1. Introduction
The occurrence and effects of hepatocellular carcinoma (HCC) are significantly influenced by various factors. These include the timing and degree of exposure to environmental and infectious risks, the accessibility of healthcare resources, and the capacity to identify and potentially treat the disease at an early stage. Alarmingly, approximately 85 percent of HCC cases are concentrated in resource-poor or medium-resource countries [1]. These geographical variations manifest in distinct incidence and mortality rates for primary liver cancer on a global scale, with the highest burden being borne by East Asia and sub-Saharan Africa (Figure 1). This is particularly pronounced in areas where medical resources are often limited. Notably, HCC constitutes an 80-90 percent of all primary liver cancer cases [1].
The pathogenesis of HCC is a multifaceted process characterized by a series of molecular dysfunctions, including cell cycle dysregulation, altered DNA methylation, chromosomal instability, immune system modulation, and microRNA dysregulation. The complex procedure involves the release of molecular intermediaries, like damage-linked molecular patterns (DAMPs) from injured or dead cells, and pathogen-linked molecular patterns (PAMPs) from viruses. These activate pattern-detecting receptors (PRRs) including TLR, CLR, NLR (Nod-like receptors), and RLR (RIG-I-like receptors) on various immune cells, ultimately resulting in an inflammatory response. Following the elimination of the external stimulus, the acute inflammatory response subsides. However, if chronic inflammation remains unresolved, it can lead to fibrosis and, eventually, cirrhosis. It is noteworthy that a significant majority (80-90%) of HCC cases are preceded by a cirrhotic event [2, 3].
Angiogenesis and blood supply play pivotal roles in cancer progression, influencing growth and metastasis. Disrupting endothelial cell responses can impede tumor growth. The growth of vascular endothelial cells and the development, progression, and dispersion of tumors are greatly impacted by the VEGFR tyrosine kinase signaling pathway, which this context significantly depends on. Sorafenib, a targeted therapy, exerts its influence on both tumor growth and angiogenesis. It achieves this by targeting the RAF/MEK/ERK pathway and receptor tyrosine kinases. Notably, Sorafenib not only induces apoptosis but also triggers iron-mediated cell death, opening up new avenues for therapeutic benefits in cancer treatment. Furthermore, Sorafenib enhances the sensitivity of melanoma cells to vemofenib through the induction of iron-mediated cell death. The synthesis of sorafenib and its use in the treatment of HCC were explored in this article [4].
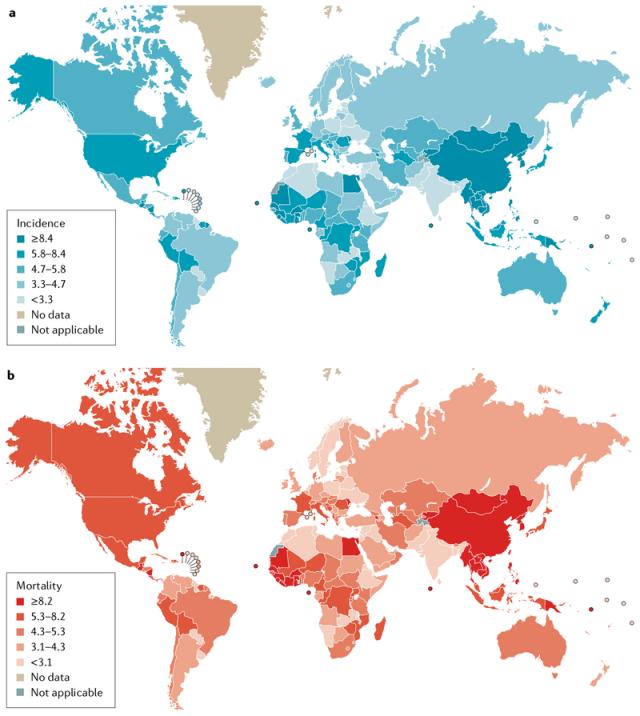
Figure 1. The incidence and mortality of primary liver cancer [1, 5].
