Alzheimer’s disease course prediction methods based on deep learning methods
Bingyi Zhang
School of Engineering Sciences, Huazhong University of Science and Technology, Wuhan, China
u202015904@hust.edu.cn
Abstract. Alzheimer 's disease (AD) is a primary disease of the nervous system with memory and cognitive impairment as the main symptoms. It is the most common type of senile dementia and seriously threatens the quality of life and life safety of the elderly. Due to the pathological characteristics of AD and the limitations of medical technology, there is currently no very effective treatment worldwide. Therefore, early prediction and diagnosis of AD has always been a research hotspot worldwide. Deep learning may finish the joint classification of several stages by mining the abundant information implicit in the visual data collected from patients. It is a hub for computer-assisted diagnosis research. Therefore, many studies have applied computer-aided methods such as deep learning to the medical image data of Alzheimer 's disease, so as to provide ideas and help for prediction and diagnosis of diseases. This paper analyzes and compares various network structures, summarizes the use of the current, comparatively new deep learning network models applied in the classification of Alzheimer's disease course, and discusses the difficulties and potential future research directions for deep learning in the field of AD diagnosis. Thus, it is very important to raise the efficiency of the clinical diagnosis of AD and the accuracy of early forecasts.
keywords: deep learning, Alzheimer’s disease, computer aided diagnosis.
1. Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease with cognitive dysfunction as the main clinical feature, and it is also a major challenge in the field of health care in the 21 st century. In 2018, the cost related to AD in the United States (including medical care, social welfare, and wage losses for patients’ families) reached $ 270 billion [1], seriously affecting the overall economy and putting enormous pressure on the U.S. health care system. AD is an irreversible and progressive brain disease characterized by cognitive decline. It is therefore essential to develop some strategies that can detect signs of the disease at an early stage in order to prevent or slow the progression of the disease [2, 3].
The traditional diagnostic methods mainly use doctors' professional knowledge and clinical experience to judge the influence of brain nerves. The diagnostic results depend on the professional quality of medical staff and the medical level of acquisition equipment, so it is inefficient and prone to missed diagnosis and misdiagnosis.
In recent years, many studies have been devoted to using computer algorithms such as machine learning and deep learning to assist in judging the course of AD. Using machine learning methods requires the definition of appropriate architecture design and preprocessing procedures [4]. Classification operations often require four steps: the extraction of features, the selection of features, the reduction of dimensions, and finally, feature-based classification algorithm selection. Compared with classical machine learning methods, one advantage of deep learning methods is that their reliability increases with the growth of the learning phase [5].
Deep learning is a method that automatically extracts and abstracts image features by constructing a deep network to classify and diagnose the three stages of subjects: normal control (NC), progressive mild cognitive impairment (MCI) and Alzheimer’s disease (AD). With the development of computer algorithms and technologies, deep learning is more and more widely used in the field of image processing and analysis. In particular, convolutional neural network (CNN ) can mine deep features of images through learning. Its various extended models have shown great application potential in the diagnosis and early prediction of AD patients [6].
This paper sorts out several latest deep learning networks extended from traditional CNN networks, introduces their principles and results, compares and analyzes them, and finally proposes possible future research and improvement directions.
2. Prediction of AD using DL
2.1. Recurrent neural network (RNN)
Recurrent neural network (RNN) is a learning method based on nonparametric sequence. The neurons in the hidden layer connect to transmit data, thus expressing the correlation between information. Because AD is a time-dependent neurodegenerative disease, RNN may obtain better prediction results than classical CNN networks since RNN can process time series data to a better extent.
LSTM is a special type of RNN. Its main idea is to introduce a gating unit into the standard RNN, which can not only solve the Vanishing gradient problem of traditional RNN, but also better analyze the temporal clinical characteristics of diseases, so as to achieve disease prediction.
The advantage of this method is that it can connect past information with current tasks; Due to the significant importance of patient time data in predicting the progression of AD, the article [7] selected LSTM as the AD prediction model.
