1. Introduction
Malignant tumors have become the leading killer threatening human health, and breast cancer has ranked first in the incidence rate of female malignant tumors [1]. Surgical procedures, radiotherapy, chemotherapy, molecular-targeted therapies, and endocrine therapy are used to treat breast cancer. Drugs that are molecularly targeted particularly aim highly-expressed molecules in tumor cells but are not or are only minimally expressed in healthy cells, thereby effectively achieving the goal of killing tumor cells. Patients with different breast cancer subtypes’ survival rates vary considerably [2]. The mortality rate of HER2-positive breast cancer is higher, followed by three negative, Luminal A, and Luminal β subtypes [3]. Breast cancer positive for Human Epidermal Growth Factor Receptor 2 (HER2, also called ERBB2) accounts for approximately 15-20% [4, 5]. Although there are anti-HER2 targeted therapies at present and sound effects have been achieved at the initial stage, many patients have experienced treatment resistance, tumor recurrence, and disease progression. Therefore, it is urgent to find new methods to treat HER2-positive breast cancer and improve the survival rate of patients [6]. HER2 has become a key target for developing HER2-positive breast cancer drugs.
Lapatinib is a drug used to treat breast cancer. The targets of lapatinib mainly include epidermal growth factor receptor (EGFR) and its mutants, HER2, and anaplastic lymphoma kinase (ALK), which are often mutated or overexpressed in tumor cells, leading to abnormalities in cell proliferation, growth, angiogenesis, and other aspects. Furthermore, lapatinib can selectively target these abnormal proteins, thereby blocking abnormal signal transduction in cells and achieving the effect of treating cancer. Its action mechanism blocks the intracellular domain of HER2 in breast cancer and plays an anti-cancer role. However, lapatinib’s poor solubility limits its clinical application. In order to enhance the anti-tumor effect of lapatinib, studies have proposed using pegylated liposome formulations of lapatinib, which can reduce tumor volume to a greater extent and significantly reduce cardiac and hepatic toxicity. The Food and Drug Administration of the United States authorized lapatinib for metastatic breast cancer treatment in 2007.
HER2 is the second member of the EGFR family, which includes four different tyrosine kinase receptors (TKRs): HER1, HER2, HER3, and HER4 [7]. HER2, unlike other members of the EGFR family, lacks a ligand-binding domain and is an orphan receptor. When other family members (especially HER1 and HER3) bind to ligands, HER2 can form heterodimers, promoting self-phosphorylation of the tyrosine kinase domain and activation [8, 9]. The unique dimeric properties of HER2 are considered practical amplifiers for other receptor signals in the EGFR family. The dimer-activated HER2 promotes cell growth, proliferation, and metastasis by regulating intracellular downstream signaling pathways such as PI3K, SRC, AKT, and MAPKs (see Figure 1) [10, 11].
Based on the above research background, the study of lapatinib aims to enhance its anti-tumor effect further and reduce its cardiotoxicity and hepatotoxicity. This paper summarizes the mechanism of action, related clinical studies, and drug resistance mechanisms of lapatinib.
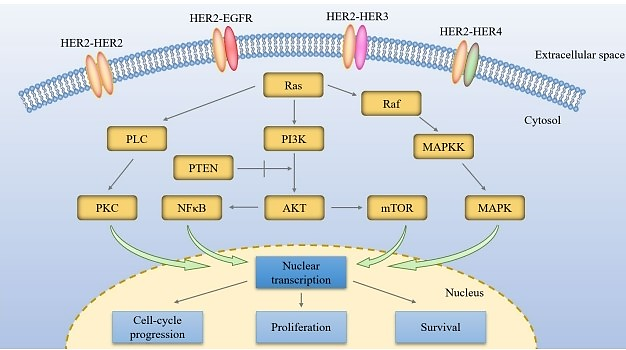
Figure 1. The HER2 receptor activates downstream signaling pathways, promoting cell growth, proliferation, and survival [12].
