1. Introduction
Lung cancer is the second most common cancer and is one of the death leading cancers in around the world [1]. Lung cancer was a relatively rare cancer type at the beginning of the 20th century. However, lung cancer cases dramatically rose in the following decades due to increased smoking among both males and females [2]. Signs and symptoms of lung cancer typically only occur in later stages as the disease is advanced, including blood coughing, breathlessness, chest infections, unexplained weight loss, bone pain, and persistent tiredness [3]. Lung cancers are commonly categorized in to two types: small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). SCLS and NSCLC develop differently and needs to be treated with different methods [4].
The EGFR gene, also known as HER1, is one of the most significant genes for the lung cancer. The EGFR is one of the receptor tyrosine kinase family, which contains four members: EGFR (HER1), ErbB2 (HER2/Neu), ErbB3 (HER3), and ErbB4 (HER4). Unlike other gene mutations that are universal to cause all different kinds of cancer, the EGFR gene mutations are highly associated with NSCLC [5]. The EGFR gene is responsible for the production of epidermal growth factor receptor (EGFR), which is a transmembrane protein that transduce signals. The EGFRs can be activated when specific ligands are bound to the receptor domain. The signal pathway is then activated within the cell, and the cell starts to proliferate and replicate. Mutations in the EGFR gene can cause lung cancer. The most common EGFR mutations in lung cancer are exon 21 L858R mutation and exon 19 del19 mutation. The mutated genes lead to false protein production, causing the production of constantly activated EGFR. Consequently, mutant cells persistently receive signals to proliferate and divide, leading to tumor growth [6].
EGFR mutation is one of the main causes of adenocarcinoma. Knowing the mechanism of EGFR mutation can lead to a better understanding of tumor growth. Moreover, studying EGFR mutation can help researchers to develop targeted therapy and personalized medicine, which potentially improves patient outcomes. There are still some research gaps in the effective treatment of lung cancer with EGFR mutation, such as the need for more research to improve treatment outcomes for patients with EGFR-mutant NSCLC and more research on acquired resistance to EGFR TKIs.hese guidelines, written in the style of a submission, show the best layout for your paper using Microsoft Word. If you don’t wish to use the Word template provided, please use the following page setup measurements.
2. Structure and activation of EGFR
The EGFRs have three domains: an EGF-binding domain, a transmembrane domain, and a tyrosine kinase (TK) domain. The EGF binding domain contains subdomains: subdomains I, II, III, and IV. Among these, subdomains II and IV are responsible for ligand binding and dimerization. The intracellular domain contains a C-terminal tail, which has several tyrosine residues that can be phosphorylated upon activation. The structure of the epidermal growth receptor has been extensively studied and is known to adopt a bilobate-fold characteristic of other protein kinase domains. In the inactivated state, the EGFR remains as two separated monomers. The two monomeric units are identical, each with an EGF-binding domain and a tyrosine kinase domain. The binding of epidermal growth factor or other EGF ligands such as transforming growth factor-α (TGF-α) and amphiregulin (AREG), leads to the activation of EGFR; the activation of EGFR causes ligan-induced dimerization, which leads to the autophosphorylation of the c-terminal tail. The phosphorylated c-terminal tail act as a binding sites for different kinds of signaling proteins, which can further conduct the signaling pathway [7]. Figure 1 indicates the structure of the EGFR. The blue monomer represents a single monomeric unit of EGFR in an inactivated state. The red molecule represents extracellular ligands. As extracellular ligands bind to the EGF binding domain of both monomers, the EGFR dimerizes and forms a unified molecule. The c-terminal tails are then phosphorylated.