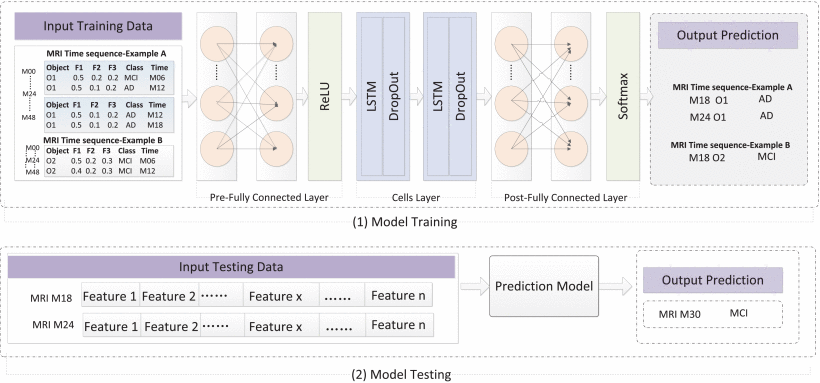
This article proposes an LSTM model that can learn long term dependencies and predict AD processes for 6 months by processing time series data. The structure of this model is shown in the following figure, including the pre-fully connected layer, the cell layer, and the post-fully connected layer, where the cell layer contains a layer of LSTM layer and a dropout wrapper. Figure 1 showed the structure of the proposed model.
Figure 1. The structure of proposed model [7].
This model was tested based on longitudinal MRI data (including 900AD, 900MCI, and 900NC) and compared with existing results. The AUCs that distinguish AD and NC, AD and MCI, were 0.935 and 0.798, respectively; But the AUC that distinguishes between NC and MCI is only 0.697, which is relatively low.
In another article [8], scientists propose a three-dimensional model based on bidirectional LSTM (BI-LSTM) plus attention mechanism. Biodirectional RNN was shown in the Figure 2.
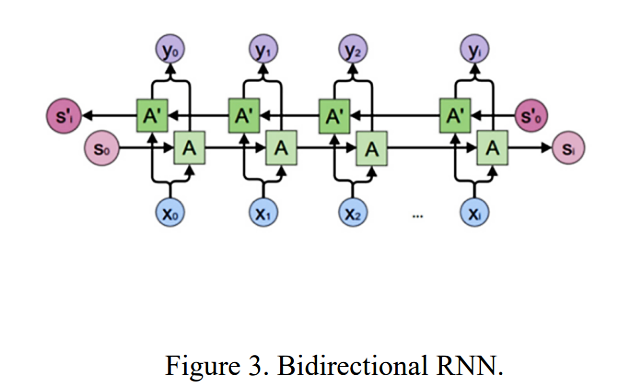
Figure 2. Biodirectional RNN [8].
The model takes patient first month, 6 months, and 12 months of CT scan data as sample input, and outputs classification predictions for NC, MCI, and AD development processes. Currently, ACC accuracy can reach over 90%. Among them, BI-LSTM proposes training sequences in both forward and backward directions, and both are linked to the output layer. Therefore, each node in input and output layers can all gain complete past as well as future information from this structure; The introduction of attention mechanism is to solve the problem of losing features in some long sentences or making short sentences mixed noise during the encoding and decoding process. However, the introduction of this mechanism will increase the computational complexity and training time of the model.
From this, it can be seen that the advantage of RNN method is that it fully extracts dynamic temporal features from follow-up data of patient time intervals and has certain data storage capabilities. However, this method also has unresolved issues, such as increasing the computational complexity of the model and thus increasing time consumption when applying follow-up data with long time intervals.
2.2. Automatic encoder (AE)
Automatic encoder is an unsupervised learning network composed of encoder and decoder; In the forward propagation process of the network, the encoder compresses and reduces the dimensionality of the feature information to remove redundant information and noise, and then the decoder reconstructs the input information through feature reconstruction; Finally, the disease progression is predicted using CNN based image information.
Article proposes a method that utilizes pre trained AE to extract image features from 3D input images [9], and then uses CNN to diagnose Alzheimer's disease. The accuracy of predicting NC/AD, MCI/AD, and MCI/NC states can reach 95%, 90%, and 92%, respectively. This method has high accuracy, but its sensitivity is lower compared to other works (92%, 94%, 86% for AD/NC, AD/MCI, MCI/NC respectively).
Article combines region of interests (ROI) information with original image to prevent loss of informative features [10]; Specifically, by registering the ROI features obtained from Automated Anatomical Labeling (AAL) with the original images of the relevant regions, a composite image is obtained. The composite image is then learned through the AE network, and the original image is learned through the CNN network. The characteristics of the two networks are combined after each convolutional layer to integrate the original image characteristics of MRI and PET with these ROI characteristics into one type of study. Figure 3 provides a proposed framework using convolutional AE and CNN to classify brain diseases.
