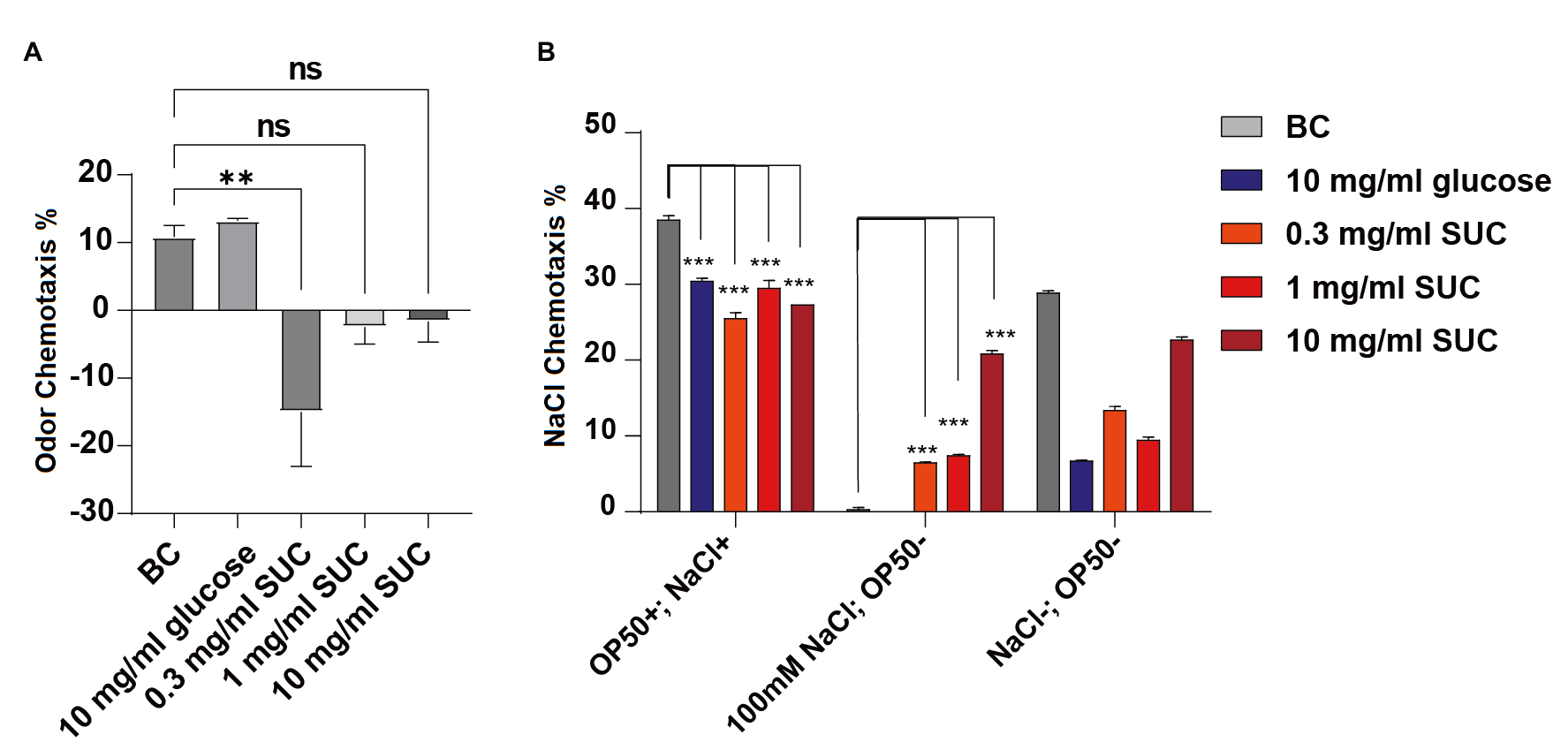
Figure 3. Effect of different concentrations of sucralose on odor chemotaxis and NaCl chemotaxis of C. elegans.
3.4. Effect of sucralose on NaCl chemotaxis in the C. elegans
To investigate the NaCl chemotaxis of C. elegans, they were transferred to NGM medium with a high concentration of NaCl and NaCl-free NGM medium, while the standard NGM medium served as the control. After a 4-hour starvation treatment, the C. elegans were transferred to chemotaxis plates, and their chemotaxis behavior was assessed.
On the standard NGM plates, the chemotaxis of C. elegans towards NaCl was significantly reduced in the treatment groups with 0.3 mg/mL sucralose, 1 mg/mL sucralose, 10 mg/mL sucralose, and the 10 mg/mL glucose control group, as compared to the blank control group (Fig. 3B, *** P < 0.001). These results suggest that sucralose treatment decreased the natural chemotaxis of C. elegans towards NaCl.
In the experimental group that combined starvation and a high concentration of 100 mM NaCl, the chemotaxis towards NaCl was significantly reduced in both the blank control group and the 10 mg/mL glucose group. Moreover, all three concentrations of sucralose showed statistical differences compared to the blank control group. This indicates that the addition of sucralose impaired the ability of C. elegans to effectively associate the high concentration of NaCl and starvation. These findings suggest that sucralose treatment negatively affected the associative learning ability of C. elegans.
\( Percentage of NaCl chemotaxis=\frac{number of C. elegans at NaCl-number of C. elegans at blank control}{total number of C. elegans×100\%} \)
3.5. Effect of sucralose on the related gene expression in the C. elegans
In order to explore the mechanism of changes in C. elegans learning and memory function induced by different concentrations of sucralose treatment, RT-PCR was used to quantify the relative expression of 11 genes known to be associated with C. elegans learning and memory (Fig. 4). These genes include the lifespan-associated PI3K gene age-1, the microtubule-binding protein related gene add-1, the neuropeptide related gene flp-34, the dopamine receptor related gene dop-1, the serotonin synthesis enzyme related gene tph-1, the calcium-binding protein related gene casy-1, and the low-glutamate receptor-related signaling molecules related gene eat-4, glr-1, nmr-1, nmr-2, and unc-43.
The results demonstrated that the relative expression of add-1, tph-1, nmr-2, and unc-43 was significantly up-regulated in the low concentration (0.3 mg/ml) sucralose group. Conversely, in the high concentration (10 mg/ml) sucralose group, the relative expression of all 11 genes was significantly down-regulated to less than 0.1-fold of the blank control group, and these differences were statistically significant. These findings indicate that the effect of sucralose treatment on the learning and memory ability of C. elegans is achieved through extensive regulation of learning and memory-related pathways.