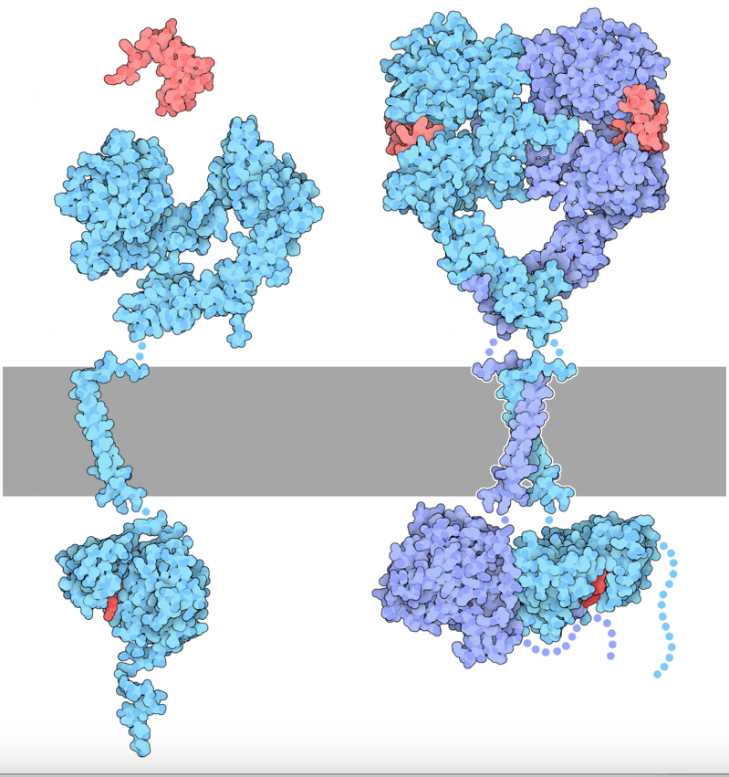
Figure 1. Structure of EGFR [8].
3. EGFR signaling pathway
3.1. MAPK/ERK pathway
As shown in figure2, the Ras/Raf/MAPK pathway conducts signals from outside the cell to the cell nucleus, and transcription is activated for cell growth. The MAPK pathway starts as the TK domain of EGFR is phosphorylated. The adaptor protein Grb2 then are able to bind to the phosphorylated c-terminal tail, allowing SOS protein to bind to the complex. The active site of SOS protein then recruits and activates Ras protein by interchanging a GDP molecule of Ras protein for a GTP molecule. The activated Ras protein then activates the RAF kinase. The RAF kinase phosphorylates and activates MEKs. The MEK then phosphorylates and activates ERK-1/2, as known as mitogen-activated protein kinase (MAPK). Activated MAPK enters the cell nucleus and stimulates the activities of several transcription factors. As a result, the protein production is increased, which further leads to cell proliferation [9].
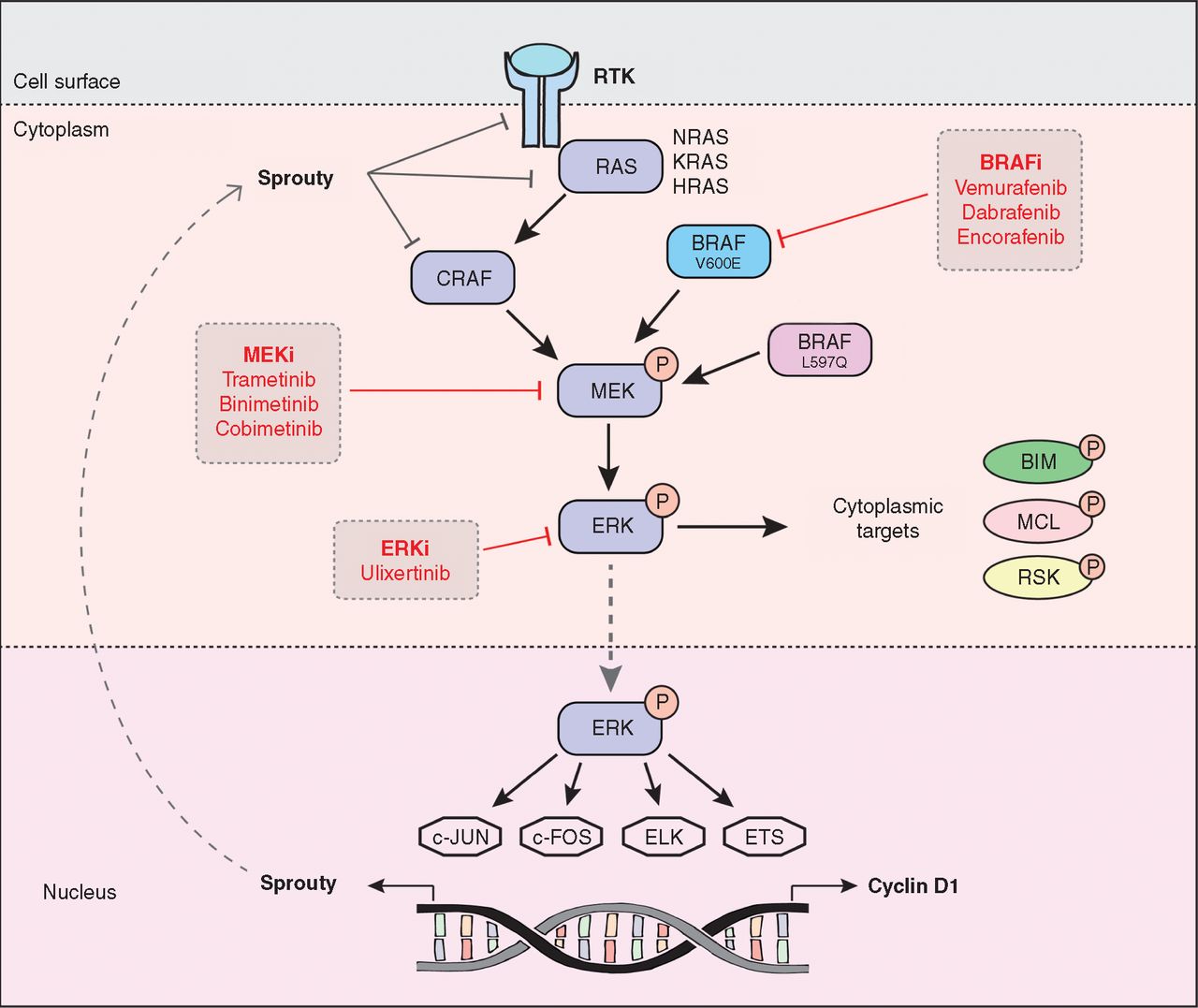
Figure 2. The MAPK/ERK Signaling Pathway [9].
3.2. PI3k/Akt/mTOR pathway
As shown in figure 3, the Phosphoinositide 3-kinase (PI3K)/Akt/mTOR pathway regulates cell metabolism, protein production, and angiogenesis. Same as the MAPK pathway, the PI3K pathway is initiated by the phosphorylation of the TK domain of EGFR. This binds to and phosphates the adaptor protein insulin receptor substrate (IRS) protein. The activated site of IRS then triggers the activation of the p85 subunit and p110 subunit of PI3K. The activated PI3K converts PI2 to PIP3. PIP3 interacts with membrane protein 3-phosphoinositide-dependent protein kinase 1 (PDK1) and PDK2, which drives the recruitment of Akt. Akt has three domains: PH domain, kinase domain and regulatory domain. The PDK1 and PDK 2 protein phosphorylate tyrosine 308 on the kinase domain and serine on the regulatory domain, activating Akt. Activated Akt can translocate from cell membrane to the cytoplasm and nucleus, activating mTOR, which is a significant regulatory molecule that could increase the expression of the growth factors and proteins, driving the replication of DNA. The PI3K/AKT/mTOR signaling pathway can then regulate cell proliferation, division, and differentiation [10].