2. Mechanism of action of Sorafenib
Sorafenib exerts its therapeutic effects by targeting various signaling pathways pivotal for cancer cell proliferation. Studies have revealed that cancer cell growth involves multiple signaling pathways, among which the RAS/MAPK signaling pathway plays a crucial role. Sorafenib operates by inhibiting this pathway, effectively counteracting the surge in cancer cell numbers during treatment. Furthermore, Sorafenib’s anti-cancer effects extend to targeting VEGFRs and PDGFRβ receptors. These receptors play a vital role in regulating angiogenesis, while PDGFRβ serves as a tyrosine kinase receptor transmitting intracellular signals. Cancer cells can exploit these receptors to promote their own growth. By targeting and inhibiting these receptors, Sorafenib disrupts their regulatory effects, depriving cancer cells of essential nutrients and oxygen. This ultimately leads to apoptosis and hinders cancer cell proliferation. Additionally, Sorafenib targets a spectrum of enzymes, including B-RAF, C-RAF, and KIT, which are commonly implicated in various cancer growth and regulatory pathways. Through inhibition of these enzymes, Sorafenib exerts its anti-cancer effects across a broader range of pathways. Lastly, Sorafenib inhibits the FOXM1 signaling pathway, which heightens the occurrence of autophagy within cancer cells. This enables cancer cells to efficiently utilize their own resources, reducing their reliance on the external environment. In this manner, Sorafenib contributes to limiting cancer cell growth and survival [6].
3. Synthetic Routes of Sorafenib
There are many synthetic routes for sorafenib, with different advantages and disadvantages. This article selects several typical routes for evaluation.
3.1. Traditional synthesis methods
Phenyl 4-chloro-3-phenylcarbamate and 4-N-methylpyridinamide are combined and dissolved in N,N-dimethylformamide to create a transparent reaction mixture (Figure 2) in the path A1. The mixture undergoes stirring at a temperature of 40-45°C for a span of 2-3 hours, after which it is cooled down to room temperature and diluted using ethyl acetate. Water, HCl and brine are then used in sequence to wash the emerged organic layer. After dilution with ethyl acetate at room temperature, it undergoes filtration and is subsequently dried under vacuum. The process results in both 4-(4-(4-(3-(4-chloro-3-(trifluoromethyl)phenyl)ureido)phenoxy)-N-methylpyridinium amide and 4-(4-(4-(3-(4-chloro-3-(trifluoromethyl)(3-(4-chloro-3-(trifluoromethyl)phenyl)ureido)phenoxy)-N-methylpyridinium carboxylate.
The synthetic route A2 of Sorafenib involved several steps (Figure 3). Initially, 4-Chloro-2-pyridinecarbonyl chloride hydrochloride (2) was derived from 2-pyridinecarboxylic acid (1) through a reaction with sulfoxide chloride, along with the presence of DMF. Subsequently, methyl 4-chloro-2-pyridinemethan-6-oate was obtained by treating with methanol (3). N-methyl-4-chloro-2-pyridinecarboxamide was synthesized by reacting methyl 4-chloro-2-pyridinecarboxylate with methylamine (4). An alternative method involved the direct reaction of 4-chloro-2-pyridinecarbonyl chloride hydrochloride (9) with a cold aqueous solution of methylamine to yield N-methyl-4-chloro-2-pyridinecarboxamide (4).
Next, the potassium salt of p-aminophenol was formed by reacting p-aminophenol with potassium tert-butoxide in DMF. This compound was subsequently reacted with N-methyl-4-chloro-2-pyridinecarboxamide (4) to produce 4-(4’-aminophenoxy)-2- pyridinecarboxamide (5). Finally, 4-(aminophenoxy)-2-(methylcarbamoyl)pyridine underwent a reaction with 4-chloro-3-trifluoromethylphenyl isocyanate in dichloromethane, or it was condensed with 4-chloro-3-trifluoromethylaniline using CDI as a condensate. These reactions resulted in the formation of Sorafenib (6) with an overall yield of 56% [7].
