1. Introduction
Endoplasmic reticulum is essential for the synthesis, folding, and modification of secretory and transmembrane proteins. Endoplasmic reticulum stress (ERS) is a significant accumulation of misfolded or unfolded proteins resulting from the impairment of this organelle's ability to fold proteins by a variety of extracellular and intracellular factors. Chronic endoplasmic reticulum stress and deficiencies in the unfolded protein response signaling pathway are rapidly being identified as essential components of human diseases such as diabetes, cancer, and neurodegenerative disorders. Consequently, the study of ER Stress has become an essential area of contemporary scientific inquiry. In multiple experimental models, a wide variety of organic and inorganic substances have been evaluated and investigated for their ability to alleviate ER stress [1].
Endoplasmic reticulum stress is an essential cellular defense mechanism shared by prokaryotes and eukaryotes. Comparable and relatively conservative in terms of evolution, both prokaryotes and eukaryotes utilize ER stress as a fundamental cellular defense mechanism [2]. Both excitation and disease play important roles in the metabolism and pathology of the entire cell and are intimately related. This paper employs the method of review and illustrates the fundamental information of the endoplasmic reticulum, one experiment about the ER Stress, the reaction mechanism of unfolded protein response (UPR), several diseases that are caused by the ER Stress, and the role of UPR in the diseases. ER Stress is the focus of journals, funding, drug research and development targets, and clinical research, and is associated with numerous diseases, tumor microenvironment, autophagy, and protein homeostasis, among others. This review contributes to the research in this field to some extent. This review provides extant research results in the field of ER Stress for future research to the following researchers by summarizing the findings of previous researchers. In addition, this review offers some instructive recommendations for future research into ER Stress.
2. An experiment related to ER stress
2.1. ER stress
In both Prokaryotes and Eukaryotes, ER is an organelle. There are two varieties of endoplasmic reticulum (ER): smooth endoplasmic reticulum (SER) and rough endoplasmic reticulum (RER). Both are attached to the outer membrane of the nucleus in the form of a flat, well-ordered membrane; the difference between them is that the letter's surface is covered with ribosomes. The largest cellular organelle, the ER, which is responsible for protein synthesis, folding, transport, and Ca2+ regulation, is vulnerable to an imbalance in its internal environment [3].
Under endoplasmic reticulum homeostasis, the BiP protein binds to the endoplasmic reticulum stress sensors, 3 ER transmembrane proteins including ATF6, IRE1, and PERK, and remains inactive. Under the condition of disease, misfolded and unfolded proteins accumulate, and BiP proteins have a higher affinity for misfolded/unfolded proteins, far exceeding the ability of molecular chaperon-assisted folding and the limit of the degradation system to remove the incorrect proteins. These ER sensors are released, activating ERS; in severe cases, this can also result in damage to other organelles, autophagy, and even cell death [4].
2.2. Experiment
The endoplasmic reticulum has a strict quality control system (ERQC) that ensures that only folded and modified mature proteins are captured by vesicles during the sorting process. The endoplasmic reticulum's capacity for quality control is limited [5]. Under normal circumstances, the quality control system is able to retrieve and identify endoplasmic reticulum proteins. When organisms are exposed to pathogens, drought, salinity, high temperature, frost, nutrient deficiency, and metal toxicity, however, they tend to produce more unfolded or misfolded proteins. In the investigation of ER Stress, some method of simulating ER Stress could be utilized. As shown in Figure 1, in the study of plant ERS response, the ER stress inducers tunicamycin (TM) and dithiothreitol (DTT) were primarily used to treat plants (cells) and induce ERS [6]. TM can inhibit both the N-glycosylation modification and the normal folding and maturation of proteins. DTT can deteriorate disulfide bonds in proteins and alter their REDOX status. Additionally, calnexin (calnexin) and calreticulin (CRT) activity may be altered, leading to ER stress, if the ER membrane calcium pump is inhibited and the ER calcium concentration is decreased.

Figure 1. A case study of endoplasmic reticulum stress under experimental conditions [4].