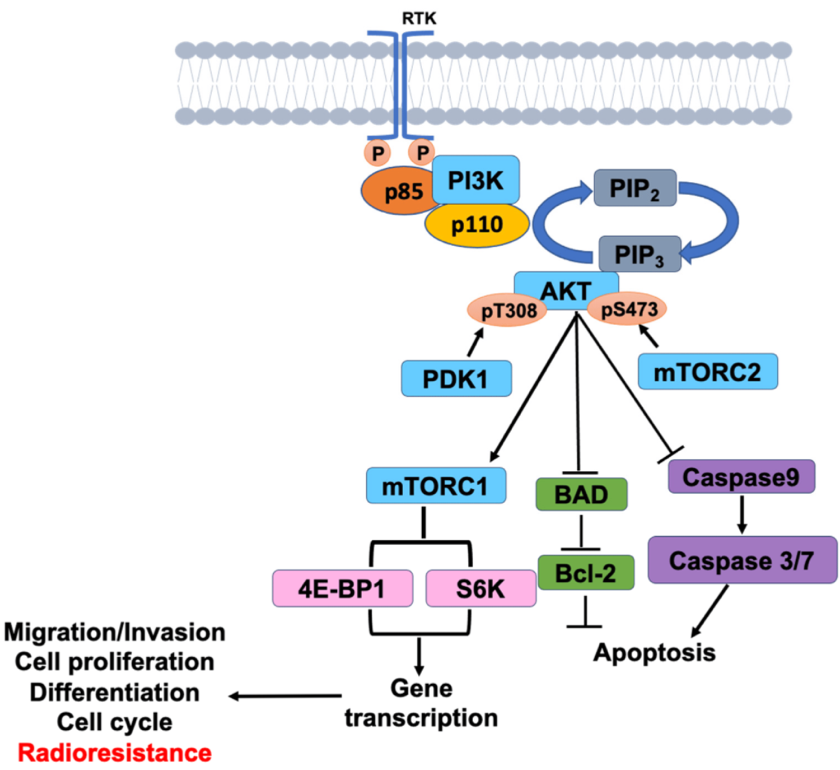
Figure 3. The PI3K/ART/mTOR Signaling Pathway [11].
3.3. JAK/STAT pathway
As shown in figure 4, the Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway regulate immunity, cell division and cell death. JAK interacts with and is phosphorylated by hormone, cytokine and growth factor receptors, including EGFR. Activated JAK proteins then phosphorylate STAT proteins. Once phosphorylated, STAT proteins dissociate from the receptor and form homodimers or heterodimers in the cytoplasm through SH2-domain–phosphotyrosine interactions. The STAT dimers then are able to move into the nucleus, where they regulate transcription processes [12]. Intriguingly, phosphorylated JAK can interact with and activate PI3K and Ras. The interaction of signaling pathways composes a signaling networks that influence each other.
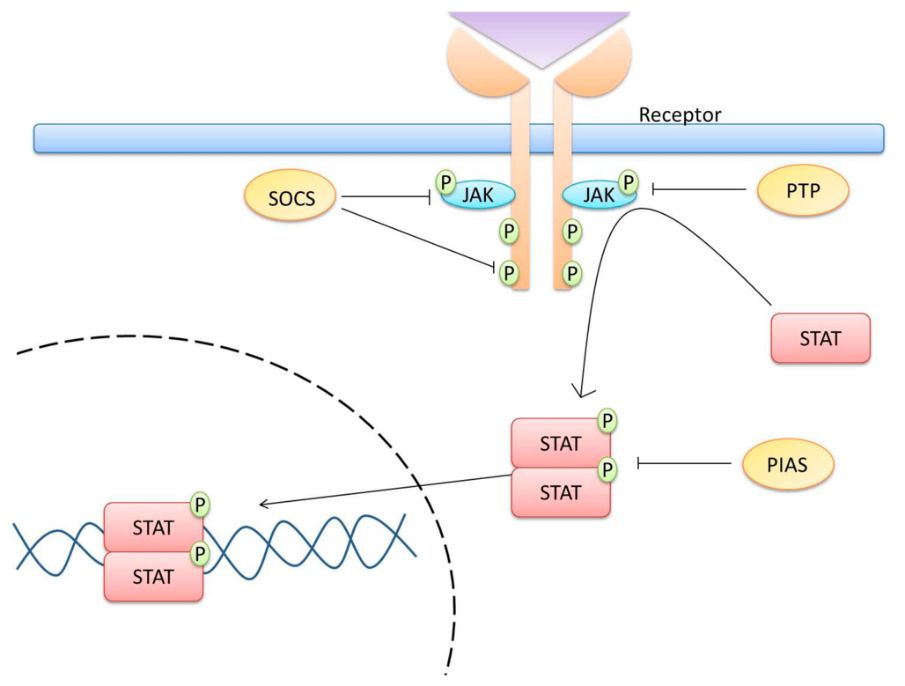
Figure 4. The JAK/STAT Signaling Pathway [13].