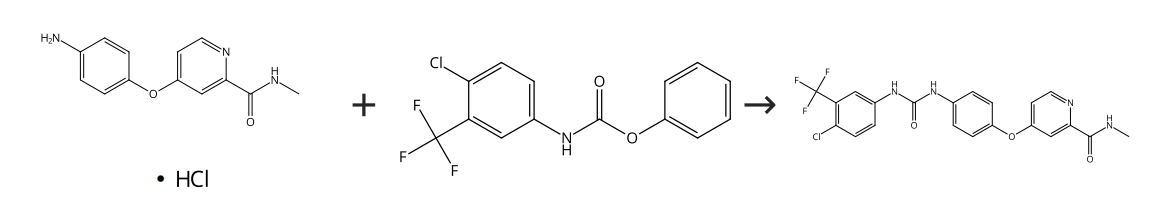
Figure 2. Sorafenib synthetic route A1 [7].
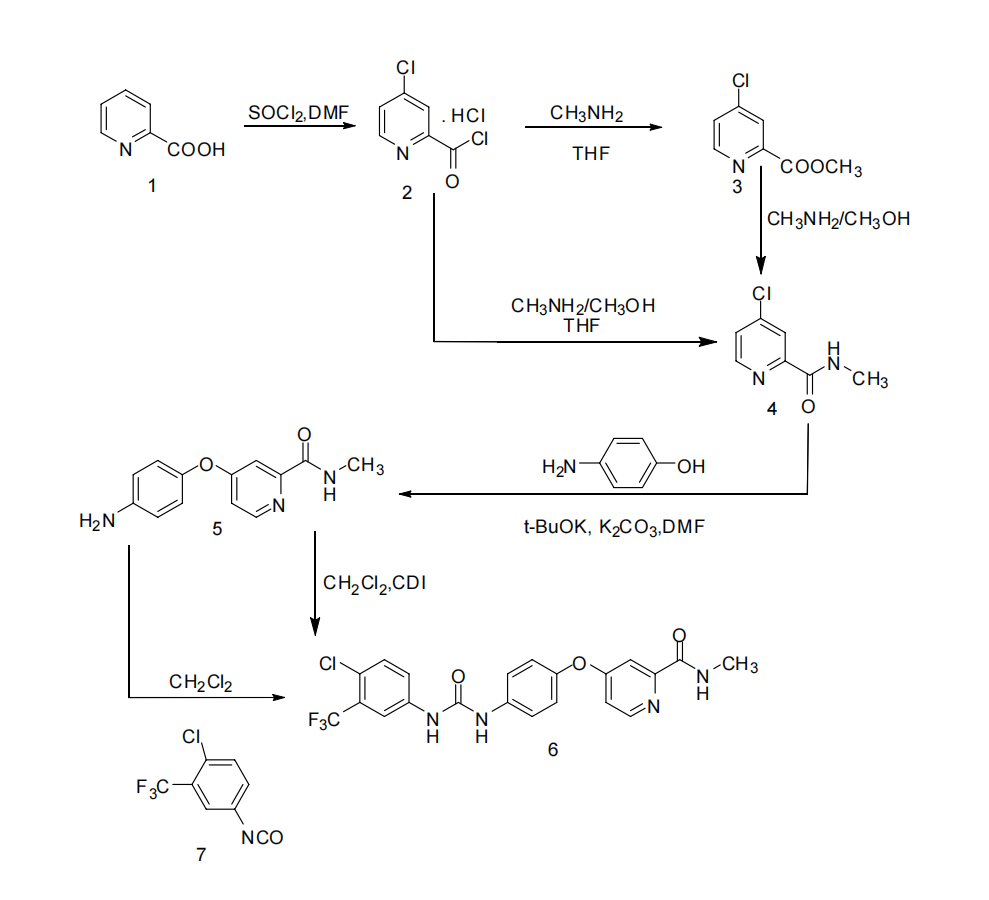
Figure 3. Sorafenib synthetic route A2 [8].