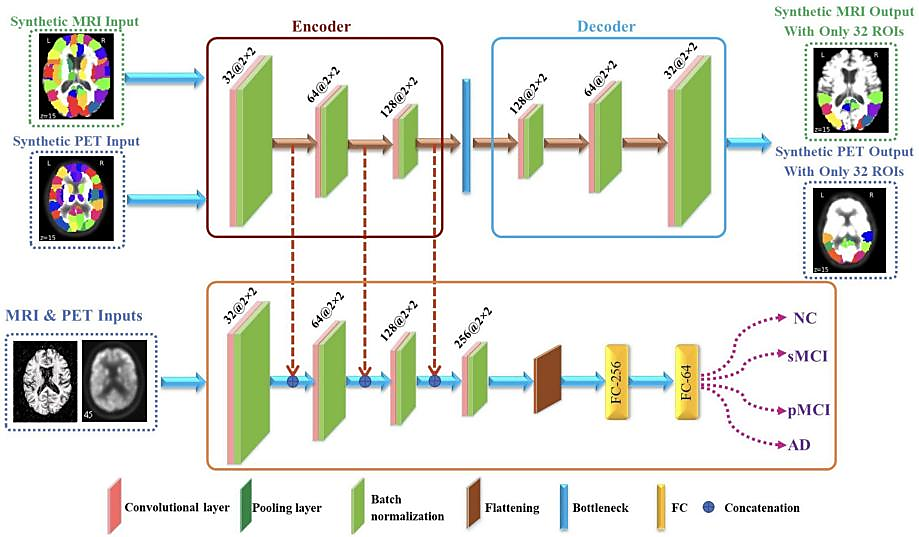
Figure 3. Convolutional auto-encoder and CNN-based proposed framework for classifying brain diseases [10].
The accuracy of this method in predicting NC/AD and MCI/AD in synthetic MRI and PET with 116 ROI features reached 97.2% and 92.31%, respectively. But this work only uses Neuroimaging data, ignoring genetic data; If neural imaging and genetic data are combined, the accuracy of AD diagnosis will be further improved; And this work did not consider the relationship between 116 ROI features.
It can be seen from the above methods that due to its Unsupervised learning; AE can solve the Hard problem of consciousness problem of model training caused by unlabeled images, and the AE stacking network can reduce the dimension of the original sequence, thus obtaining more effective feature information. However, the current difficulties of this method lie in the high computational complexity and difficulty in image registration when processing high-dimensional multimodal data, which often implies high computational complexity and long computational time.
2.3. Deep belief network (DBN)
The Deep belief network (DBN), similar to the above AE, is also a Unsupervised learning network, which consists of a visual layer, a classification layer, and several hidden layers. The hidden layer is composed of multiple Restricted Boltzmann machine (RBMs). There are symmetric connections between layers but no nodes in the layer. In the DBN training process, one layer of RBM is trained each time, and then the output of the current RBM is used as the input for the next layer of RBM training until all layers are trained. Finally, the walk sleep algorithm is used for tuning.
In article scientists proposes a multitask learning framework based on DBN, as shown in the Figure 4[11]. And introduce dropout technology and zero masking strategy to improve the robustness and generalization ability of the model. The model achieved 98.6%, 96.7%, and 91.9% accuracy in NC/AD, NC/pMCI, and pMCI/AD classification, respectively.
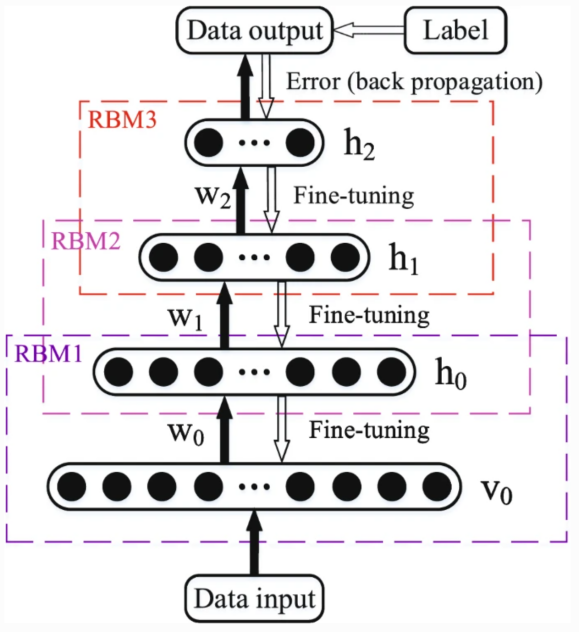
Figure 4. The schematic diagram of a DBN [11].