4. Typical EGFR mutations
Common EGFR mutations are EGFR L858R mutation and EGFR del19 mutation, which accounts for 85% of the EGFR mutation. The L858R mutation is a single nucleotide mutation in the exon 21 in the TK domain of EGFR gene. This mutation leads to a missense mutation -a nucleotide change from thymine to guanine, which further influences the protein synthesis substituting leucine to arginine at codon 858. The L858R mutation increases the activity of the EGFR kinase. Research has shown that L858R mutated EGFR kinase is fifty times more active than a normal EGFR kinase due to the disruption of stabilization of the inactive EGFR monomers, so that kinase maintains in a constitutively active state. EGFR L858R mutation also enhances the binding of inhibitors to the kinase, resulting induced cell invasion and metastasis. EGFR del19 mutation is caused by the deletion in exon 19, which destabilizes the inactive EGFR monomeric structure, leading increased receptor dimerization. Del19 mutation also locks the kinase domain in an active conformation, causing the continuous activation of EGFR signaling pathways and cell proliferation [14].
5. Current treatment strategies
The current treatment for lung cancer caused by EGFR mutation includes but is not limited to surgery, radiotherapy, chemotherapy, targeted therapy, immunotherapy and combination therapy. To this article's extent, targeted therapy will be primarily discussed due to the particularity of EGFR mutation. EGFR tyrosine kinase inhibitor (TKI) is a type of targeted therapy that is used as a treatment for NSCLC. TKI can specifically inhibit the TK domain of EGFR in order to prevent tumor cell growth. TKI has three generations along development, each with different characteristics [15]. The choice of TKI often depends on the patient's condition and resistance to the medicine. Figure 5 shows the chemical structures of TIKs.
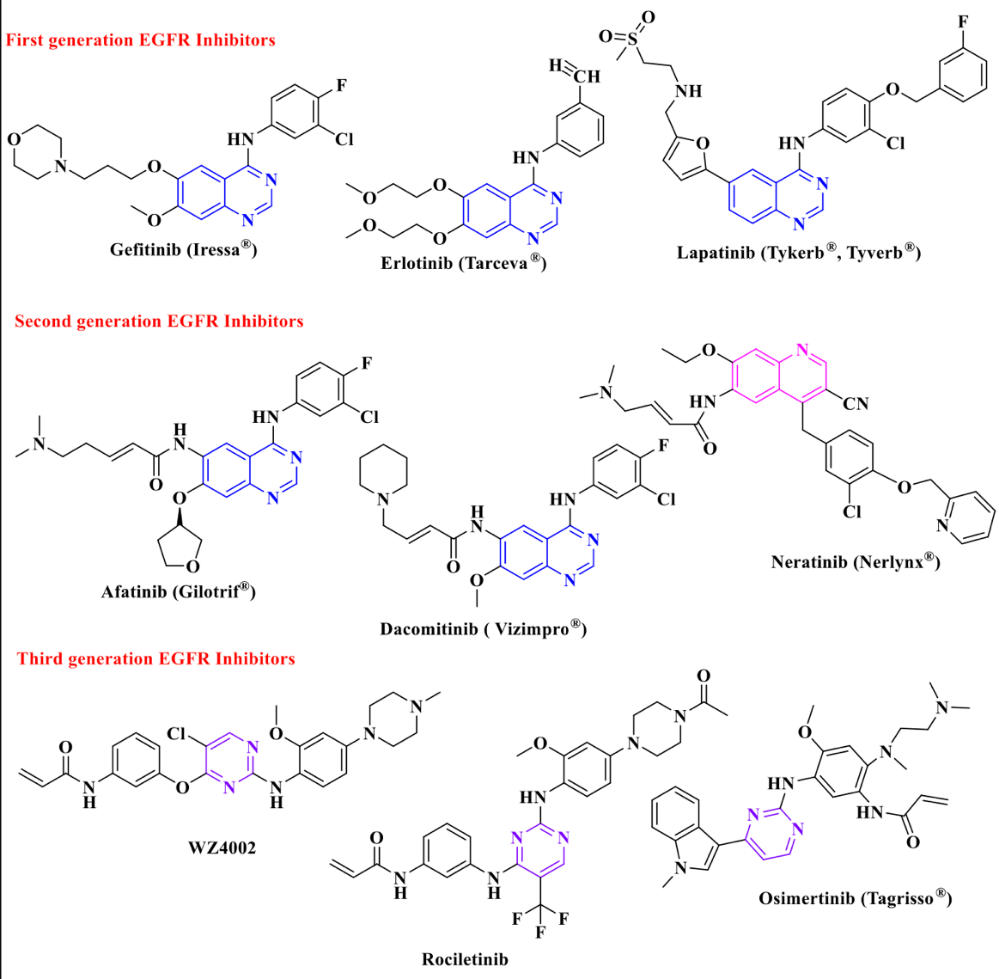
Figure 5. Chemical Structures of TIKs [15].