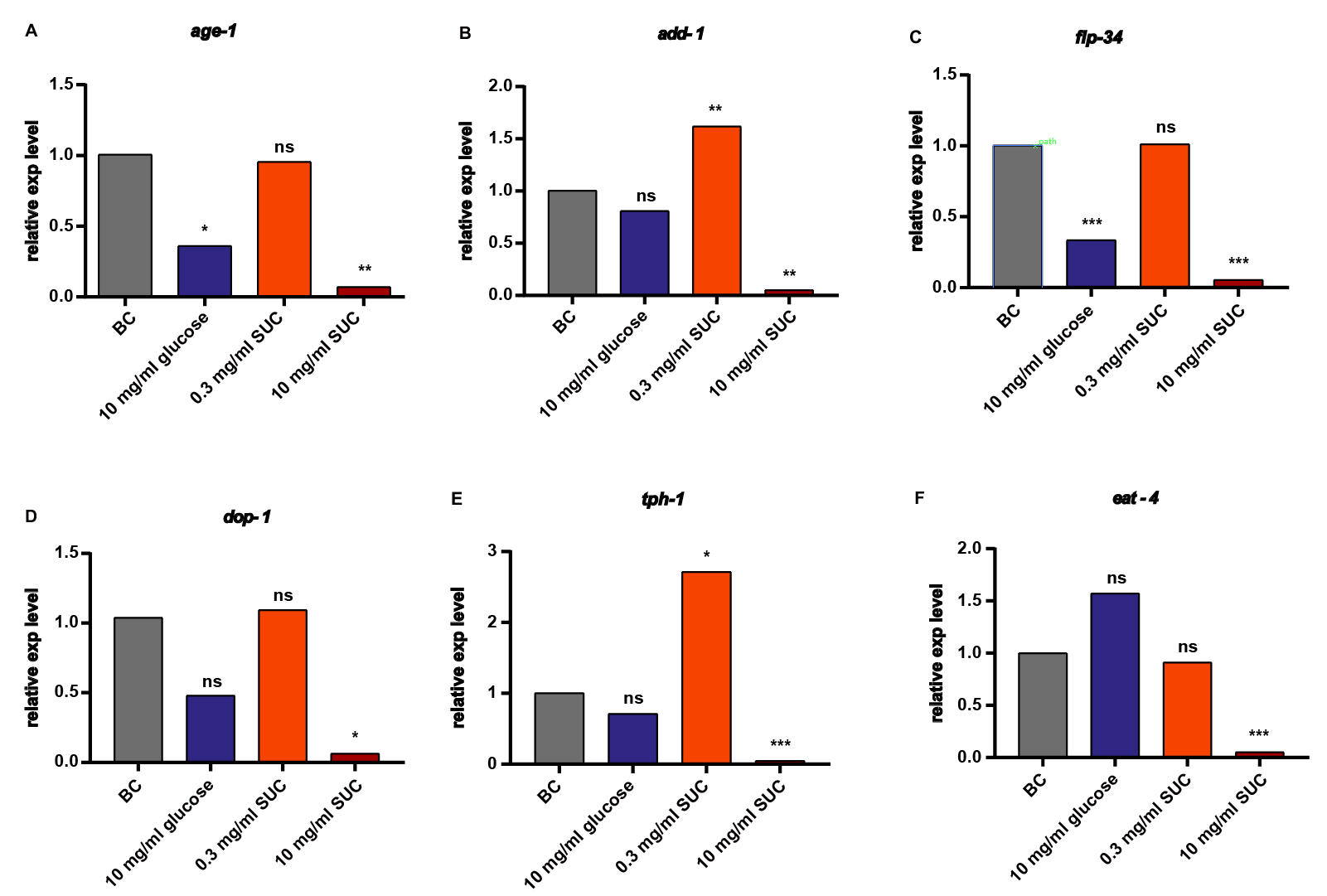
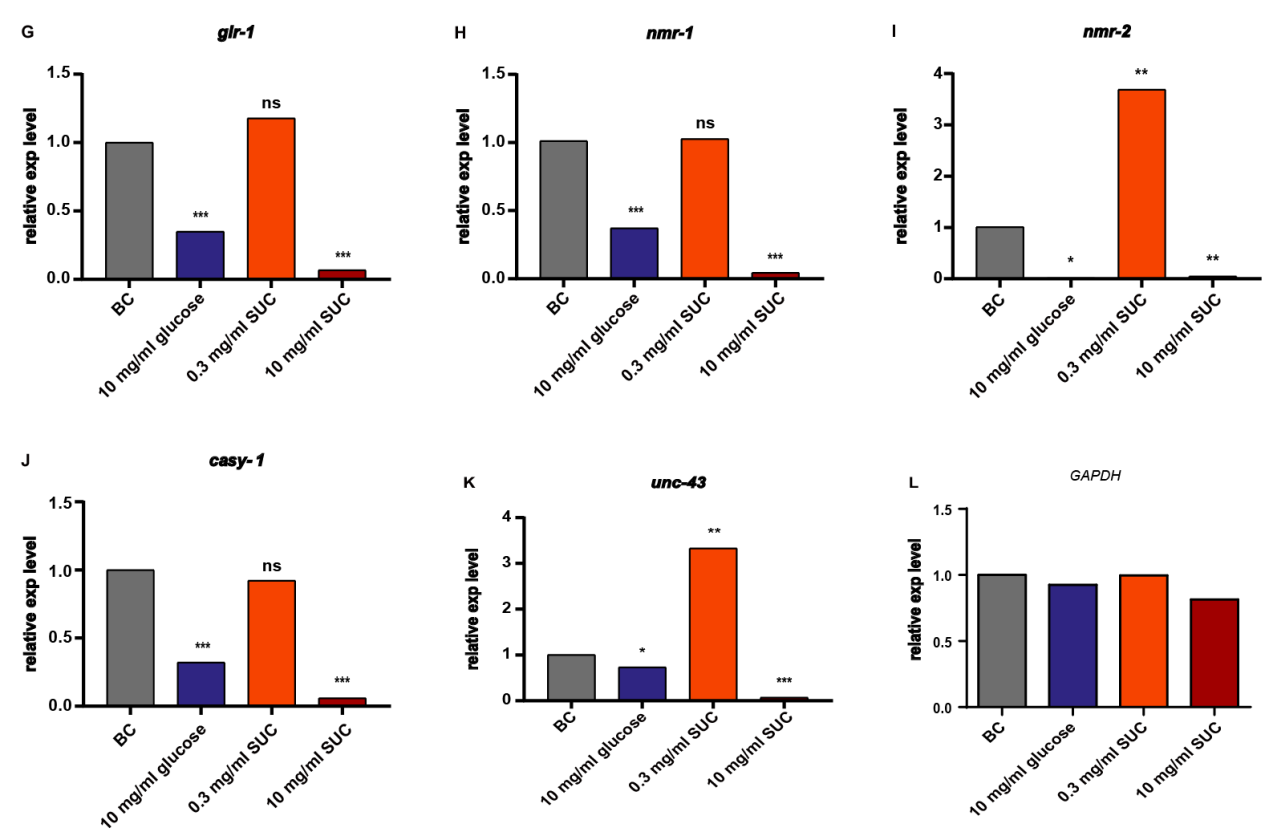
Figure 4. Analysis of the effect of different concentrations of sucralose on the relative expression of genes. age-1 (A), add-1 (B), flp-34 (C), dop-1 (D), tph-1 (E), eat-4 (F), glr-1 (G), nmr-1 (H), nmr-2 (I), casy-1 (J), unc-43 (K), all of the above genes were expressed by using GAPDH (L) as an internal reference, and the relative expression of all the above genes with blank control group (BC) as a baseline. * P < 0.05, ** P < 0.01, *** P < 0.001.