3. Methods to relieve ER stress-unfolded protein response(UPR)
The ERS consists primarily of three signaling pathways: the unfolded protein response, the endoplasmic reticulum excess response and the sterol cascade reaction. UPR is a cytoprotective mechanism activated by the dissociated receptor protein when ERS occurs. It is mediated by three endoplasmic reticulum transmembrane sensing proteins: inositol demand factor 1(IRE1), protein kinase R-like endoplasmic reticulum kinase (PERK) and activated transcription factor 6 (ATF6). As depicted in the images below, these three classical UPR pathways play a crucial role in mitigating ER Stress and preserving intracellular environment homeostasis. Now, the author would like to discuss the activation of these three pathways.
The UPR response can partially ameliorate the ER's burden and damage, restore the ER's protein homeostasis, and repair the ER's equilibrium. Processing the accumulated proteins in the ER, such as by increasing the expression of genes associated with folding enzymes or molecular chaperones (such as BiP), promotes protein folding in the ER. The ER-associated Protein Degradation System (ERAD) is activated in response to improperly folded proteins. The second is to reduce the rate at which mRNA is translated into proteins, thereby preventing further protein accumulation in the ER. UPR, a crucial adaptive cellular stress response [7], contributes to the development of medication and treatment resistance.
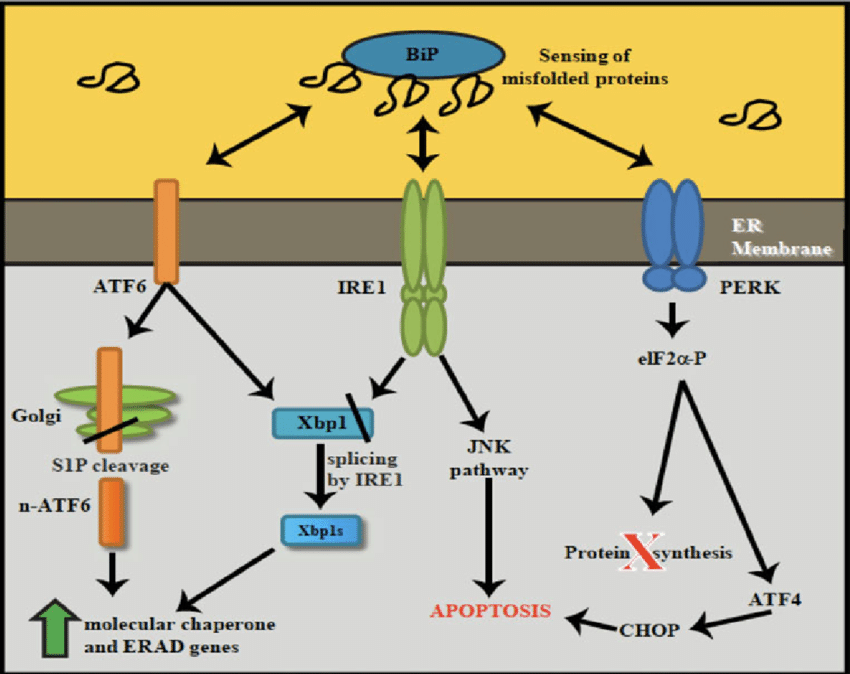
Figure 2. A schematic representation of UPR signaling pathway [7].
4. Endoplasmic reticulum apoptosis and diseases
Many diseases have been linked to apoptosis, which can be triggered by stress in the endoplasmic reticulum. Diseases of the nervous system, heart, blood vessels, and the immune system, as well as cancer and diabetes, are the primary targets of research [8].
Neurodegenerative disorders come first. The death of neurons is at the root of a group of diseases called neurodegenerative disorders. For instance, Alzheimer's illness has been associated to endoplasmic reticulum stress. Endoplasmic reticulum stress can contribute to the accumulation of A and Tau, two key pathogenic features of Alzheimer's disease.
In addition to Alzheimer's disease, Parkinson's disease (PD) is another prevalent neurodegenerative disorder that primarily affects the elderly (those over the age of 60) and typically manifests as tremors or clumsiness in one limb that eventually spread to the other. The main causes of the disease include aging, genetics, and the environment. Misfolding and aggregation of -synuclein in the cytoplasm are caused by these mechanisms [9]. Excessive stress causes death of dopaminergic neurons, while endoplasmic reticulum stress aids in the correct folding of unfolded and misfolded proteins, decreasing synthesis of insoluble -synuclein. Parkinson's disease is a result [8].