5.1. First generation EGFR TKI
First generation TKIs, such as gefitinib, lapatinib, and erlotinib, have been used extensively in the treatment of NSCLC. The first generation TKIs stick to the EGFR TK domain reversibly and inhibit the binding of ATP to the TK domain in order to interrupt cell proliferation and stimulate apoptosis, inhibiting the growth of cancer cells. However, majority of the NSCLC patients have grown resistant to the first-generation TKIs and the chance of recurrence is usually high due to the point mutation of T790M, most commonly.
5.2. Second generation EGFR TKI
Dasatinib, nilotinib, and afatinib are typical second generation TKIs. Second generation TKIs were mainly developed to overcome the resistance that occurs in first-generation TKIs. Second generation TKIs are generally more potent and can achieve quicker responses. Different to the first generation, second generation TKIs bind to the TK domain irreversibly, overcoming T790M or other secondary mutations caused by the resistance of first generation TKIs. However, the efficacy is limited because of the dose-limited toxicity, and it has a higher risk of side effects, particularly cardiovascular complications. Therefore, the use of second-generation TKIs is often more nuanced and may depend on patient’s age and medical condition [16].
5.3. Third generation EGFR TKI
The third generation TKIs are the most recent treatment strategy. Typical drugs approved by FDA are ponatinib and osimertinib, in which osimertinib is the most commonly used medicine in the treatment of NSCLC. They are particularly effective against both unmutated EGFR and T790M resistance variants. However, acquired resistance to third generation TKIs also occurs in clinical. The C797S mutation in exon 20 is a typical and representative resistance mutation identified. The C797S mutation eliminates the binding ability of TKIs, leading to resistance. On the other hand, the efficacy of third generation TKIS is also limited to patients who have already developed resistance to the first and second generations of TKIs [7].
In conclusion, EGFR TKIs are relatively more selective and effective treatment of NSCLC. Regarding toxicity, the third generation TKIs have shown a decreased rate of severe morbidity compared to several first and second generation TKIs [17]. However, imatinib, a first-generation TKI, has shown significantly lower toxicity, and it may be a better option for NSCLC patients with comorbidities [18]. Efficacy wise, the third generation TKIs are more effective than earlier generations due to the new mechanisms against cancer cell gene mutations. However, resistance development has been shown in all generations of TKIs. The tumor cell gene mutates all the time. Novel medicines and treatments are needed to be done as soon as possible.
5.4. TKI Resistance in lung cancer
Resistance to TKIs is a crucial obstacle for researchers to develop new generation TKIs or other types of targeted drugs. Two types of TKI resistance are commonly seen in the literature: intrinsic and acquired. Around 25% of the cases are intrinsic resistance due to the lack of initial response to TKI treatment of the patient, while acquired resistance is caused by the development of resistance a period after the treatment. Acquired resistance can occur due to secondary alterations within the gene, which detaches the previous inhibitor from the receptor, resulting reactivation of the signaling pathway or activation of alternative signaling pathways [19].
The T790M mutation in the EGFR gene is one of the most common causes of resistance. A missense mutation changes methionine to threonine at site 790 in exon 20 of the EGFR gene, which decreases the efficacy of the treatment by increasing the ATP affinity to a level that more closely resembles wild-type EGFR. Approximately 60% of recurrences of cancer are caused by T790M mutation [14].
The C797S mutation is another common resistance mechanism, particularly to osimertinib. C797S mutation occurs as the cysteine at codon is substituted by serine, invalidating the binding between osimertinib and EGFR, thereby conferring resistance to the drug. As mentioned, the emergence of the C797S mutation can result in resistance to all current EGFR TKIs, but most commonly happens to third generation TKIs such as osimertinib [20].