3.2. Synthesis of sorafenib intermediate
3.2.1. Synthesis of 4-chloro-2-pyridinecarbonyl chloride hydrochloride. In route B1, a one-step direct condensation process was employed to obtain N-methyl-4-chloro-2-pyridinecarboxamide (Figure 4). This was achieved by directly condensing N-methyl-4-chloro-2-pyridinecarboxamide with 4-chloropyridine, catalyzed by FeSO4 and concentrated hydrochloric acid in the presence of HCONHMe and hydrogen peroxide. This route yielded a product with an 81% yield. However, it should be noted that this route is associated with higher risks, as the reaction is more difficult to control, making it less suitable for industrial-scale production [8].
In route B2, 4-Chloro-2-pyridinecarbonyl chloride hydrochloride was prepared by adding chlorobenzene as the reaction solvent along with NaBr as a catalyst (Figure 5). This mixture was maintained in a toluene solution without further treatment. Subsequently, an aqueous solution of methylamine was slowly added at -5°C after cooling. Finally, the product was obtained through acidic dissolution and alkaline precipitation, resulting in a yield of 87%. This route not only improved the yield and purity of the product but also featured easily controllable reaction conditions and simple operations, making it highly suitable for industrial production.
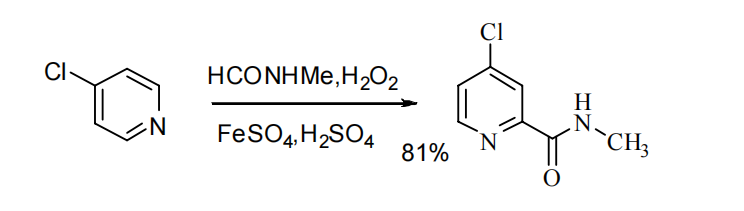
Figure 4. Sorafenib intermediate synthetic route B1 [8].
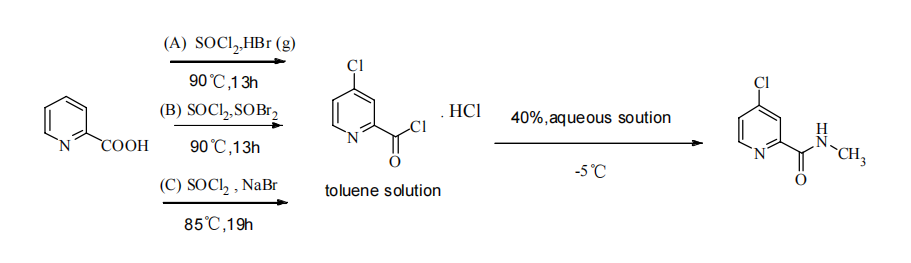
Figure 5. Sorafenib intermediate synthetic route B2 [8].
3.2.2. Synthesis of 4-(aminophenoxy)-2-(methylcarbamoyl)pyridine. Two methods were compared in the synthesis of this intermediate (Figure 6). In Method 1, 4-(aminophenoxy)-2-(methylcarbamoyl) pyridine was synthesized using Williamson’s method. This involved condensing p-aminophenol with potassium tert-butoxide in DMF as a solvent, forming p-aminophenol salt. The subsequent condensation with N-methyl (4-chloro-2-pyridinyl) formamide, catalyzed by anhydrous K2CO3, yielded the desired product with a yield of 85%. While this route is easier to operate, it employs high boiling point solvents like DMF and DMSO, which can be challenging to recover and are prone to oxidation during the reaction. Method 2 utilized phase transfer catalysis. The process involved mixing p-aminophenol, solid sodium hydroxide, a 45% sodium hydroxide solution, and the phase transfer catalyst tetrabutylammonium bisulfate in THF. Afterwards, the mixture was heated to 65°C and kept at this temperature for a total of 6 hours. This yielded 4-(aminophenoxy)-2-(methylcarbamoyl) pyridine with a 74% yield. In 2008, Zhao et al. improved this method by replacing the relatively expensive tetrabutylammonium bisulfate with polyethylene glycol 600, still achieving a 72% yield [8].