3.6. Homology analysis of sucralose-regulated genes associated with learning and memory in C. elegans and human genes
In this study, we used the NCBI-Homologene tool to find out whether the 11 learning memory-related genes regulated by sucralose had homologous genes in humans and the percentage of homology. We found that nmr-2, age-1, add-1, dop-1, tph-1, eat-4, glr-1, nmr-1, casy-1 and unc- 43 are all homologous in humans, with homology ranging from 30% to 60% (Table 4). This suggests that these sucralose-regulated genes in C. elegans may be similarly affected by sucralose in humans.
Table 4. Homology analysis of C. elegans and human genes.
Gene_C. elegans | Gene_H. sapiens | Homology | Similarities |
nmr-2 | GRIN2D | yes | 36% |
age-1 | PIK3CA | yes | 30% |
add-1 | ADD1 | yes | 37% |
flp-34 | no | ||
dop-1 | ADRA2B | yes | 42% |
tph-1 | TPH2 | yes | 54% |
eat-4 | SLC17A7 | yes | 54% |
glr-1 | GRIA3 | yes | 38% |
nmr-1 | GRIN1 | yes | 33% |
casy-1 | CLSTN1 | yes | 30% |
unc-43 | CAMK2D | yes | 60% |
4. Discussion
4.1. Effects of different concentration of sucralose on the learning memory function of C. elegans
To better align with the types of sugar substitutes commonly used in sugar-substituted foods available in the market, the study initially examined six popular sugar-substituted beverages from three companies. Through field observations conducted in the supermarkets in Beijing, it was identified that these beverages utilized sucralose as a sweetener to varying degrees. Based on this observation, the study chose sucralose in combination with the biological model of C. elegans to explore the effects of sugar substitutes on learning and memory functions and their mechanisms of action.
In this study, we initially tested the effect of sucralose on the evasive response of C. elegans using a tap stimulus assay. The results showed that a high concentration of sucralose significantly prolonged the time of evasive response. This indicates that high concentrations of sucralose treatment can reduce the sensitivity of C. elegans to tap stimulus, ultimately leading to a significant reduction in the mechanosensory-related non-associative learning ability of C. elegans. The results of the chemotaxis assays, including the benzaldehyde odor chemotaxis experiment and NaCl chemotaxis ability, revealed that low concentrations of sucralose inhibited odor chemotaxis in C. elegans. Furthermore, all three concentrations of sucralose treatments inhibited NaCl chemotaxis, suggesting that sucralose treatments significantly reduced the C. elegans’ innate chemotaxis response to the inducer. Additionally, the NaCl chemotaxis experiments and starvation tests demonstrated that different concentrations of sucralose treatments impaired the associative learning ability of C. elegans. This finding indicates that the addition of sucralose reduced the associative learning ability of C. elegans.
Combined neuropsychological testing and EEG/qEEG analysis in humans revealed that long-term supplementation with sucralose significantly reduced overall memory, encoding memory, and executive function. This suggests that prolonged consumption of sucralose has an adverse effect on learning and memory functions [29]. Oytun et al. conducted a study on rats, treating them with various types of artificial sweeteners including sucralose over an extended period. Their findings indicated that long-term consumption of artificial sweeteners impaired cognitive ability and hippocampal integrity [30]. In previous studies, the concentration of sugar substitutes has also been explored to some extent. Jiao et al. investigated the effects of different concentrations of sucralose on various aspects of C. elegans, such as lifespan, egg-laying, swallowing, and locomotion. They found that sucralose extended the lifespan of C. elegans in a dose-dependent manner, did not affect egg-laying or swallowing frequency, and had a positive effect on locomotion at low concentrations. However, high concentrations of sucralose had adverse effects on the locomotion of C. elegans [31]. Another study examined the effect of sucralose on C. elegans lifespan and locomotor activity. The findings showed that low concentrations of sucralose (0.03-0.3 mg/mL) significantly extended nematode lifespan and improved locomotor activity. However, high concentrations of sucralose (10 mg/mL) had a tendency to shorten nematode lifespan, although the effect was not statistically significant [32]. These studies suggest a dose-dependent effect of sucralose on various aspects of C. elegans’ life activities. In this study, we observed differences in the effects of different concentrations of sucralose on learning and memory. For instance, the effects of high and low concentrations of sucralose on non-associative learning behaviors differed.
4.2. The inhibition of learning and memory functions in C. elegans by sucralose may be mediated through mechanisms such as glutamate receptor-related signaling pathways
In terms of mechanisms, the current research has not definitively established the specific mechanisms underlying the impairment of learning and memory functions caused by sugar substitutes. However, some studies have suggested that the damage to dopamine neurons may play a role in this impairment [33-35]. Furthermore, it has been reported that the effect of a high carbohydrate diet on the learning ability of C. elegans is regulated by different gene networks [36]
To investigate the genetic mechanism underlying the regulation of C. elegans learning memory by sucralose, this study employed RT-qPCR technology to analyze 11 genes known to be associated with C. elegans learning memory. The goal was to explore how different concentrations of sucralose treatment affect the learning memory function of C. elegans and elucidate the underlying mechanisms involved. The genes included age-1 (PI3K gene associated with lifespan), add-1 (microtubule-binding protein), flp-34 (neuropeptide), dop-1 (dopamine receptor), tph-1 (serotonin synthesis enzyme), casy-1 (calcium-synthesizing protein), as well as glutamate receptor-related signaling molecules eat-4, glr-1, nmr-1, nmr-2, and unc-43. The results revealed significant up-regulation of add-1, tph-1, nmr-2, and unc-43 gene expressions in the low concentration (0.3 mg/mL) sucralose treatment group. Notably, nmr-2 showed a 3.69-fold up-regulation compared to the blank control group. In contrast, the high concentration (10 mg/mL) sucralose treatment group exhibited significant down-regulation of all 11 genes to less than 0.1-fold compared to the blank control group. These differences were statistically significant. These findings suggest that sucralose treatment affects the learning memory ability of C. elegans through extensive regulation of pathways associated with learning and memory.