5.5. Fourth generation TKIs
The fourth generation TKIs are new class of medicine developed to overcome resistance to earlier generations of TKIs. BT-176, OBX02-011, BBT-207, BDTX-1535, BLU-945, and Bi-732 are several drugs that are being developed and have shown promising efficacy in preclinical trials. Most of them have shown high potentials in selecting and overcoming resistance caused by C797S mutation [21].
5.6. Immunotherapies
Immunotherapy combined with TKIs is also a promising solution to the high resistance rate of TKIs. Immunotherapy can help TKI to function even under high resistance due to the tumor mutation. The combination of Afatinib and cetuximab, EAI045 and cetuximab, Brigatinib and cetuximab are widely researched [8].
6. Conclusion
As the technology progresses, novel treatments, including targeted therapy, have been developed. Understanding more about the function of EGFRs and EGFR signaling pathway machinery can lead to more approaches to treating lung cancer. Currently, TKI resistance is a crucial challenge in treating lung cancer. Understanding the molecular and genetic mechanisms of TKI resistance is the key to the development of novel therapeutic strategies. In some cases, reintroducing first-generation TKIs has shown to be effective in cases of T790M loss. However, this approach is still under investigation, and more clinical trials are needed to ensure the result. On the other hand, further research of new drugs and combinatorial therapies is required to improve lung cancer treatment outcomes.
References
[1]. Xia C et al 2022 Cancer statistics in China and United States, 2022: Profiles, trends, and determinants Chinese Med J-Peking 135 5 pp 584–590
[2]. Siddiqui F et al 05 Sep 2023 2023 Lung Cancer, National Library of Medicine.
[3]. NHS 05 Sep 2023 2022 Lung cancer NHS choices.
[4]. Centers for Disease Control and Prevention 05 Sep 2023 2023 What is lung cancer?
[5]. O’Leary C et al. 2020 ‘(EGFR)-mutated Non-Small-Cell Lung Cancer (NSCLC) Pharmaceuticals 13 10 p 273
[6]. American Lung Association 04 Sep 2023 2022 EGFR and lung cancer American Lung Association
[7]. Singh M et al 2018 Targeting non-small cell lung cancer with small-molecule EGFR tyrosine kinase inhibitors Drug Discov Today 23 3 pp 745–753
[8]. Goodsell D 15 Sep 2023 2010 PDB101: Molecule of the month: Epidermal growth factor RCSB
[9]. Smalley I et al 2018 ERK inhibition: A new front in the war against MAPK pathway–driven cancers? Cancer Discov 8 2 pp 140–142
[10]. Yang J et al 2019 Targeting PI3K in cancer: Mechanisms and advances in clinical trials Mol Cancer 18 1
[11]. Su Y-C et al 2022 Targeting PI3K/AKT/mtor signaling pathway as a radiosensitization in head and neck squamous cell carcinomas Int J Mol Sci 23 24 p 15749
[12]. Erdogan F et al 2022 Jak‐Stat Core Cancer pathway: An integrative cancer interactome analysis J Cell Mol Med 26 7 pp 2049–2062
[13]. Gutiérrez-Hoya A et al 2020 Role of the JAK/STAT pathway in cervical cancer: Its relationship with HPV E6/E7 oncoproteins Cells 9 10 p 2297
[14]. Li Y et al 2023 ‘Toward the next generation EGFR inhibitors: An overview of osimertinib resistance mediated by EGFR mutations in non-small cell lung cancer Cell Commun Signal 21 1
[15]. Othman I M M et al 2021 New pyrimidine and pyrazole-based compounds as potential EGFR inhibitors: Synthesis, anticancer, Antimicrobial Evaluation and Computational Studies Bioorg Chem 114 p 105078
[16]. Westover D et al 2018 Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors Ann Oncol 29 pp i10–i19
[17]. Hao Y et al 2023 Comparison of efficacy and safety of second‐ and third‐generation for non‐small‐cell lung cancer with uncommon mutations Cancer Med 12 15 pp 15903–15911
[18]. Vener C et al 2020 First-line Imatinib vs second- and third-generation TKIS for chronic-phase CML: A systematic review and meta-analysis Blood Adv 4 12 pp 2723–2735
[19]. Cooper A J et al 2022 Third-generation EGFR and ALK inhibitors: Mechanisms of resistance and management Nat Rev Clin Oncol 19 8 pp 499–514
[20]. Osoegawa A et al 2021 High incidence of c797s mutation in patients with long treatment history of EGFR tyrosine kinase inhibitors including osimertinib JTO Clinical and Research Reports 2 7 p 100191
[21]. Lim SM et al 2023 BBT-176, a Novel Fourth-Generation Tyrosine Kinase Inhibitor for Osimertinib-Resistant EGFR Mutations in Non-Small Cell Lung Cancer. Clin Cancer Res Aug 15 29 16 3004-3016
Cite this article
Zhao,S. (2023). Progress on the EGFR pathway drives targeted therapy for NSCLC. Theoretical and Natural Science,17,280-287.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Xia C et al 2022 Cancer statistics in China and United States, 2022: Profiles, trends, and determinants Chinese Med J-Peking 135 5 pp 584–590
[2]. Siddiqui F et al 05 Sep 2023 2023 Lung Cancer, National Library of Medicine.