The advantage of the above model is that the learning method of this multi-task has the advantage of learning several related tasks at one time. Therefore, it can not only reflect the differences and links between the different tasks, but also improve the effectiveness of the learning model by summarizing the possible common characteristics of several tasks; And the RBM method of layer-by-layer training gives the whole DBN a better initial weight value, which solves the problem of slow Rate of convergence of the deep level neural network.
2.4. Combination of multiple networks
Multi network fusion is an architecture that combines two or more different types of networks, divided into cascading and integrating. Although single network deep learning methods have the advantages of fast training speed and low computational cost, they often cannot fully learn deeper data features for complex AD neural influence information. Therefore, in the past few years, many scientists have conducted research on multi network AD diagnosis. And, the base classifier structure was shown in the Figure 5.
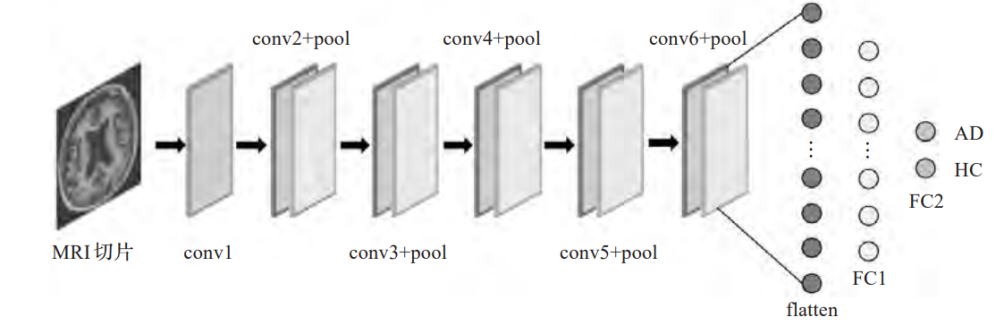
Figure 5. Base classifier structure [12].
In article, scientists select MRI 2D slices from three dimensional planes for training [12] and use the obtained CNN ensemble classifier for AD classification. This method can effectively utilize feature information from different directions in the same brain area.
There are also learning methods based on 3D image blocks, such as that in article [13]. Take 27 image blocks along the cross-sectional direction and input them into a multi-layer integrated 3D CNN network to extract features, with an NC/AD classification accuracy of 97.77%. These multi network integration methods can obtain more comprehensive information on brain tissue lesions, effectively solving the problem of losing details in a single network model; At the same time, the combination of multiple classifiers for Ensemble learning can prevent over fitting to a certain extent. However, this method also has certain problems, such as the integration of multiple brain region-based classifiers resulting in high computational and annotation costs, long computational time, and high difficulty in network design.
3. Discussion
The various networks of deep learning provide diverse and efficient network models for AD assisted diagnosis, achieving high-precision classification of the course of AD. This chapter will summarize the main ideas, advantages, and disadvantages of the above methods.
Recurrent neural network (RNN) mainly uses the interconnection of neurons in the hidden layer to transmit data and express the correlation between data. It can use the regular follow-up data of AD patients to establish a time correlation model. Its advantage lies in its ability to process random length time series information, and it can predict the progression of AD patients by analyzing clinical data on the time series; However, the problem lies in the high difficulty of training and implementation, and the large amount of computation and time when applying data with a long-time span.
Automatic encoder (AE) utilizes encoders and decoders to reconstruct input data, allowing complex and abstract features to be learned from brain image data without the need for precise label information. This feature can to some extent solve the problem of obtaining a large amount of annotated training data for functional imaging with a small clinical application range, but model design still faces the problems of complex calculation, long operation time, and difficult parameter adjustment.
The characteristic of Deep Confidence Network (DBN) is that its hidden layer is constructed by multiple RBMs, with symmetric connection constraints between each layer, and no connections among neurons within the layers. This structure improves the problems of limited medical annotation data and inaccurate annotation, but often requires multimodal complex problems based on MRI and PET. If the parameters are not selected appropriately, it will lead to difficulty in setting the optimal solution and low network classification accuracy.
