1. Introduction
Type 2 Diabetes Mellitus (T2DM) is a paramount health worry worldwide with millions of people suffering due to this condition. In T2DM patients either the body fails to produce ample insulin or cannot utilize it effectively leading to heightened blood sugar levels. The incidence of T2DM has substantially increased over recent years due to various factors like sedentary lifestyles obesity alongside genetic factors that contribute to the disease. Resultantly there are severe complications like nerve damage and cardiovascular diseases.
This research intricately explores T2DMs pathophysiology encompassing all relevant aspects as disease causes, symptoms, prevention strategies and available treatment options in detail while highlighting the heterogeneity of this disease. The primary objective aims at early diagnosis and emphasizes lifestyle modification as a cornerstone for better disease management. Furthermore we studied various current treatments methods that not only increases insulin sensitivity but also prevents progressive pancreatic β cell failure traits- distinctive characteristics in most Type 2 Diabetes Mellitus sufferers. Despite its severe global public health implications there is yet a substantial knowledge gap in fully understanding its underlying pathophysiology^1 as well as implementing effective prevention/therapy strategies; thus our research study undertakes the task towards bridging these gaps bringing us one step closer towards mitigating Type 2 Diabetes Mellituss impact on peoples daily lives.
As per a recent IDF report around 463 million adults aged between 20 79 years globally were living with diabetes in 2019; one third were undiagnosed—implying nearly around 232 million people worldwide. Furthermore over 90% of these cases consisted of persons diagnosed with Type 2 Diabetes Mellitus (T2DM) one among three primary types alongside Type 1 or gestational diabetes. A novel study predicted an alarming increase of approximately592 million diabetics globally by2035 particularly due to type 2 diabetes mellitus indicating an urgent need for greater control mechanisms [1]. The increasing prevalence of diabetes mellitus particularly T2DM is predominantly due to modern lifestyle choices, such as easy access to high calorie foods and limited opportunities for exercise owing to an extensive reliance on cars or television watching- habits [2] that coincide with several risks factors for T2DM like adiposity or obesity (BMI≥30 kg/m2) sedentary behavior whilst experiencing poor sleep patterns [3]. Adiposity creates metabolic abnormalities leading to insulin resistance wherein there exists an inverse linear relationship between BMI and age at diagnosis [3]. Multiple pathways underlie this pathological process, involving both cell-autonomous mechanisms and inter-organ communication [4].
Its worth mentioning that although environmental factors have an essential role in developing T2DM in individuals Genetically inherited genes are also crucial; polygenic features play a vital part- most loci increase insulin secretion risk while some decrease insulin action in this type of diabetes [4]. The chance of siblings having the disease is higher when two siblings are affected rather than one This genetic information should be considered as only providing glucose intolerance prevention rather than definite progression towards body system derangement mainly depending on external aspects mentioned above.
2. Pathophysiology of T2DM
The root causes of type 2 diabetes mellitus (T2DM) are primarily attributed to highly inefficient pancreatic β cell secreted insulin alongside reduced sensitivity to insulin amongst tissues sensitive to it.
Before looking into specific mechanisms linked with these two factors lets first examine the beta cell physiology briefly. These β cells synthesize pre-proinsulin which undergoes a conformational change aided by several proteins within the endoplasmic reticulum (ER) when maturing towards proinsulin production detailed under (Figure 1).
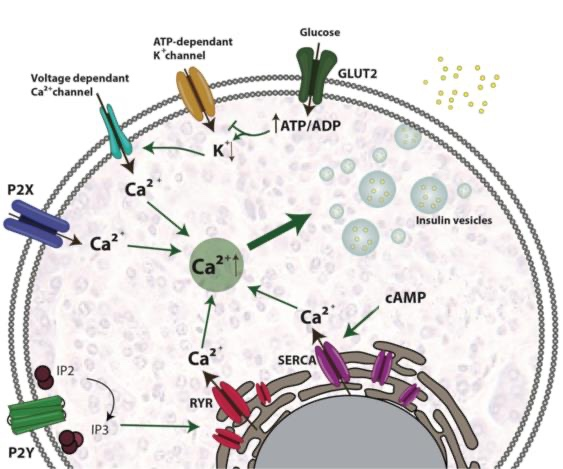
Figure 1. Beta-cell physiology [4].