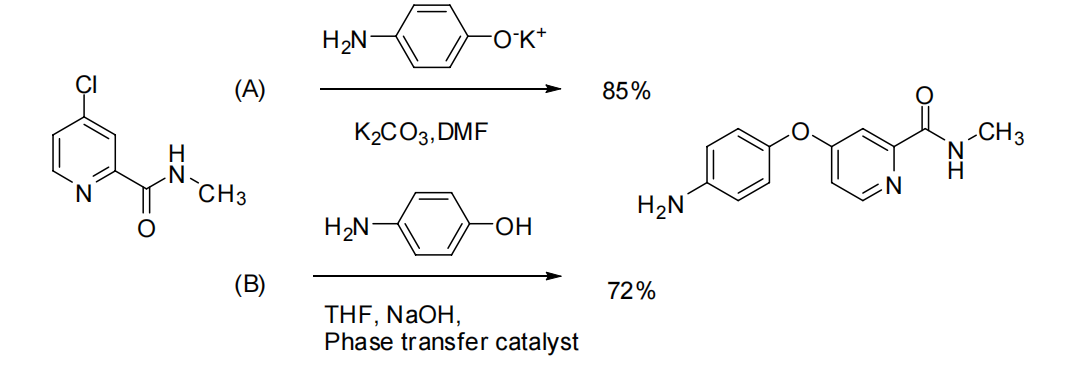
Figure 6. Sorafenib intermediate synthetic route C [8].
3.2.3. Synthesis of 4-chloro-3-(trifluoromethyl)benzene isocyanate. The preparation of 3-Trifluoromethyl-4-chloroaniline hydrochloride involved refluxing 3-Trifluoromethyl-4-chloroaniline hydrochloride with bis(phosgene) gas in toluene for 16 hours, yielding 86% (Figure 7). However, this process was lengthier and produced more symmetrized urea by-products. In 2010, Zhang Q. W. et al. enhanced the process by introducing diisopropylethyl amine (DIEA) to the solvent methylene chloride, resulting in a slight increase in yield. They also experimented with replacing methylene chloride with a greener solvent, 2-methyltetrahydrofuran, which proved to be more effective. They adopted a one-pot method instead of using dichloromethane, leading to better results with an 85% yield [8].
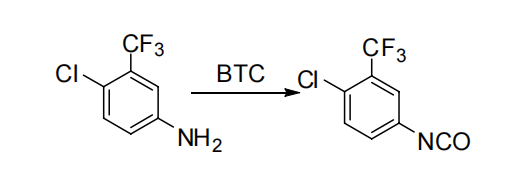
Figure 7. Sorafenib intermediate synthetic route D [8].
3.3. Total synthesis of sorafenib
A total synthetic route for sorafenib has been explored, providing important improvements in the production of the drug (Figure 8) [8].
Method (A) involves the reaction of 3-trifluoromethyl-4-chloroaniline with N, N’-carbonyldiimidazole (CDI) to produce the active intermediate N -(3-trifluoromethyl-4-chlorophenyl)-1H-imidazole-1-carboxamide, which is condensed with 4-(aminophenoxy)-2-(methylcarbamoyl)pyridine to give the final product sorafenib. However, this method has disadvantages, as N, N’-carbonyldiimidazole (CDI) is unstable and highly susceptible to hydrolysis. Moreover, it is too expensive for use in industrial production. Method (B) involves the condensation of 3-trifluoromethyl-4-chlorophenyl isocyanate with 4-(aminophenoxy)-2-(methylcarbamoyl)pyridine to produce sorafenib. However, this method has its own set of drawbacks. The preparation of 3-trifluoromethyl-4-chlorophenyl isocyanate is complex, involving a long reaction time, and separation and purification can be challenging. Additionally, 3-trifluoromethyl-4-chlorophenyl isocyanate is unstable and prone to generating symmetrical urea by-products.