2. Target receptor in breast cancer treatment
2.1. EGFR
EGFR is a receptor tyrosine kinase on the surface of tumor cells [7]. When EGFR binds to its ligand, various signaling pathways that promote cell growth and proliferation are initiated. EGFR gene mutations may be contained in some tumor cells, causing undue pathway activation and speeding up tumor progression. Lapatinib achieves therapeutic effects by selectively inhibiting the activation of EGFR and its mutants and inhibiting the growth of tumor cells.
2.2. HER2
As a receptor tyrosine kinase, HER2 plays a distinct role than EFGR in tumor cell survival and proliferation [8]. HER2 overexpression or mutation is common in many leukemias, breast cancers, and other tumors. Lapatinib can bind to HER2, inhibit its activation, and block the transmission of related signaling pathways, thereby preventing the proliferation and survival of tumor cells and playing a therapeutic role.
2.3. ALK
ALK mutations are widely found in lung and malignant tumors [8]. These mutations activate the ALK protein, leading to inflammation and abnormal proliferation. One of the mechanisms of action of lapatinib is to inhibit the activation of ALK, preventing normal proliferation and survival of tumor cells, thereby achieving anti-tumor effects.
3. Mechanism of lapatinib in breast cancer treatment
Lapatinib is an efficient tyrosine kinase inhibitor, and its molecular mechanism of action mainly involves the interaction with HER2 receptors and the regulation of signal transduction pathways [9]. Lapatinib can bind to the HER2 receptor, blocking the interaction between HER2 and its downstream signaling molecules. In breast cancer, the HER2 receptor is essential; its overexpression is associated with the disease’s onset and progression. Lapatinib can selectively bind to HER2 receptors, blocking the transmission of HER2 signals and thereby preventing the growth and spread of cancer cells.
Lapatinib exerts its effect by inhibiting the phosphorylation process of HER2 receptors [7]. The phosphorylation of HER2 receptors is a crucial step in their activation, activating signaling pathways at the downstream and facilitating cell survival and proliferation. Lapatinib binds to the extracellular domain of the HER2 receptor, blocking its phosphorylation process and thereby inhibiting the activation of downstream signaling. Lapatinib can also play a role by promoting the internalization and degradation of HER2 receptors [10]. The expression level of HER2 receptors on cell membranes is closely related to tumor invasion and metastasis. One of the mechanisms of action of lapatinib is to promote the internalization and degradation of the HER2 receptor and reduce its expression level on the cell membrane, thus inhibiting the invasion and metastasis of breast cancer cells. In addition to affecting the activity and expression level of HER2 receptors, lapatinib can also regulate other signaling pathways. The occurrence and development of breast cancer involve abnormal activation of multiple signal pathways, including the Ras/MAPK and PI3K/Akt pathways (Figure 1). Through modulating these signaling pathways’ activities, lapatinib can inhibit tumor cell proliferation and survival. Specifically, lapatinib can inhibit the activation of the Ras/MAPK pathway and reduce cell proliferation ability. At the same time, it can also inhibit the activation of the PI3K/Akt pathway and cell viability [12].
Moreover, lapatinib can induce cell apoptosis [12]. Important in malignant tumor treatment, apoptosis is the normal process of cell self-destruction. By hindering HER2 signaling pathway activation, lapatinib induces apoptosis in breast cancer cells, leading to the tumor cells’ demise.
Lapatinib can also inhibit tumor angiogenesis. Tumor cells obtain sufficient nutrients and oxygen through angiogenesis, promoting tumor growth and diffusion. Lapatinib reduces the dependence of tumor cells on angiogenesis by inhibiting the expression and activity of angiogenic factors such as VEGF, thereby inhibiting the angiogenesis process of tumors.
Therefore, the pharmacological effects of lapatinib mainly include blocking the HER2 signaling pathway, inhibiting the activation of downstream signaling molecules, inducing cell apoptosis, and inhibiting tumor angiogenesis. As a tyrosine kinase inhibitor, lapatinib treats breast cancer through the inhibition of HER2 receptor internalization and phosphorylation and by modulating several signaling pathways’ activities. Such mechanisms enable lapatinib to be a crucial drug in breast cancer treatment, and it offers a vital treatment alternative to breast cancer patients who are HER-2 positive.