Among the 11 genes analyzed, the changes observed in glutamatergic neuron-related genes were more prominent compared to the dopamine neuron-related gene dop-1. Specifically, the expression levels of genes such as nmr-1/2, which encode NMDA-type glutamate receptor subunits, were significantly decreased in the high concentration sucralose group. This suggests that high concentrations of sucralose may lead to impaired non-associated as well as associated learning and memory functions in C. elegans by damaging glutamatergic neurons. Interestingly, low concentrations of sucralose were found to promote the expression of certain relevant genes. This finding may seem contradictory to the impaired odor chemotaxis observed in C. elegans. However, it is possible that the increased expression of add-1 promotes the expression of glr-1, resulting in an excessive increase in the content of the AMPA-type glutamate receptor [37]. This, in turn, raises the threshold of glutamate signaling levels required to activate the receptor, ultimately impairing the learning memory function of C. elegans.
There are existing findings regarding the regulation of learning memory by the remaining genes in question (wormbase.org). For instance, the lifespan-related gene age-1 has been shown to influence intracellular signaling and metabolic regulation by modulating the activity of the insulin/PI3K signaling pathway, which in turn affects learning behavior. Mutations in tph-1, a gene responsible for serotonin synthesis, have been found to impact the synthesis of 5-HT (serotonin) in C. elegans, thereby affecting their ability to learn odor-food associations. The gene unc-43, which encodes a Ca2+/Calmodulin-dependent protein kinase, has been implicated in PA14 aversive learning. In the present study, the regulation of these pathways by sucralose was investigated, and it was observed that gene expression was significantly down-regulated, especially when high concentrations of sucralose were applied.
4.3. Effect of sucralose on human learning memory function and its mechanism
Human studies have demonstrated that long-term consumption of sucralose can lead to significant decreases in overall memory, encoded memory, and executive functioning [29]. Long-term or excessive consumption of sugar substitutes may contribute to metabolic disorders, increased nighttime sweet intake, and a decline in learning and memory capacity. [38, 39]. Animal experiments with immature rats have demonstrated that the long-term habitual intake of low-calorie sweeteners can have lasting effects on glucose regulation, sugar-driven behaviors, and memory processes that rely on the hippocampus. These effects may be attributed, at least in part, to changes in the expression of nutrient transporters, sweet taste receptors, and central gene pathways [40]
In this study, it was observed that sucralose can impact the learning and memory capacity of C. elegans by broadly regulating a network of genes associated with learning and memory. Several of the learning and memory-related genes affected by sucralose, such as nmr-2, age-1, add-1, dop-1, tph-1, eat-4, glr-1, nmr-1, casy-1, and unc-43, have homologous counterparts in humans. This finding lays the groundwork for applying the research findings to explore the effects and mechanisms of sugar substitute consumption in humans. For example, the mouse homolog of nmr-2, Grin2d, has been identified as a subunit of the NMDA receptor in hippocampal interneurons, playing a role in excitatory synaptic transmission [41]. The human homolog of the dop-1 gene in C. elegans, known as ADRA2B, has been associated with certain cognitive effects when mutated in humans. Male carriers of this gene mutation exhibit impaired recognition memory under stress conditions, while female carriers show enhanced long-term memory, increased emotional memory, greater amygdala response to emotional stimuli, and increased intrusion of trauma memories, making them more susceptible to post-traumatic stress disorder [42, 43]. Therefore, the high concentration of sucralose proposed in this study, based on the effects observed in C. elegans, may potentially impair their non-associative and associative learning and memory abilities by causing damage to glutamatergic neurons. This finding may also provide insights into the safety and mechanisms of sugar substitute consumption in humans, given the high genetic homology between C. elegans and humans.
4.4. Potential neurodevelopmental harms of sugar-substituted beverages in the adolescent population
Sugar-substituted beverages have gained popularity as an alternative to sugar-sweetened beverages, particularly among individuals who are health-conscious or seeking to reduce their sugar intake. According to a report from the Centers for Disease Control and Prevention (CDC), approximately one-fifth of the U.S. population consumed sugar-substituted beverages on a daily basis during the period of 2009-2010. The percentage of women consuming these beverages increased from 17.8% to 21.2%, while for men, it increased from 13.9% to 19.0% between 1999-2000 and 2009-2010. The growing influence of sugar-substituted beverage advertising has contributed to the increasing market share of these beverages [10]. However, research indicates that only women, the elderly, and individuals with disabilities possess some knowledge about sugar substitutes, while young people have limited awareness [44]. Despite the numerous benefits of sugar substitutes compared to caloric sweeteners, such as their potential contributions to weight control, blood glucose management, reduced dental problems, and expanded beverage choices, this study uncovers a concerning finding. It highlights that the consumption of sugar substitutes can have adverse effects on learning and memory functions, particularly when used in excessive amounts. These findings raise concerns about the potential hazards of sugar substitutes for adolescent neurodevelopment.
5. Conclusion
In this study, the developmental stage of C. elegans was chosen as the experimental model to investigate the impact of three different concentrations of sucralose on non-associative and associative learning abilities. In the non-associative learning experiment, C. elegans treated with high concentrations of sucralose exhibited a significant prolongation in withdrawal reaction time, while those treated with low concentrations of sucralose showed a significant reduction in odor chemotaxis. In the associative learning experiment, different concentrations of sucralose adversely affected the associative learning ability of C. elegans. To understand the underlying regulatory mechanisms, gene expression changes of 11 C. elegans genes associated with learning and memory were analyzed using RT-qPCR after sucralose treatment. The results showed that low concentrations of sucralose significantly up-regulated the relative expression of four gene groups, namely add-1, tph-1, nmr-2, and unc-43, while high concentrations of sucralose led to a significant down-regulation of the relative expression of all the genes, particularly the glutamate receptor signaling pathway represented by nmr-2.
Overall, the study suggests that high concentrations of sucralose can diminish the learning and memory abilities of C. elegans by extensively modulating pathways related to learning and memory. Since homology analysis revealed that 10 out of the 11 genes have counterparts in humans, it is plausible that human consumption of sucralose may also impact the expression levels of these learning and memory-related genes. Consequently, the study highlights the potential harm of sucralose intake in the adolescent population and provides a theoretical foundation for further investigating the effects of sucralose on learning and memory in both mammalian experimental animals and humans.