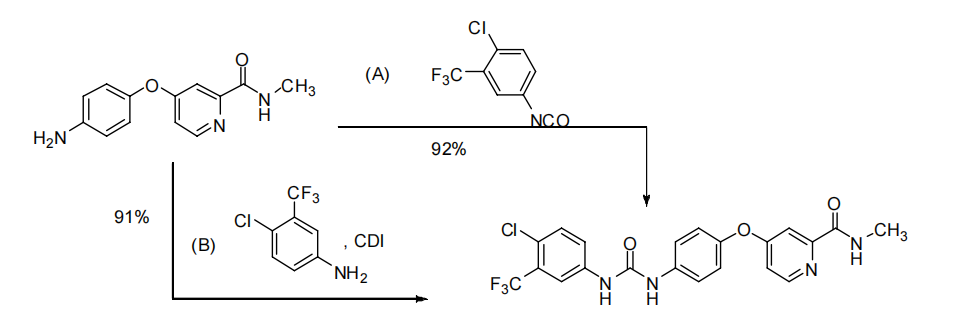
Figure 8. Total synthesis of sorafenib [8].
4. Current application and tolerance research of Sorafenib
HCC presents a significant global health challenge, and while sorafenib has shown efficacy in treating advanced HCC, the issue of drug resistance is a concerning factor. Resistance to sorafenib can take two forms: primary, where HCC is inherently unresponsive to sorafenib, and acquired, where initial sensitivity develops into resistance during treatment. The elements influencing these forms of resistance, not yet fully understood, are present in both liver cancer cells and the tumor microenvironment. Within liver cancer cells, factors like cell autophagy, epithelial-mesenchymal transition (EMT), iron death blockage, and tumor-initiating cells play a role in resistance to sorafenib. In the tumor microenvironment, factors such as exocytosis and vasculature selection also contribute to resistance.
4.1. MiR-3
In the study of Xiaofeng Tang et al, they successfully detected the expression of miR-3 in HCC cell lines with different sensitivities to sorafenib by reverse transcription quantitative PCR. Afterwards, they transfected miR-3 inhibitors, miR-3 mimics and seven (FBW7) short interfering RNAs (siRNAs) containing F-box and WD repeat domains into HCC cells as a way to regulate the expression levels of miR-3 and FBW7. Cell growth was assessed by mixing ethynyl deoxyuridine (EdU) and using Cell Counting Kit-8. Meanwhile, the expression of FBW7 protein was observed by protein blotting. The expression of miR-3 was elevated in the environment of HCC cells with sorafenib resistance, a phenomenon that suggests that miR-3 may be a new therapeutic strategy against sorafenib resistance.
Sorafenib induces hypoxia, and sorafenib treatment leads to a reduction in the number of tumour vessels and pericyte depletion, followed by hypoxia, triggering EMT and resistance to sorafenib. SIH contributes to the colonisation and stabilisation of HIF-1α and HIF-α in the core and produces the subsequent effects of vascular development and oncogene recording, which facilitates the adaptation of HCC cells to survive in a sorafenib-enriched environment. Sorafenib, in turn, triggered the conversion of HIF-1α to a HIF-α-dependent pathway, effectively enhancing this adaptation and providing great flexibility. Overall, the HIF family showed enhanced resistance to sorafenib and enhanced particle catabolism of HIF proteins in oxygen-deprived environments, thereby restoring the sensitivity of HCC cells to sorafenib. The SIH and HIF families, from the viewpoint of CSC, could amplify the stemness attribute of HCC cells either by raising the expression of genes regulating stemness and stem cell markers, or by lowering the expression of AR. As previously mentioned, the efficiency of sorafenib in HCC can be significantly improved by using potent HIF- inhibitors or AR inhibitors.