4. The synthetic routes of lapatinib
4.1. Synthetic route 1
Figure 2 shows that 4-chloro-6-iodoquinazoline (6) was synthesized from 6-iodoquinazolone (5) and thionyl chloride. Then, it reacted with 3-chloro-4-((3-fluorophenyl)methoxy)aniline (7) to obtain K(3-chloro-4-((3-fluorophenyl)methoxy)phenyl)-6-iodo-4-quinazolimine (2). N-(3-chloro-4-(3-fluorobenzyloxy)phenyl)-6-((5-formyl) furan-2-yl)-4-quinazolin amine (3) was synthesized by Suzuki coupling reaction with 5-formylfuran-2-boric acid catalyzed by palladium carbon. Lapatinib base was obtained by substitution reaction with 2-methylsulfonyl ethylamine hydrochloride, reduction with sodium triacetoxyborohydride, and reaction with p-toluenesulfonic acid to obtain lapatinib di-p-toluenesulfonic acid (4). Lapatinib di-p-toluenesulfonic acid monohydrate (1) was refined by tetrahydrofuran/water [13].
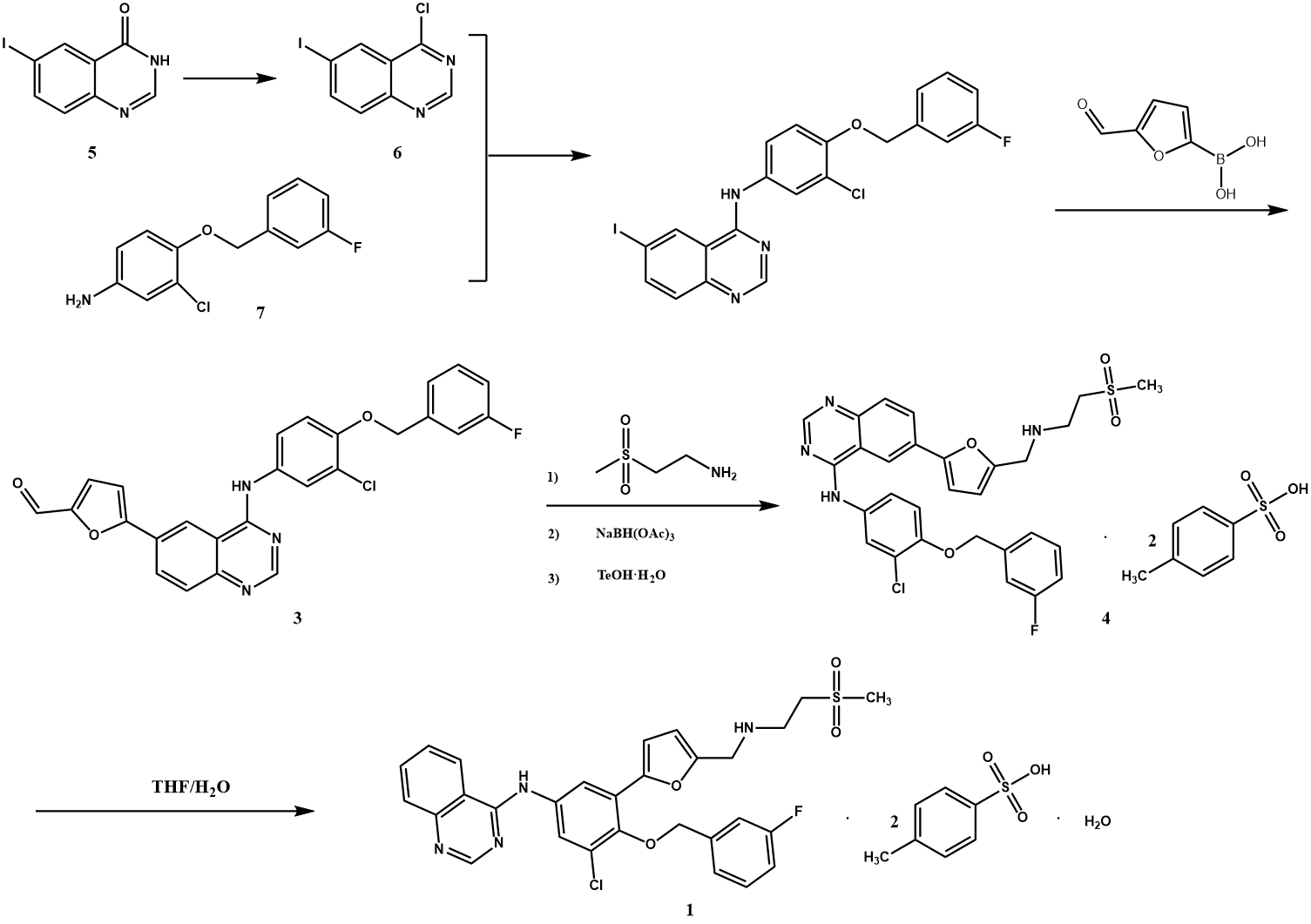
Figure 2. Synthetic route 1 [13].