Multi network fusion utilizes two or more networks to collaborate together, enabling multi-level feature fusion expression of data and further improving the accuracy of recognition and diagnosis. But its current problem lies in the high difficulty of network design, complex parameters, high computational requirements, and strong dependency correlation between networks.
Based on this, potential improvement directions for future deep learning in AD assisted diagnosis and prediction include:
1) The accuracy of multi classification diagnostic models in discriminating MCI is relatively low, and the differentiation and diagnosis of sMCI and pMCI are very important. The accuracy of NC/AD classification in existing studies can reach 99%, but the classification accuracy of sMCI and pMCI is generally low due to small differences between samples. In the future, the accuracy of early diagnosis of AD can be improved by fusing different biomarker data, specifically by adding other biomarkers such as clinical diagnostic data and gene data on the basis of neuroimaging.
2) At present, high-quality medical imaging data is scarce, so it is impossible to obtain enough image samples for training and testing. Since the Cranial nerves imaging data of AD has extremely high dimensions, complex structure, and the number of extracted features is often far greater than the number of samples, the lack of samples is likely to lead to over fitting of the model. In the future, we can further study the deep learning methods of high-order small samples, such as Unsupervised learning, Transfer learning, data augmentation, etc.
3) The current problem faced by multimodal models is the presence of semantic conflicts or missing modalities in multimodal data. For semantic conflict issues, algorithms can be continuously improved in subsequent research to capture hierarchical correlations between modalities and improve the fusion performance of feature fusion algorithms for complex data; In response to modal loss, the information complementarity between modalities can be utilized well, and the feature weights of rich and missing modalities in the network can be balanced.
4. Conclusion
In conclusion, the relevant work of deep-net models in the classification and diagnosis of Alzheimer's disease progression is presented and analysed. At present, due to the serious risk of Alzheimer's disease to the safety of elderly people worldwide, early prediction of Alzheimer's disease is the focus of attention in various countries around the world. Deep-net method offers many diverse and effective calculation models for the auxiliary diagnosis of Alzheimer's disease, including recurrent neural network (RNN), automatic encoder (AE), deep-trust network (DBN), multi-network fusion and other methods that can achieve AD disease course prediction and classification accuracy can reach 99%. Medical staff may use these computer classification forecasts for better patient care. In addition, the article presents some relevant research findings and progress on the above models and makes appropriate recommendations on current shortcomings. Finally, the article anticipates challenges in this area and future developments, providing references to researchers and developers of related technologies. I believe that the continuous optimisation and improvement of methods of deep learning will ensure more accurate and effective diagnostic methods for clinical diagnosis and early prediction of Alzheimer's disease at a later stage.
References
[1]. Alzheimer's Association. 2018 Alzheimer's disease facts and figures. Alzheimer's Dementia 14, 367–429. (2018)
[2]. Galvin, J. E. “Prevention of Alzheimer's disease: lessons learned and applied.” J. Am. Geriatr. Soc. 65, 2128–2133. (2017).
[3]. Schelke, M. W., Attia, P., Palenchar, D. J., Kaplan, B., Mureb, M., Ganzer, C. A., et al. “Mechanisms of risk reduction in the clinical practice of Alzheimer's disease prevention.” Front. Aging Neurosci. 10:96. (2018).
[4]. Lu, D., and Weng, Q. “A survey of image classification methods and techniques for improving classification performance.” Int. J. Remote Sens. 28, 823–870. (2007).
[5]. Shastry, K.A.; Vijayakumar, V.; V, M.K.M.; B A, M.; B N, C. “Deep Learning Techniques for the Effective Prediction of Alzheimer’s Disease: A Comprehensive Review.” Healthcare 2022, 10, 1842.
[6]. Lecun, Y., Bengio, Y., and Hinton, G. “Deep learning.” Nature 521:436. (2015).
[7]. Hong, X. et al., “Predicting Alzheimer’s Disease Using LSTM,” in IEEE Access, vol. 7, pp. 80893-80901, (2019)
[8]. Pan, Q., Wang, S. and Zhang, J. “Prediction of Alzheimer’s Disease Based on Bidirectional LSTM.” Journal of Physics: Conference Series. 1187. 052030. (2019).