4.2. SCAP
Using the cholesterol sensor SCAP can promote sorafenib resistance by modulating autophagy in HCC, and the expression and subsequent cholesterol deposition of the cholesterol sensor SCAP correlates with the course of resistance to sorafenib. Overexpressed CAP is present in HCC tissues and HCC cell lines with sorafenib resistance, but inhibition of SCAP increases sorafenib susceptibility in HCC cells that are sorafenib-resistant. Additionally, it was discovered that sorafenib resistance was linked to decreased autophagy, and decreased autophagy was associated with decreased AMPK activity. Stilbene’s clinical significance lies in the fact that it is a SCAP inhibitor that reverses acquired resistance to sorafenib in both vivo and in vitro [9].
4.3. SOR nanocarriers
Numerous new SOR nanocarriers have been developed by researchers to overcome drug resistance in HCC. The range of nanoparticles (NPs) used for drug delivery is typically from 5-100 nm. A significant increase in publications regarding the use of NPs to treat Hepatocellular Carcinoma (HCC) has been observed over the last few years, which indicates significant advancements in this approach [10]. The release efficiency and bioavailability of SOR-NPs (SOR-NPs) are high, and they actively target tumour tissues. The solubility of SOR is enhanced by the small diameter and large specific surface area of SOR-NPs. The delivery of SOR-NPs to the targeted tumor tissues can be facilitated by altering their properties. Moreover, the zeta potential and other characteristics of NPs can be tailored to enhance cellular responses. The therapeutic efficiency of SOR-NPs is improved by a high absolute zeta potential, which is indicative of high surface charge density, in cancer cells. Furthermore, NPs effectively decrease the therapeutic dose and frequency of administration by controlling drug release. Chemotherapeutic drugs’ cytotoxicity and degradation rate are reduced by NPs. In addition, magnetic fields are used to deliver drug-carrying NPs to tumor tissues in vivo, and the acidic tumor environment can cause drug release. SOR-NPs are capable of treating cancer by bypassing the physical and physiological barriers that inhibit conventional drugs. Nanotechnology has the capacity to modify cancer cell resistance to anticancer drugs and overcome MDR [11].
4.4. Combination medication
A synergistic anti-cancer effect can be produced by the combination of Sorafenib and Quercetin. By reducing HCC cell viability, blocking cell cycle progression, and promoting apoptosis and necrosis, it reduces HCC cell viability. The use of a combination of sorafenib and quercetin was more successful in hindering the progression of cancer, restoring key gene expression levels, and maintaining liver structural integrity compared to singular therapy. It has been concluded that using sorafenib and quercetin together can be a promising anti-cancer strategy for improved treatment outcomes [12].
5. Conclusion
HCC has become a global issue, and it is urgent and necessary to explore its treatment options. As an effective therapeutic drug, sorafenib is an excellent option for the treatment of HCC. However, sorafenib is not without its drawbacks. Firstly, it is a long-term medication associated with a range of adverse reactions, imposing pressure on extended usage. Prolonged use also leads to the development of drug resistance, and escalating the dosage further exacerbates adverse reactions in patients. Secondly, the raw materials for sorafenib are expensive, and the preparation process is cumbersome and time-consuming. In many synthesis routes, specialized methods like ionic liquids are required, and there are challenges in recycling and processing. Finally, sorafenib cannot completely cure HCC, although it has demonstrated excellent performance in the treatment of other tumors. There is an urgent need to explore options to enhance the performance of sorafenib, including improving its therapeutic effect and minimizing adverse reactions. Simultaneously, there is room for improvement in the synthetic route of sorafenib, and a low-cost and efficient solution is needed to replace the current method. More research needs to be devoted to these aspects to bring more benefits to HCC patients.