4.2. Synthetic route 2
In one study, 4-hydroxy-6-iodoquinazoline was synthesized by cyclization of 2-amino-5-iodobenzoic acid with formamidine acetate (Figure 3) [14]. After chlorination, it was condensed with 3-chloro-4-[(3-fluorophenyl)methoxy]aniline (4) to obtain n-[3-chloro-4-[(3-fluorophenyl) methoxy]phenyl]-6-iodo-4-quinazoline amine (5). The solubility of 4-hydroxy (chloro)-6-iodoquinazoline was poor, and excessive thionyl chloride or phosphorus oxychloride was required during chlorination, which was not easy to post-treatment and has great environmental pollution. In this study, (1) was prepared by the route shown in Figure 2 concerning relevant literature, and the reaction conditions were optimized; 2-amino-5-iodobenzonitrile (2) was obtained by iodization with 2-aminobenzylnitrile as raw material. (2) was condensed with N, N-dimethylformamide dimethylacetal (DMF·DMA) to obtain (3). After the excess DMF·DMA was evaporated under reduced pressure, glacial acetic acid and (4) were directly added for dimroth rearrangement to obtain (5) in 69.6% yield (based on 2-aminobenzylnitrile). (5) then reacted with 5-formylfuran-2-boric acid by Suzuki coupling reaction to obtain n-[3-chloro-4-[(3-fluorophenyl)methoxy]phenyl]-6-[(5-formyl)furan-2-yl]-4-quinazolin amine (6). (6) then reacted with 2-(methanesulfonyl) ethylamine (7) to obtain lapatinib (8), finally forming a salt with p-toluenesulfonic acid to obtain (1). This improved route avoided using thionyl chloride or phosphorus oxychloride and could reduce environmental pollution. The total yield was 10% (based on 2-aminobenzylnitrile).