After this transition phase proinsulin gets transported from the ER into immature secretory vesicles within the Golgi apparatus (GA) splitting into C peptide and insulin. Insulin once mature is stored in granules until release becomes triggered primarily due to high levels of glucose although other factors like amino acids, fatty acids, or hormones can also stimulate it. When glucose levels surge in circulation β cells directly take up glucose by means of GLUT2 transporter acting as a glucose sensor. Glucose metabolism induces ATP/ADP ratio build up within cell space that consequently leads to a halting of ATP dependent potassium channels on plasma membranes due to membrane depolarization and opening up voltage dependent Ca2+ channels resulting in Ca2+ influx. Elevated intracellular Ca2+ concentrations trigger fusion of storage vesicles containing insulin with plasma membranes ultimately leading to insulin exocytosis.
Due to physical inactivity and obesity mainly characterized by an impairment affecting insulins function towards normal glucose uptake or a restraint of liver based glucose production - certain individuals experience insulin resistance manifesting long before T2DM onset. Once tissues display insulin resistance there occurs a feedback mechanism stimulating typically normal islet cells signaling them that elevated amounts insulin are required (Figure 2).
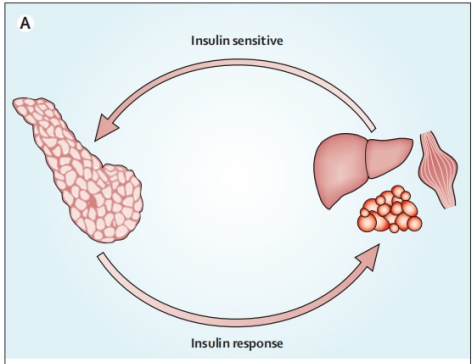
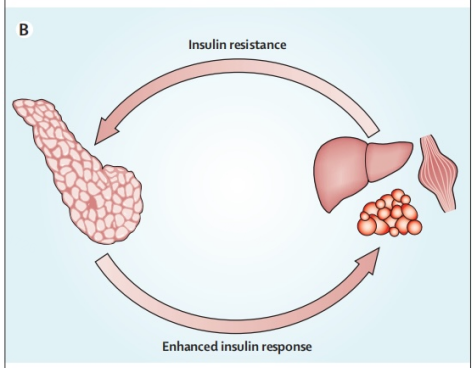
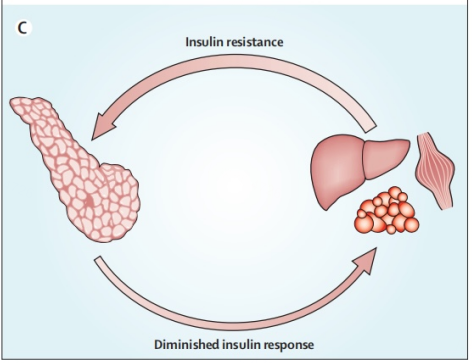
Figure 2. Feedback loop [5].
However when pancreatic β-cells cannot manage compensations attributed with insulin resistance this marks the point in which type 2 diabetes mellitus (T2DM) disease manifestation commences. In essence β-cell dysfunction or even failures occur due to primarily hyperinsulinaemia; it's sustainable compensation through exhaustion leads to existing inefficiency with production of insulin output necessary for adequate functioning. This phenomenon often occurs within an excessive nutritional state that mirrors obesity and is characterized by symptoms including hyperglycemia and hyperlipidemia, which lead one towards a more predisposed state towards chronic inflammation [5]. Genetic differences signify that some β-cells may be more vulnerable to toxic pressures induced from inflammation stress, metabolic/oxidative stress, ER stress or amyloid stress; processes that have a potential risk of leading towards loss in integrity with respect to islet cells. For instance oxidative stresses induced by excess lipids and sugar (hyperglycemia /hyperlipidemia) leads to ROS generation that inhibits calcium ion mobilization whilst activating proapoptotic signals; additionally an increased amount of Free Fatty Acids (FFAs) or elevated glucose-cascade activities overflows resulting in ER stress unforlded protein responses induces apoptosis [2].