References
[1]. Forner A, Reig M and Bruix J 2018 Lancet 391 10127
[2]. Chidambaranathan-Reghupaty S, Fisher PB and Sarkar D 2021 Adv. Cancer Res. 149 1
[3]. Li Q, Chen, K, Zhang, T, Jiang, D, Chen, L, Jiang, J, Zhang, C and Li, S 2023 Eur. J. Pharmacol 955 175913
[4]. Li Y, Yang W, Zheng Y, Dai W, Ji J, Wu L, Cheng Z, Zhang J, Li J, Xu X, Wu J, Yang M, Feng J and Guo C 2023 J. Exp. Clin. Cancer Res. 42 6
[5]. Yang J D, Hainaut P, Gores G J, Amadou A, Plymoth A and Roberts L R 2019 Nat. Rev. Gastro. Hepat. 16 589
[6]. Xia S, Pan Y, Liang Y, Xu J and Cai X 2020 E. Bio. Med. 51 102610
[7]. Breitler S, Oldenhuis N J, Fors B P and Buchwald S L 2011 Org. Lett. 13 3262-326
[8]. Sun C 2011 Study on the synthesis process of sorafenib (Harbin: Heilongjiang University)
[9]. Li D, Yao Y, Rao Y, Huang X, Wei L, You Z, Zheng G, Hou X, Su Y, Varghese Z, Moorhead JF, Chen Y and Ruan XZ 2022 J. Exp. Clin. Cancer Res. 41 116
[10]. Cheng Z, Jiang W and Jin D 2020 Bba-Rev. Cancer 1874 188382
[11]. Kong F-H, Ye Q-F, Miao X-Y, Liu X, Huang S-Q, Xiong, L Wen, Y and Zhang Z-J 2021 Theranostics 11 5464–5490
[12]. Abdu S, Juaid N, Amin A, Moulay M and Miled N 2022 Molecules 27 8082
Cite this article
Xu,Z. (2023). The synthetic routes of sorafenib and its applications on hepatocellular carcinoma. Theoretical and Natural Science,17,19-26.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Forner A, Reig M and Bruix J 2018 Lancet 391 10127
[2]. Chidambaranathan-Reghupaty S, Fisher PB and Sarkar D 2021 Adv. Cancer Res. 149 1
[3]. Li Q, Chen, K, Zhang, T, Jiang, D, Chen, L, Jiang, J, Zhang, C and Li, S 2023 Eur. J. Pharmacol 955 175913
[4]. Li Y, Yang W, Zheng Y, Dai W, Ji J, Wu L, Cheng Z, Zhang J, Li J, Xu X, Wu J, Yang M, Feng J and Guo C 2023 J. Exp. Clin. Cancer Res. 42 6
[5]. Yang J D, Hainaut P, Gores G J, Amadou A, Plymoth A and Roberts L R 2019 Nat. Rev. Gastro. Hepat. 16 589
[6]. Xia S, Pan Y, Liang Y, Xu J and Cai X 2020 E. Bio. Med. 51 102610
[7]. Breitler S, Oldenhuis N J, Fors B P and Buchwald S L 2011 Org. Lett. 13 3262-326
[8]. Sun C 2011 Study on the synthesis process of sorafenib (Harbin: Heilongjiang University)
[9]. Li D, Yao Y, Rao Y, Huang X, Wei L, You Z, Zheng G, Hou X, Su Y, Varghese Z, Moorhead JF, Chen Y and Ruan XZ 2022 J. Exp. Clin. Cancer Res. 41 116
[10]. Cheng Z, Jiang W and Jin D 2020 Bba-Rev. Cancer 1874 188382
[11]. Kong F-H, Ye Q-F, Miao X-Y, Liu X, Huang S-Q, Xiong, L Wen, Y and Zhang Z-J 2021 Theranostics 11 5464–5490
[12]. Abdu S, Juaid N, Amin A, Moulay M and Miled N 2022 Molecules 27 8082