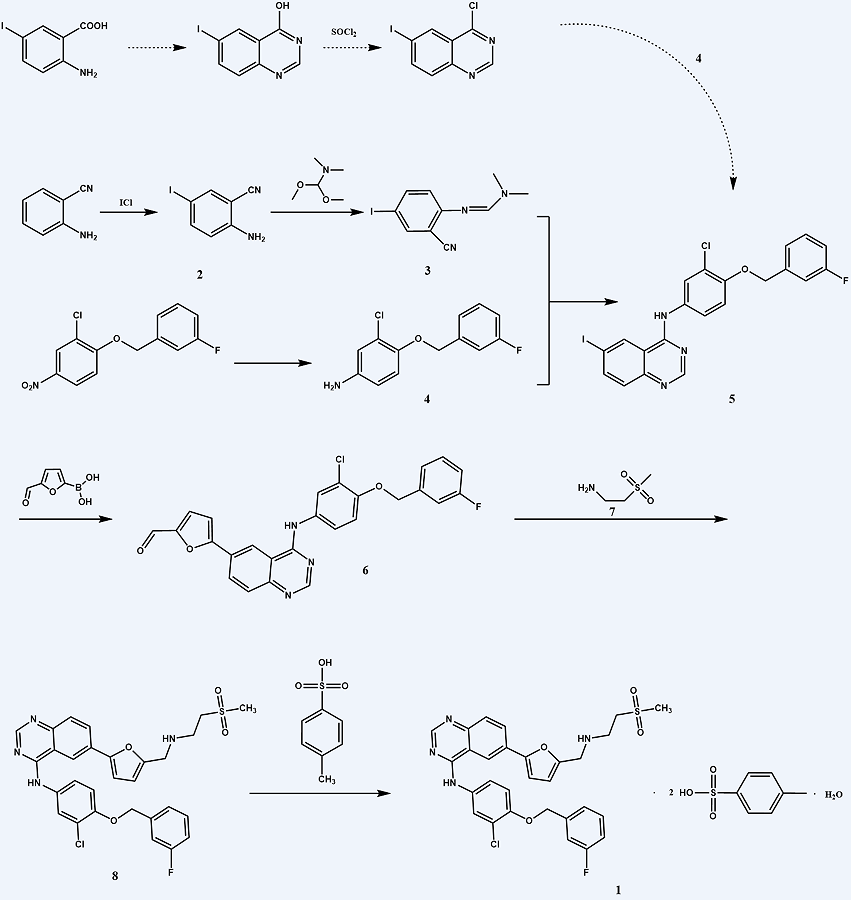
Figure 3. Synthetic route 2 [14].
5. Applications of lapatinib in breast cancer treatment
Regarding breast cancer treatment, the application of lapatinib varies, covering treatment stages, use modes, and combined use with other drugs. As a targeted drug for breast cancer, lapatinib demonstrates notable therapeutic efficacy. Notably, it substantially improves patient survival rates and overall outcomes. Concurrent lapatinib administration in clinical trials has consistently resulted in substantially prolonged survival without progression and overall survival incidences than separate agents for chemotherapy [15]. It highlights lapatinib’s significance in such treatment. In treating breast cancer that is HER2-positive, lapatinib also demonstrates encouraging results. By selectively inhibiting the activation of the HER2 receptor, lapatinib effectively hinders cancer cell proliferation and metastasis [16]. Clinical trial data indicate that patients benefiting from combination chemotherapy involving lapatinib experience notably enhanced effectiveness compared to those receiving single-agent chemotherapy [17]. At the same time, lapatinib can be used as a first-line treatment option. For patients with early HER2-positive breast cancer, lapatinib combined with other chemotherapy drugs can be used as a first-line treatment plan to eliminate early tumor cells and reduce the risk of tumor recurrence and metastasis [18]. Additionally, lapatinib is frequently used to treat advanced breast cancer. Patients with advanced breast cancer frequently combine lapatinib with endocrine therapy or chemotherapy medications to promote the drug’s therapeutic consequence. One research discovered how combining lapatinib can substantially improve efficacy on advanced breast cancer patients, prolong patient longevity, and slow the development of the disease. Lapatinib has therefore become indispensable for advanced breast cancer treatment.
In addition, the application of lapatinib includes various situations, such as neoadjuvant therapy and adjuvant therapy. Lapatinib is administered prior to breast cancer surgery to mitigate tumor mass and halt its progression, thereby making surgery more effective. Lapatinib can likewise be administered alongside other medications, such as chemotherapy and endocrine therapy drugs to enhance treatment effectiveness and reduce patient tolerance to side effects [19].
It is important to note that patient differences also affect the therapeutic effect of lapatinib. Factors such as different subtypes of breast cancer, the patient’s condition, and gene mutations will affect the therapeutic effect of lapatinib. Therefore, in clinical applications, careful consideration should be given to the individual characteristics of patients and genetic testing results to develop personalized treatment plans and improve treatment effectiveness.
Generally, in breast cancer treatment, there is an intense and extensive lapatinib application, and an individualized treatment plan can be designed according to the patients’ specific conditions. Whether it is early breast cancer or late breast cancer, applying lapatinib can provide effective treatment strategies and bring hope to patients. However, although lapatinib has shown promising efficacy in treating breast cancer, attention should be paid to its adverse reactions and management to ensure patients’ safety and quality of life.