Acknowledgments
I am grateful for the support and guidance I received throughout this scientific research endeavor. I owe my success to the mentors, relatives, and colleagues who have helped me along the way. Firstly, I express my heartfelt thanks to my supervisor, Mr. Wang Junwei, for his invaluable guidance in thesis writing and insightful suggestions for revision. I am also thankful to Prof. Zhang Xiucheng’s team at China Agricultural University for their enthusiastic collaboration in the lab, specifically in the cultivation, synchronization, and learning memory experiments of C. elegans. I extend my gratitude to He Qin for his encouragement and positive cooperation. Lastly, I want to acknowledge my parents for their selfless support and encouragement, allowing me to focus on my research and handle challenges with ease. To all those who have contributed to my journey, I sincerely appreciate your assistance and generosity.
References
[1]. E. M. Navarrete-Munoz et al., “Sweet-beverage consumption and risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC),” American Journal of Clinical Nutrition, vol. 104, no. 3, pp. 760-768, Sep 2016, doi: 10.3945/ajcn.116.130963.
[2]. J. J. Anderson et al., “The associations of sugar-sweetened, artificially sweetened and naturally sweet juices with all-cause mortality in 198,285 UK Biobank participants: a prospective cohort study,” Bmc Medicine, vol. 18, no. 1, Apr 2020, Art no. 97, doi: 10.1186/s12916-020-01554-5.
[3]. D. Martyn et al., “Low-/No-Calorie Sweeteners: A Review of Global Intakes,” Nutrients, vol. 10, no. 3, Mar 2018, Art no. 357, doi: 10.3390/nu10030357.
[4]. K. Noda, K. Nakayama, and T. Oku, “Serum Glucose and Insulin Levels and Erythritol Balance after Oral-Administration of Erythritol in Healthy-Subjects,” European Journal of Clinical Nutrition, vol. 48, no. 4, pp. 286-292, Apr 1994. [Online]. Available: <Go to ISI>://WOS:A1994NG43700008.
[5]. M. Libik-Konieczny et al., “Steviol glycosides profile inStevia rebaudianaBertoni hairy roots cultured under oxidative stress-inducing conditions,” Applied Microbiology and Biotechnology, vol. 104, no. 13, pp. 5929-5941, Jul 2020, doi: 10.1007/s00253-020-10661-5.
[6]. C. Gardana, P. Simonetti, E. Canzi, R. Zanchi, and P. Pietta, “Metabolism of stevioside and rebaudioside A from Stevia rebaudiana extracts by human microflora,” Journal of Agricultural and Food Chemistry, vol. 51, no. 22, pp. 6618-6622, Oct 2003, doi: 10.1021/jf0303619.
[7]. K. Rycerz and J. E. Jaworska-Adamu, “Effects of aspartame metabolites on astrocytes and neurons,” Folia Neuropathologica, vol. 51, no. 1, pp. 10-17, 2013, doi: 10.5114/fn.2013.34191.
[8]. E. Gardner, “ALTERNATIVE SUGARS Sucralose,” British Dental Journal, vol. 224, no. 1, pp. 5-5, Jan 2018, doi: 10.1038/sj.bdj.2018.15.
[9]. S. G. Wood, B. A. John, and D. R. Hawkins, “The pharmacokinetics and metabolism of sucralose in the dog,” Food and Chemical Toxicology, vol. 38, pp. S99-S106, 2000. [Online]. Available: <Go to ISI>://WOS:000088118800010.
[10]. G. V. Research. “Diet Soft Drinks Market Size, Share & Trends Analysis Report By Distribution Channel (Supermarkets & General Merchandise, Online), By Region, And Segment Forecasts.” https://www.grandviewresearch.com/industry-analysis/diet-soft-drinks-market (accessed September 10, 2023).
[11]. China Baogao. “China Sugar Free Drinks Industry Development Status Research and Investment Trend Forecast Report(2022-2029.” https://www.chinabaogao.com/baogao/202210/ 614514.html (accessed September 10, 2023).
[12]. M. Witkowski et al., “The artificial sweetener erythritol and cardiovascular event risk,” Nature Medicine, 2023 Feb 2023, doi: 10.1038/s41591-023-02223-9.
[13]. E. Harris, “Experts Disagree About Aspartame’s “Possibly Carcinogenic” Status,” Jama-Journal of the American Medical Association, 2023 Jul 2023, doi: 10.1001/jama.2023.13132.
[14]. Lazarov and C. Hollands, “Hippocampal neurogenesis: Learning to remember,” Progress in Neurobiology, vol. 138, pp. 1-18, Mar-May 2016, doi: 10.1016/j.pneurobio.2015.12.006.
[15]. Erbas et al., “Evaluation of long-term effects of artificial sweeteners on rat brain: a biochemical, behavioral, and histological study,” Journal of Biochemical and Molecular Toxicology, vol. 32, no. 6, Jun 2018, Art no. e22053, doi: 10.1002/jbt.22053.
[16]. M. A. Lebda, K. M. Sadek, and Y. S. El-Sayed, “Aspartame and Soft Drink-Mediated Neurotoxicity in Rats: Implication of Oxidative Stress, Apoptotic Signaling Pathways, Electrolytes and Hormonal Levels,” Metabolic Brain Disease, vol. 32, no. 5, pp. 1639-1647, Oct 2017, doi: 10.1007/s11011-017-0052-y.
[17]. X. Li, G. P. Dong, G. X. Han, L. P. Du, and M. Y. Li, “Zebrafish Behavioral Phenomics Links Artificial Sweetener Aspartame to Behavioral Toxicity and Neurotransmitter Homeostasis,” Journal of Agricultural and Food Chemistry, vol. 69, no. 50, pp. 15393-15402, Dec 2021, doi: 10.1021/acs.jafc.1c06077.
[18]. J. P. Finn and G. H. Lord, “Neurotoxicity studies on sucralose and its hydrolysis products with special reference to histopathologic and ultrastructural changes,” Food and Chemical Toxicology, vol. 38, pp. S7-S17, 2000. [Online]. Available: <Go to ISI>://WOS:000088118800003.
[19]. F. Yang et al., “Food nutritional evaluation: Caenorhabditis elegans as a model organism,” Shipin Kexue / Food Science, vol. 40, no. 11, pp. 268-276, 2019. [Online]. Available: <Go to ISI>://CABI:20193357634.
[20]. N. Zhao, C. Ren, H. Liu, and C. Zhang, “Current progress on the methods for studying learning behavior of Caenorhabditis elegans,” Journal of Northwest A & F University - Natural Science Edition, vol. 37, no. 11, pp. 55-61, 2009. [Online]. Available: <Go to ISI>://CABI:20093354966.