Outside of the scope β-cell dysfunction another consequential variable can be observed based on lower masses attributed amongst affected cells (assumedly from increased apoptisis). Results noted amongst a group weight-matched participants containing both those affected and unaffected with type-2 diabetes indicated reductions up-to 60% between affected groups versus healthy counterparts; subjects exhibiting prediabetic traits showed almost up-t0 40% decreases of beta-cell mass as well [3]. The overall pathophysiology of T2DM is shown in Figure 3.
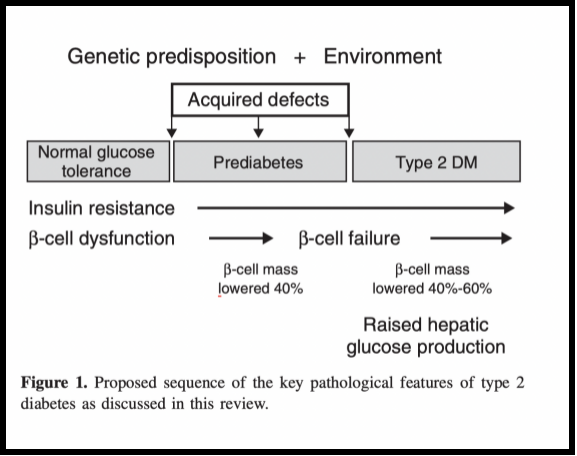
Figure 3. Key pathological features of T2DM [2].
3. Symptoms of T2DM
Type 2 diabetes disrupts normal biological functions, causing various microvascular issues that hamper day-to-day life in many ways. Factors such as high glucose levels over an extended period paired with genetics pose a severe risk during such complications like nephropathy and retinopathy. These issues reflect the gravity of unchecked high glucose levels on overall health. In addition to these implications, macrovascular challenges frequently arise among Type 2 diabetics -leading to peripheral vascular disease and related atherosclerosis risks [6].
Yet another obstacle faced by individuals with Type 2 diabetes is hypertension, which is visible two-three times more than usual following several causes - disruptions on innate circadian rhythms leading to intense nocturnal blood pressure troubles, disturbed blood flow autoregulation mechanisms; stiffness in large arteries triggering spikes in intracellular sodium concentrations; sensitivity impacts involving the hormone angiotensin II-while insulin resistance or endothelial dysfunction fuel this fire attributing core values involved sources ranging up from predisposition for obesity even further towards genetic makeup influence [3].
4. Prevention methods for T2DM
Although one permanently has their genetic makeup, environmental factors are adjustable in terms of preventing T2DM onset. Strategies such as identifying those considered with pre-diabetes for implementing lifestyle changes such as diet coupled properly-prescribed use of antidiabetic or anti-obesity medication come off greatly positive in addressing the ailment. Since at this early stage known as impaired glucose tolerance/ prediabetes reversing the effect on insulin resistance becomes particularly visible when getting patients back closer-than-ever-to-normal level blood glucose. Therefore, early awareness is beneficial [3].
5. Treatments
Treating type 2 diabetes requires effective means to boost insulin sensitivity while avoiding progressive pancreatic βcell failure along with inhibiting arising complications from this condition. Modified insulins have been created with promptly responsive qualities replicating post-meal insulin effects whilst having prolonged effects meaning less frequent administration undertakings [6].
Various drugs act on different body parts; classic organ systems corresponding to pancreas, liver, muscle & adipose tissue had been targeted for years but there has been significant recent focus on non-classic target areas like kidney, intestine & brain for treatment options.
Medications reliant on gastrointestinal functions include α-glucosidase inhibitors that slow down complex carbohydrate disintegration rate within gastrointestinal tracts leading to slower glucose absorption; pramlintide that slows down stomach emptying to reduce swift glucose absorption; bile-acid-binding resin colesevelam that intervenes in cholesterol levels & modifies release of other gastrointestinal peptides ensuing lower plasma glucose amounts [6].