[9]. Rohollah Hedayati, Mohammad Khedmati, Mehran Taghipour-Gorjikolaie, “Deep feature extraction method based on ensemble of convolutional auto encoders: Application to Alzheimer’s disease diagnosis”, Biomedical Signal Processing and Control, Volume 66, 102397, ISSN 1746-8094 (2021)
[10]. Abdelaziz M, Wang T, Elazab A. “Fusing Multimodal and Anatomical Volumes of Interest Features Using Convolutional Auto-Encoder and Convolutional Neural Networks for Alzheimer's Disease Diagnosis.” Front Aging Neurosci. 2022 Apr 28;14:812870.
[11]. Zeng, N., Li, H. & Peng, Y. “A new deep belief network-based multi-task learning for diagnosis of Alzheimer’s disease.” Neural Comput & Applic 35, 11599–11610 (2023).
[12]. Zeng A, Jia L, Pan D, Song X. “Early prognosis of Alzheimer's disease based on convolutional neural networks and ensemble learning”. Journal of Biomedical Engineering, 2019 Oct;36(5):711-719.
[13]. Raju, M., Gopi, V.P., Anitha, V.S. et al. “Multi-class diagnosis of Alzheimer’s disease using cascaded three dimensional-convolutional neural network”. Phys Eng Sci Med 43, 1219–1228 (2020).
Cite this article
Zhang,B. (2023). Alzheimer’s disease course prediction methods based on deep learning methods. Theoretical and Natural Science,22,157-163.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Alzheimer's Association. 2018 Alzheimer's disease facts and figures. Alzheimer's Dementia 14, 367–429. (2018)
[2]. Galvin, J. E. “Prevention of Alzheimer's disease: lessons learned and applied.” J. Am. Geriatr. Soc. 65, 2128–2133. (2017).
[3]. Schelke, M. W., Attia, P., Palenchar, D. J., Kaplan, B., Mureb, M., Ganzer, C. A., et al. “Mechanisms of risk reduction in the clinical practice of Alzheimer's disease prevention.” Front. Aging Neurosci. 10:96. (2018).
[4]. Lu, D., and Weng, Q. “A survey of image classification methods and techniques for improving classification performance.” Int. J. Remote Sens. 28, 823–870. (2007).
[5]. Shastry, K.A.; Vijayakumar, V.; V, M.K.M.; B A, M.; B N, C. “Deep Learning Techniques for the Effective Prediction of Alzheimer’s Disease: A Comprehensive Review.” Healthcare 2022, 10, 1842.
[6]. Lecun, Y., Bengio, Y., and Hinton, G. “Deep learning.” Nature 521:436. (2015).
[7]. Hong, X. et al., “Predicting Alzheimer’s Disease Using LSTM,” in IEEE Access, vol. 7, pp. 80893-80901, (2019)
[8]. Pan, Q., Wang, S. and Zhang, J. “Prediction of Alzheimer’s Disease Based on Bidirectional LSTM.” Journal of Physics: Conference Series. 1187. 052030. (2019).
[9]. Rohollah Hedayati, Mohammad Khedmati, Mehran Taghipour-Gorjikolaie, “Deep feature extraction method based on ensemble of convolutional auto encoders: Application to Alzheimer’s disease diagnosis”, Biomedical Signal Processing and Control, Volume 66, 102397, ISSN 1746-8094 (2021)
[10]. Abdelaziz M, Wang T, Elazab A. “Fusing Multimodal and Anatomical Volumes of Interest Features Using Convolutional Auto-Encoder and Convolutional Neural Networks for Alzheimer's Disease Diagnosis.” Front Aging Neurosci. 2022 Apr 28;14:812870.
[11]. Zeng, N., Li, H. & Peng, Y. “A new deep belief network-based multi-task learning for diagnosis of Alzheimer’s disease.” Neural Comput & Applic 35, 11599–11610 (2023).
[12]. Zeng A, Jia L, Pan D, Song X. “Early prognosis of Alzheimer's disease based on convolutional neural networks and ensemble learning”. Journal of Biomedical Engineering, 2019 Oct;36(5):711-719.
[13]. Raju, M., Gopi, V.P., Anitha, V.S. et al. “Multi-class diagnosis of Alzheimer’s disease using cascaded three dimensional-convolutional neural network”. Phys Eng Sci Med 43, 1219–1228 (2020).