[21]. C. H. Rankin, C. D. O. Beck, and C. M. Chiba, “CAENORHABDITIS-ELEGANS - A NEW MODEL SYSTEM FOR THE STUDY OF LEARNING AND MEMORY,” Behavioural Brain Research, vol. 37, no. 1, pp. 89-92, Feb 1990, doi: 10.1016/0166-4328(90)90074-o.
[22]. Y. Wang et al., “Aesculin offers increased resistance against oxidative stress and protective effects against A beta-induced neurotoxicity in Caenorhabditis elegans,” European Journal of Pharmacology, vol. 917, Feb 2022, Art no. 174755, doi: 10.1016/j.ejphar.2022.174755.
[23]. C. I. Bargmann, E. Hartwieg, and H. R. Horvitz, “ODORANT-SELECTIVE GENES AND NEURONS MEDIATE OLFACTION IN C-ELEGANS,” Cell, vol. 74, no. 3, pp. 515-527, Aug 1993, doi: 10.1016/0092-8674(93)80053-h.
[24]. R. Chandra et al., “Sleep is required to consolidate odor memory and remodel olfactory synapses,” Cell, vol. 186, no. 13, pp. 2911-+, Jun 2023, doi: 10.1016/j.cell.2023.05.006.
[25]. V. Raj and A. Thekkuveettil, “Dopamine plays a critical role in the olfactory adaptive learning pathway in Caenorhabditis elegans,” Journal of Neuroscience Research, vol. 100, no. 11, pp. 2028-2043, Nov 2022, doi: 10.1002/jnr.25112.
[26]. C. H. Rankin, C. D. Beck, and C. M. Chiba, “Caenorhabditis elegans: a new model system for the study of learning and memory,” Behav Brain Res, vol. 37, no. 1, pp. 89-92, Feb 12 1990, doi: 10.1016/0166-4328(90)90074-o.
[27]. Y. Wang et al., “Aesculin offers increased resistance against oxidative stress and protective effects against Abeta-induced neurotoxicity in Caenorhabditis elegans,” Eur J Pharmacol, vol. 917, p. 174755, Feb 15 2022, doi: 10.1016/j.ejphar.2022.174755.
[28]. C. I. Bargmann, “Chemosensation in C. elegans,” WormBook : the online review of C. elegans biology, pp. 1-29, Oct 25 2006, doi: 10.1895/wormbook.1.123.1.
[29]. M. S. Lopez-Meza, G. Otero-Ojeda, J. A. Estrada, F. J. Esquivel-Hernandez, and I. Contreras, “The impact of nutritive and non-nutritive sweeteners on the central nervous system: preliminary study,” Nutritional neuroscience, vol. 25, no. 8, pp. 1623-1632, Aug 2022, doi: 10.1080/1028415X.2021.1885239.
[30]. Erbas et al., “Evaluation of long-term effects of artificial sweeteners on rat brain: a biochemical, behavioral, and histological study,” Journal of biochemical and molecular toxicology, vol. 32, no. 6, p. e22053, Jun 2018, doi: 10.1002/jbt.22053.
[31]. Jiao Yan, “Using nematodes as a model to study the consumption safety of three commonly used sugar substitutes,” M.S., Jilin University, 2018.
[32]. M. Zhang et al., “Aspartame and sucralose extend the lifespan and improve the health status of C. elegans,” Food & function, vol. 12, no. 20, pp. 9912-9921, Oct 19 2021, doi: 10.1039/d1fo01579f.
[33]. N. F. Salaya-Velazquez, L. A. Lopez-Mucino, S. Mejia-Chavez, P. Sanchez-Aparicio, A. A. Dominguez-Guadarrama, and A. Venebra-Munoz, “Anandamide and sucralose change DeltaFosB expression in the reward system,” Neuroreport, vol. 31, no. 3, pp. 240-244, Feb 5 2020, doi: 10.1097/WNR.0000000000001400.
[34]. C. Salim, N. Thadathil, M. Muralidhara, and P. S. Rajini, “Insights on the age dependent neurodegeneration induced by Monocrotophos, (an organophosphorous insecticide) in Caenorhabditis elegans fed high glucose: Evidence in wild and transgenic strains,” Comparative biochemistry and physiology. Toxicology & pharmacology : CBP, vol. 211, pp. 15-24, Sep 2018, doi: 10.1016/j.cbpc.2018.05.002.
[35]. Lingli Zhang, “A study on the relationship and mechanism between dietary intake pattern and individual behavior,” PhD, Shanghai Jiao Tong University, 2019.
[36]. N. Guo, J. Wang, and X. Wang, “Effect of starvation and high-carbohydrate diet on learning ability of Caenorhabditis elegans,” Heliyon, vol. 5, no. 3, p. e01289, Mar 2019, doi: 10.1016/j.heliyon.2019.e01289.
[37]. V. Vukojevic et al., “A role for alpha-adducin (ADD-1) in nematode and human memory,” The EMBO journal, vol. 31, no. 6, pp. 1453-66, Mar 21 2012, doi: 10.1038/emboj.2012.14.
[38]. S. E. Swithers, “Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements,” Trends in endocrinology and metabolism: TEM, vol. 24, no. 9, pp. 431-41, Sep 2013, doi: 10.1016/j.tem.2013.05.005.
[39]. S. L. Casperson, L. Johnson, and J. N. Roemmich, “The relative reinforcing value of sweet versus savory snack foods after consumption of sugar- or non-nutritive sweetened beverages,” Appetite, vol. 112, pp. 143-149, May 1 2017, doi: 10.1016/j.appet.2017.01.028.
[40]. L. Tsan et al., “Early-life low-calorie sweetener consumption disrupts glucose regulation, sugar-motivated behavior, and memory function in rats,” JCI insight, vol. 7, no. 20, Oct 24 2022, doi: 10.1172/jci.insight.157714.
[41]. R. E. Perszyk et al., “GluN2D-Containing N-methyl-d-Aspartate Receptors Mediate Synaptic Transmission in Hippocampal Interneurons and Regulate Interneuron Activity,” Molecular pharmacology, vol. 90, no. 6, pp. 689-702, Dec 2016, doi: 10.1124/mol.116.105130.
[42]. P. R. Zoladz et al., “ADRA2B deletion variant influences time-dependent effects of pre-learning stress on long-term memory,” Neurobiology of learning and memory, vol. 140, pp. 71-81, Apr 2017, doi: 10.1016/j.nlm.2017.02.014.
[43]. P. R. Zoladz et al., “ADRA2B deletion variant selectively predicts stress-induced enhancement of long-term memory in females,” Psychoneuroendocrinology, vol. 48, pp. 111-22, Oct 2014, doi: 10.1016/j.psyneuen.2014.06.012.