[3]. NHS 05 Sep 2023 2022 Lung cancer NHS choices.
[4]. Centers for Disease Control and Prevention 05 Sep 2023 2023 What is lung cancer?
[5]. O’Leary C et al. 2020 ‘(EGFR)-mutated Non-Small-Cell Lung Cancer (NSCLC) Pharmaceuticals 13 10 p 273
[6]. American Lung Association 04 Sep 2023 2022 EGFR and lung cancer American Lung Association
[7]. Singh M et al 2018 Targeting non-small cell lung cancer with small-molecule EGFR tyrosine kinase inhibitors Drug Discov Today 23 3 pp 745–753
[8]. Goodsell D 15 Sep 2023 2010 PDB101: Molecule of the month: Epidermal growth factor RCSB
[9]. Smalley I et al 2018 ERK inhibition: A new front in the war against MAPK pathway–driven cancers? Cancer Discov 8 2 pp 140–142
[10]. Yang J et al 2019 Targeting PI3K in cancer: Mechanisms and advances in clinical trials Mol Cancer 18 1
[11]. Su Y-C et al 2022 Targeting PI3K/AKT/mtor signaling pathway as a radiosensitization in head and neck squamous cell carcinomas Int J Mol Sci 23 24 p 15749
[12]. Erdogan F et al 2022 Jak‐Stat Core Cancer pathway: An integrative cancer interactome analysis J Cell Mol Med 26 7 pp 2049–2062
[13]. Gutiérrez-Hoya A et al 2020 Role of the JAK/STAT pathway in cervical cancer: Its relationship with HPV E6/E7 oncoproteins Cells 9 10 p 2297
[14]. Li Y et al 2023 ‘Toward the next generation EGFR inhibitors: An overview of osimertinib resistance mediated by EGFR mutations in non-small cell lung cancer Cell Commun Signal 21 1
[15]. Othman I M M et al 2021 New pyrimidine and pyrazole-based compounds as potential EGFR inhibitors: Synthesis, anticancer, Antimicrobial Evaluation and Computational Studies Bioorg Chem 114 p 105078
[16]. Westover D et al 2018 Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors Ann Oncol 29 pp i10–i19
[17]. Hao Y et al 2023 Comparison of efficacy and safety of second‐ and third‐generation for non‐small‐cell lung cancer with uncommon mutations Cancer Med 12 15 pp 15903–15911
[18]. Vener C et al 2020 First-line Imatinib vs second- and third-generation TKIS for chronic-phase CML: A systematic review and meta-analysis Blood Adv 4 12 pp 2723–2735
[19]. Cooper A J et al 2022 Third-generation EGFR and ALK inhibitors: Mechanisms of resistance and management Nat Rev Clin Oncol 19 8 pp 499–514
[20]. Osoegawa A et al 2021 High incidence of c797s mutation in patients with long treatment history of EGFR tyrosine kinase inhibitors including osimertinib JTO Clinical and Research Reports 2 7 p 100191
[21]. Lim SM et al 2023 BBT-176, a Novel Fourth-Generation Tyrosine Kinase Inhibitor for Osimertinib-Resistant EGFR Mutations in Non-Small Cell Lung Cancer. Clin Cancer Res Aug 15 29 16 3004-3016