6. Conclusion
Lapatinib is a promising targeted drug for breast cancer treatment, demonstrating notable therapeutic efficacy. Clinical trials have shown that its administration significantly increases patient and overall survival rates. Patients obtaining chemotherapy alongside lapatinib had substantially prolonged their survival without cancer progression as well as overall survival than those obtaining chemotherapy alone. These findings substantiate lapatinib’s substantial impact on breast cancer treatment outcomes. Furthermore, lapatinib exhibits particular efficacy in addressing HER2-positive breast cancer cases. By selectively inhibiting the activation of the HER2 receptor, lapatinib effectively hinders cancer cell proliferation and metastasis. Clinical trials reinforce this observation, indicating that patients benefiting from a combination chemotherapy regimen involving lapatinib exhibit significantly higher response rates than those on single chemotherapy drugs.
While significant strides have been made in understanding lapatinib’s mechanisms of action, areas still require further exploration. A comprehensive comprehension of the full spectrum of signaling pathways and molecular mechanisms influenced by lapatinib is essential. Moreover, the clinical application of lapatinib necessitates additional research data to validate its efficacy and safety and assess patient tolerance and responsiveness to treatment. Future research efforts should encompass a multifaceted approach. It includes conducting experiments such as gene expression profiling and protein interaction network analysis to thoroughly investigate the intricate mechanisms in breast cancer cells under lapatinib influence. Furthermore, designing and executing comprehensive animal experiments and clinical trials will be pivotal in validating the efficacy and safety of lapatinib across diverse patient populations. Exploring potential combinations of lapatinib with other drugs, including chemotherapy agents or immunotherapy drugs, will be instrumental in enhancing treatment effectiveness while minimizing potential side effects. Finally, investigating lapatinib’s application potential in preventing breast cancer occurrence and recurrence and its role in screening and early diagnosis holds promise for more effective breast cancer management strategies.
In conclusion, future research should deepen the understanding of lapatinib’s mechanism of action and further validate its efficacy and safety. In addition, research into combined therapies and preventative mechanisms can enable the development of more efficient breast cancer prevention and treatment strategies.
References
[1]. Alwan N A S 2016 J. Glob. Oncol. 2(5) 255
[2]. Anjum R and Blenis J 2008 Nat. Rev. Mol. Cell Biol. 9 747
[3]. Lara R, Seckl M J and Pardo O E 2013 Cancer Res. 73 5301
[4]. Romeo Y and Roux P P 2011 Expert Opin. Ther. Targets 15 5
[5]. Zheng B, Jeong J H, Asara J M, Yuan Y Y, Granter S R, Chin L and Cantley L C 2009 Mol. Cell 33 237
[6]. Cho Y Y 2017 Arch. Pharm. Res. 40 291
[7]. David J P, Mehic D, Bakiri L, Schilling A F, Priemel M, Idarraga M H, Reschke M O, Hoffmann O, Amling M and Wagner E F 2005 J. Clin. Invest. 115 664
[8]. Cho Y Y, He Z, Zhang Y, Choi H S, Zhu F, Choi B Y, Kang B S, Ma W Y, Bode A M and Dong Z 2005 Cancer Res. 65 3596