[44]. C. Miller, J. Dono, M. Scully, B. Morley, and K. Ettridge, “Adolescents’ knowledge and beliefs regarding health risks of soda and diet soda consumption,” Public health nutrition, vol. 25, no. 11, pp. 3044-3053, Nov 2022, doi: 10.1017/S1368980022001719.
Cite this article
Yang,H. (2023). Effects of sucralose on learning and memory in Caenorhabditis Elegans. Theoretical and Natural Science,16,25-41.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. E. M. Navarrete-Munoz et al., “Sweet-beverage consumption and risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC),” American Journal of Clinical Nutrition, vol. 104, no. 3, pp. 760-768, Sep 2016, doi: 10.3945/ajcn.116.130963.
[2]. J. J. Anderson et al., “The associations of sugar-sweetened, artificially sweetened and naturally sweet juices with all-cause mortality in 198,285 UK Biobank participants: a prospective cohort study,” Bmc Medicine, vol. 18, no. 1, Apr 2020, Art no. 97, doi: 10.1186/s12916-020-01554-5.
[3]. D. Martyn et al., “Low-/No-Calorie Sweeteners: A Review of Global Intakes,” Nutrients, vol. 10, no. 3, Mar 2018, Art no. 357, doi: 10.3390/nu10030357.
[4]. K. Noda, K. Nakayama, and T. Oku, “Serum Glucose and Insulin Levels and Erythritol Balance after Oral-Administration of Erythritol in Healthy-Subjects,” European Journal of Clinical Nutrition, vol. 48, no. 4, pp. 286-292, Apr 1994. [Online]. Available: <Go to ISI>://WOS:A1994NG43700008.
[5]. M. Libik-Konieczny et al., “Steviol glycosides profile inStevia rebaudianaBertoni hairy roots cultured under oxidative stress-inducing conditions,” Applied Microbiology and Biotechnology, vol. 104, no. 13, pp. 5929-5941, Jul 2020, doi: 10.1007/s00253-020-10661-5.
[6]. C. Gardana, P. Simonetti, E. Canzi, R. Zanchi, and P. Pietta, “Metabolism of stevioside and rebaudioside A from Stevia rebaudiana extracts by human microflora,” Journal of Agricultural and Food Chemistry, vol. 51, no. 22, pp. 6618-6622, Oct 2003, doi: 10.1021/jf0303619.
[7]. K. Rycerz and J. E. Jaworska-Adamu, “Effects of aspartame metabolites on astrocytes and neurons,” Folia Neuropathologica, vol. 51, no. 1, pp. 10-17, 2013, doi: 10.5114/fn.2013.34191.
[8]. E. Gardner, “ALTERNATIVE SUGARS Sucralose,” British Dental Journal, vol. 224, no. 1, pp. 5-5, Jan 2018, doi: 10.1038/sj.bdj.2018.15.
[9]. S. G. Wood, B. A. John, and D. R. Hawkins, “The pharmacokinetics and metabolism of sucralose in the dog,” Food and Chemical Toxicology, vol. 38, pp. S99-S106, 2000. [Online]. Available: <Go to ISI>://WOS:000088118800010.
[10]. G. V. Research. “Diet Soft Drinks Market Size, Share & Trends Analysis Report By Distribution Channel (Supermarkets & General Merchandise, Online), By Region, And Segment Forecasts.” https://www.grandviewresearch.com/industry-analysis/diet-soft-drinks-market (accessed September 10, 2023).
[11]. China Baogao. “China Sugar Free Drinks Industry Development Status Research and Investment Trend Forecast Report(2022-2029.” https://www.chinabaogao.com/baogao/202210/ 614514.html (accessed September 10, 2023).
[12]. M. Witkowski et al., “The artificial sweetener erythritol and cardiovascular event risk,” Nature Medicine, 2023 Feb 2023, doi: 10.1038/s41591-023-02223-9.
[13]. E. Harris, “Experts Disagree About Aspartame’s “Possibly Carcinogenic” Status,” Jama-Journal of the American Medical Association, 2023 Jul 2023, doi: 10.1001/jama.2023.13132.
[14]. Lazarov and C. Hollands, “Hippocampal neurogenesis: Learning to remember,” Progress in Neurobiology, vol. 138, pp. 1-18, Mar-May 2016, doi: 10.1016/j.pneurobio.2015.12.006.
[15]. Erbas et al., “Evaluation of long-term effects of artificial sweeteners on rat brain: a biochemical, behavioral, and histological study,” Journal of Biochemical and Molecular Toxicology, vol. 32, no. 6, Jun 2018, Art no. e22053, doi: 10.1002/jbt.22053.
[16]. M. A. Lebda, K. M. Sadek, and Y. S. El-Sayed, “Aspartame and Soft Drink-Mediated Neurotoxicity in Rats: Implication of Oxidative Stress, Apoptotic Signaling Pathways, Electrolytes and Hormonal Levels,” Metabolic Brain Disease, vol. 32, no. 5, pp. 1639-1647, Oct 2017, doi: 10.1007/s11011-017-0052-y.
[17]. X. Li, G. P. Dong, G. X. Han, L. P. Du, and M. Y. Li, “Zebrafish Behavioral Phenomics Links Artificial Sweetener Aspartame to Behavioral Toxicity and Neurotransmitter Homeostasis,” Journal of Agricultural and Food Chemistry, vol. 69, no. 50, pp. 15393-15402, Dec 2021, doi: 10.1021/acs.jafc.1c06077.
[18]. J. P. Finn and G. H. Lord, “Neurotoxicity studies on sucralose and its hydrolysis products with special reference to histopathologic and ultrastructural changes,” Food and Chemical Toxicology, vol. 38, pp. S7-S17, 2000. [Online]. Available: <Go to ISI>://WOS:000088118800003.
[19]. F. Yang et al., “Food nutritional evaluation: Caenorhabditis elegans as a model organism,” Shipin Kexue / Food Science, vol. 40, no. 11, pp. 268-276, 2019. [Online]. Available: <Go to ISI>://CABI:20193357634.
[20]. N. Zhao, C. Ren, H. Liu, and C. Zhang, “Current progress on the methods for studying learning behavior of Caenorhabditis elegans,” Journal of Northwest A & F University - Natural Science Edition, vol. 37, no. 11, pp. 55-61, 2009. [Online]. Available: <Go to ISI>://CABI:20093354966.
[21]. C. H. Rankin, C. D. O. Beck, and C. M. Chiba, “CAENORHABDITIS-ELEGANS - A NEW MODEL SYSTEM FOR THE STUDY OF LEARNING AND MEMORY,” Behavioural Brain Research, vol. 37, no. 1, pp. 89-92, Feb 1990, doi: 10.1016/0166-4328(90)90074-o.