Sodium-glucose co-transporter 2 (SGLT2) regulates glucose reabsorption from urine passages passing through S1 and S2 segments of proximal tubule.Inhibitors such as dapagliflozin and canagliflozin promote urinary glucose excretion reducing plasma glucose levels, blood pressure, and body weight. However, these drugs have side-effects such as a five-fold increase in the risk of genital mycotic infections, higher possibilities for lower urinary tract infections along with unexpected increments in LDL or HDL cholesterol levels leading to cardiovascular ailments. When considering prescription drugs intended to have an impact on the central nervous system bromocriptine comes to mind as an example of an approved option. Specifically acting as a dopamine receptor agonist, one way it can influence physiology includes restoring circular rhythm – thereby aiding with glucose metabolism regulation.
6. Conclusion
Type 2 Diabetes Mellitus represents an intricate medical challenge explored by scientists for decades now. In light of these investigations emerged various treatments and preventive measures that address different aspects of this complex disorder effectively. Successful preventative strategies primarily advocate lifestyle modifications such as dietary adjustments for healthier meal regimens; adequately engaging in physical activity; maintaining healthy body weight standards--all geared towards significantly reducing risks associated with this ailment's onset or progression. Notably too is early diagnosis during pre-diabetic stages where impaired glucose levels occur--an intervention observed to positively impact on its development trajectory. Approaches adopted for managing insulin sensitivity enhancement or stopping progressive pancreatic β-cell failure involve drug therapies ranging from variously adjusted insulins drugs targeting disparate organ systems on which T2DM acts. Innovative therapeutic techniques, such as gastrointestinal medication effect, Sodium-glucose co-transporter 2 inhibitors (SGLT2), and drugs that operate at the CNS level, signify exciting options for managing T2DM. Despite possible side effects, these treatment options demonstrate a great potential to positively reduce diabetes prevalence with significant reduction benefits achievable in the future by engaging research teams, healthcare providers and patients alike.
References
[1]. International Diabetes Federation. IDF Diabetes Atlas 6th Edition. IDF [online], (2013) http://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf .
[2]. DeFronzo, Ralph A. "Pathogenesis of type 2 diabetes mellitus." Medical clinics 88.4: 787-835 (2004).
[3]. DeFronzo, Ralph A., et al. "Type 2 diabetes mellitus." Nature reviews Disease primers 1.1: 1-22(2015).
[4]. Galicia-Garcia, Unai, et al. "Pathophysiology of type 2 diabetes mellitus." International journal of molecular sciences 21.17: 6275 (2020).
[5]. Kahn, Steven E., Mark E. Cooper, and Stefano Del Prato. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. The Lancet 383.9922: 1068-1083 (2014).
[6]. Lebovitz, Harold E. Type 2 diabetes: an overview. Clinical chemistry 45.8: 1339-1345 (1999).
Cite this article
Wang,M. (2023). The pathology of type 2 diabetes mellitus and relevant treatments. Theoretical and Natural Science,22,1-6.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. International Diabetes Federation. IDF Diabetes Atlas 6th Edition. IDF [online], (2013) http://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf .
[2]. DeFronzo, Ralph A. "Pathogenesis of type 2 diabetes mellitus." Medical clinics 88.4: 787-835 (2004).
[3]. DeFronzo, Ralph A., et al. "Type 2 diabetes mellitus." Nature reviews Disease primers 1.1: 1-22(2015).
[4]. Galicia-Garcia, Unai, et al. "Pathophysiology of type 2 diabetes mellitus." International journal of molecular sciences 21.17: 6275 (2020).
[5]. Kahn, Steven E., Mark E. Cooper, and Stefano Del Prato. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. The Lancet 383.9922: 1068-1083 (2014).
[6]. Lebovitz, Harold E. Type 2 diabetes: an overview. Clinical chemistry 45.8: 1339-1345 (1999).