[9]. Li D, Jin L, Alesi G N, Kim Y M, Fan J, Seo J H, Wang D, Tucker M, Gu T L and Lee B H 2013 J. Biol. Chem. 288 32528
[10]. Lim H C, Xie L, Zhang W, Li R, Chen Z C, Wu G Z, Cui S S, Tan E K and Zeng L 2013 PLoS One 8 e74334
[11]. Cho Y Y, Yao K, Bode A M, Bergen H R, Madden B J, Oh S M, Ermakova S, Kang B S, Choi H S and Shim J H 2007 J. Biol. Chem. 282 8380
[12]. Shi X M 2022 Research on the mechanism of RSK2 promoting HER2 positive breast cancer cell proliferation and metastasis (Changsha: Central South University)
[13]. Zhang Q W, Zhou H Y and You Q D 2010 J. China Pharm. Univ. 41 317
[14]. Khabnadideh S, Pez D, Musso A, Brun R, Pérez L M R, González-Pacanowska D and Gilbert I H 2005 Bioorgan. Med. Chem. 13 2637
[15]. Wang J and Xu B 2019 Nature 4(1) 34
[16]. Wissner A, Fraser H L, Ingalls C L, Dushin R G, Floyd M B, Cheung K, Nittoli T, Ravi M R, Tan X and Loganzo F 2007 Bioorgan. Med. Chem. 15 3635
[17]. Fischer R W and Misun M 2001 Org. Proc. Res. Dev. 5 581
[18]. Mcclure M S, Osterhout M H, Roschangar F and Sacchetti M J 2007 Quinazoline ditosylate salt compounds (U.S. Patent No. 7,157,466)
[19]. Ji X, Wang W W, Xu G H, Li F and Yao S C 2009 Chin. J. Pharm. Industry 40 801
Cite this article
Wang,Y. (2023). The mechanism of lapatinib and its application in breast cancer. Theoretical and Natural Science,17,41-47.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Alwan N A S 2016 J. Glob. Oncol. 2(5) 255
[2]. Anjum R and Blenis J 2008 Nat. Rev. Mol. Cell Biol. 9 747
[3]. Lara R, Seckl M J and Pardo O E 2013 Cancer Res. 73 5301
[4]. Romeo Y and Roux P P 2011 Expert Opin. Ther. Targets 15 5
[5]. Zheng B, Jeong J H, Asara J M, Yuan Y Y, Granter S R, Chin L and Cantley L C 2009 Mol. Cell 33 237
[6]. Cho Y Y 2017 Arch. Pharm. Res. 40 291
[7]. David J P, Mehic D, Bakiri L, Schilling A F, Priemel M, Idarraga M H, Reschke M O, Hoffmann O, Amling M and Wagner E F 2005 J. Clin. Invest. 115 664
[8]. Cho Y Y, He Z, Zhang Y, Choi H S, Zhu F, Choi B Y, Kang B S, Ma W Y, Bode A M and Dong Z 2005 Cancer Res. 65 3596
[9]. Li D, Jin L, Alesi G N, Kim Y M, Fan J, Seo J H, Wang D, Tucker M, Gu T L and Lee B H 2013 J. Biol. Chem. 288 32528
[10]. Lim H C, Xie L, Zhang W, Li R, Chen Z C, Wu G Z, Cui S S, Tan E K and Zeng L 2013 PLoS One 8 e74334
[11]. Cho Y Y, Yao K, Bode A M, Bergen H R, Madden B J, Oh S M, Ermakova S, Kang B S, Choi H S and Shim J H 2007 J. Biol. Chem. 282 8380
[12]. Shi X M 2022 Research on the mechanism of RSK2 promoting HER2 positive breast cancer cell proliferation and metastasis (Changsha: Central South University)
[13]. Zhang Q W, Zhou H Y and You Q D 2010 J. China Pharm. Univ. 41 317
[14]. Khabnadideh S, Pez D, Musso A, Brun R, Pérez L M R, González-Pacanowska D and Gilbert I H 2005 Bioorgan. Med. Chem. 13 2637
[15]. Wang J and Xu B 2019 Nature 4(1) 34
[16]. Wissner A, Fraser H L, Ingalls C L, Dushin R G, Floyd M B, Cheung K, Nittoli T, Ravi M R, Tan X and Loganzo F 2007 Bioorgan. Med. Chem. 15 3635
[17]. Fischer R W and Misun M 2001 Org. Proc. Res. Dev. 5 581
[18]. Mcclure M S, Osterhout M H, Roschangar F and Sacchetti M J 2007 Quinazoline ditosylate salt compounds (U.S. Patent No. 7,157,466)
[19]. Ji X, Wang W W, Xu G H, Li F and Yao S C 2009 Chin. J. Pharm. Industry 40 801