[22]. Y. Wang et al., “Aesculin offers increased resistance against oxidative stress and protective effects against A beta-induced neurotoxicity in Caenorhabditis elegans,” European Journal of Pharmacology, vol. 917, Feb 2022, Art no. 174755, doi: 10.1016/j.ejphar.2022.174755.
[23]. C. I. Bargmann, E. Hartwieg, and H. R. Horvitz, “ODORANT-SELECTIVE GENES AND NEURONS MEDIATE OLFACTION IN C-ELEGANS,” Cell, vol. 74, no. 3, pp. 515-527, Aug 1993, doi: 10.1016/0092-8674(93)80053-h.
[24]. R. Chandra et al., “Sleep is required to consolidate odor memory and remodel olfactory synapses,” Cell, vol. 186, no. 13, pp. 2911-+, Jun 2023, doi: 10.1016/j.cell.2023.05.006.
[25]. V. Raj and A. Thekkuveettil, “Dopamine plays a critical role in the olfactory adaptive learning pathway in Caenorhabditis elegans,” Journal of Neuroscience Research, vol. 100, no. 11, pp. 2028-2043, Nov 2022, doi: 10.1002/jnr.25112.
[26]. C. H. Rankin, C. D. Beck, and C. M. Chiba, “Caenorhabditis elegans: a new model system for the study of learning and memory,” Behav Brain Res, vol. 37, no. 1, pp. 89-92, Feb 12 1990, doi: 10.1016/0166-4328(90)90074-o.
[27]. Y. Wang et al., “Aesculin offers increased resistance against oxidative stress and protective effects against Abeta-induced neurotoxicity in Caenorhabditis elegans,” Eur J Pharmacol, vol. 917, p. 174755, Feb 15 2022, doi: 10.1016/j.ejphar.2022.174755.
[28]. C. I. Bargmann, “Chemosensation in C. elegans,” WormBook : the online review of C. elegans biology, pp. 1-29, Oct 25 2006, doi: 10.1895/wormbook.1.123.1.
[29]. M. S. Lopez-Meza, G. Otero-Ojeda, J. A. Estrada, F. J. Esquivel-Hernandez, and I. Contreras, “The impact of nutritive and non-nutritive sweeteners on the central nervous system: preliminary study,” Nutritional neuroscience, vol. 25, no. 8, pp. 1623-1632, Aug 2022, doi: 10.1080/1028415X.2021.1885239.
[30]. Erbas et al., “Evaluation of long-term effects of artificial sweeteners on rat brain: a biochemical, behavioral, and histological study,” Journal of biochemical and molecular toxicology, vol. 32, no. 6, p. e22053, Jun 2018, doi: 10.1002/jbt.22053.
[31]. Jiao Yan, “Using nematodes as a model to study the consumption safety of three commonly used sugar substitutes,” M.S., Jilin University, 2018.
[32]. M. Zhang et al., “Aspartame and sucralose extend the lifespan and improve the health status of C. elegans,” Food & function, vol. 12, no. 20, pp. 9912-9921, Oct 19 2021, doi: 10.1039/d1fo01579f.
[33]. N. F. Salaya-Velazquez, L. A. Lopez-Mucino, S. Mejia-Chavez, P. Sanchez-Aparicio, A. A. Dominguez-Guadarrama, and A. Venebra-Munoz, “Anandamide and sucralose change DeltaFosB expression in the reward system,” Neuroreport, vol. 31, no. 3, pp. 240-244, Feb 5 2020, doi: 10.1097/WNR.0000000000001400.
[34]. C. Salim, N. Thadathil, M. Muralidhara, and P. S. Rajini, “Insights on the age dependent neurodegeneration induced by Monocrotophos, (an organophosphorous insecticide) in Caenorhabditis elegans fed high glucose: Evidence in wild and transgenic strains,” Comparative biochemistry and physiology. Toxicology & pharmacology : CBP, vol. 211, pp. 15-24, Sep 2018, doi: 10.1016/j.cbpc.2018.05.002.
[35]. Lingli Zhang, “A study on the relationship and mechanism between dietary intake pattern and individual behavior,” PhD, Shanghai Jiao Tong University, 2019.
[36]. N. Guo, J. Wang, and X. Wang, “Effect of starvation and high-carbohydrate diet on learning ability of Caenorhabditis elegans,” Heliyon, vol. 5, no. 3, p. e01289, Mar 2019, doi: 10.1016/j.heliyon.2019.e01289.
[37]. V. Vukojevic et al., “A role for alpha-adducin (ADD-1) in nematode and human memory,” The EMBO journal, vol. 31, no. 6, pp. 1453-66, Mar 21 2012, doi: 10.1038/emboj.2012.14.
[38]. S. E. Swithers, “Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements,” Trends in endocrinology and metabolism: TEM, vol. 24, no. 9, pp. 431-41, Sep 2013, doi: 10.1016/j.tem.2013.05.005.
[39]. S. L. Casperson, L. Johnson, and J. N. Roemmich, “The relative reinforcing value of sweet versus savory snack foods after consumption of sugar- or non-nutritive sweetened beverages,” Appetite, vol. 112, pp. 143-149, May 1 2017, doi: 10.1016/j.appet.2017.01.028.
[40]. L. Tsan et al., “Early-life low-calorie sweetener consumption disrupts glucose regulation, sugar-motivated behavior, and memory function in rats,” JCI insight, vol. 7, no. 20, Oct 24 2022, doi: 10.1172/jci.insight.157714.
[41]. R. E. Perszyk et al., “GluN2D-Containing N-methyl-d-Aspartate Receptors Mediate Synaptic Transmission in Hippocampal Interneurons and Regulate Interneuron Activity,” Molecular pharmacology, vol. 90, no. 6, pp. 689-702, Dec 2016, doi: 10.1124/mol.116.105130.
[42]. P. R. Zoladz et al., “ADRA2B deletion variant influences time-dependent effects of pre-learning stress on long-term memory,” Neurobiology of learning and memory, vol. 140, pp. 71-81, Apr 2017, doi: 10.1016/j.nlm.2017.02.014.
[43]. P. R. Zoladz et al., “ADRA2B deletion variant selectively predicts stress-induced enhancement of long-term memory in females,” Psychoneuroendocrinology, vol. 48, pp. 111-22, Oct 2014, doi: 10.1016/j.psyneuen.2014.06.012.
[44]. C. Miller, J. Dono, M. Scully, B. Morley, and K. Ettridge, “Adolescents’ knowledge and beliefs regarding health risks of soda and diet soda consumption,” Public health nutrition, vol. 25, no. 11, pp. 3044-3053, Nov 2022, doi: 10.1017/S1368980022001719.