The second is heart disease. The most common cause of heart disease and stroke is atherosclerosis. Atherosclerosis has its pathological basis in abnormalities in lipid metabolism, and it manifests itself in the thickening and hardening of the artery wall and narrowing of the vascular cavity. Atherosclerosis lesions begin in the intima and are characterized by lipid and complex carbohydrate accumulation, bleeding, and thrombosis. Human and animal atherosclerotic lesions have been discovered to contain ERS markers, especially at more advanced stages of the illness, suggesting a role for ERS in atherosclerosis development [10].
The elevated blood sugar associated with the metabolic condition known as diabetes. Increasing evidence suggests that cell dysfunction driven by endoplasmic reticulum stress contributes to the onset and progression of diabetes mellitus. The endoplasmic reticulum homeostasis signaling pathway (ERS) plays a role in islet beta cell homeostasis. The breakdown of endoplasmic reticulum homeostasis can lead to islet beta cell death or an autoimmune reaction, both of which can result in diabetes, however moderate ERS pairings are helpful for restoring the intracellular environment. Insulin insufficiency is caused by a lack of beta cells, which is a hallmark of type 1 diabetes (T1DM). In type 1 diabetes, osmotic immune cells cause ERS in beta cells by the production of proinflammatory cytokines that destroy beta cells. In the autoimmune response that causes TIDM, nitric oxide (NO) is produced as an inflammatory mediator. Excessive levels of NO have been found to trigger apoptosis in cells in a CHOP-dependent manner [11]. As a result, NO is critically involved in T1DM+ cell death.
Finally, cancer is linked to ER stress as well. Cancer is a disease that develops when cells divide uncontrollably. Cancer cells prefer to thrive in high-stress, high-inflammatory environments. The intense conditions trigger a stress reaction in the ER, which is known as ER stress. These reactions set off a cascade of signaling channels that ultimately alter cellular metabolism and proliferation and promote tumor growth. For instance, there has been a lot of research focus on NASH-related liver cancer recently. ERS has been linked to steatosis, steatohepatitis, and nonalcoholic fatty liver disease in several studies. Other research has shown that steatohepatitis, which can raise the risk of liver cancer, can be caused by the combination of ERS and a high-fat diet. NASH may speed the development of liver cancer by increasing fat synthesis and activating the sterol-mediated element binding protein (SREBP) [12].
With the discovery of the components of the unfolded protein response (UPR) and the elucidation of its mechanisms, genetic studies have demonstrated that its signaling plays a crucial role in the pathogenesis of many diseases, particularly those involving highly secreted cell types. We discovered that PPP1R15A (an anta gonistic PERK phosphatase; also known as GADD34) protects renal tubules from ER stress-induced injury [13]. Recent research has linked UPR to the occurrence and progression of numerous diseases, including cancer, diabetes, neurodegenerative disorders, etc. The cells in tumor tissues are exposed to a severe environment, including hypoxia, nutrient deficiency, and acidity, as well as metabolic changes in tumor cells, damage to genome integrity, and peroxide accumulation, all of which can induce ER stress and UPR. According to studies, tumor cells utilize UPR to decrease endoplasmic reticulum pressure and aid cell survival [13].
5. Conclusion
Endoplasmic reticulum stress can be caused by an accumulation of folded or misfolded proteins in the endoplasmic reticulum as well as a disruption in cellular homeostasis. The primary effect of endoplasmic reticulum stress is apoptosis, which causes tissue and organ injury. In the current library of compounds for controlling ER stress and UPR, there are a number of useful tool compounds and even a few medications that have been approved for use in humans under a variety of clinical conditions, but there are still issues and limitations. This conclusively demonstrates the viability of revealing the pathogenesis, progression, and treatment mechanisms of diseases through the lens of endoplasmic reticulum stress. Cancer, cardiovascular disease, neurodegenerative disease, and metabolic disease are all closely associated with ERS, which is a common research hotspot. Numerous medications and substances have been studied as potential novel treatments for a variety of disorders, and the results of these studies have led to the development of new therapeutic options. Future research could lead to the discovery of additional effective treatments for endoplasmic reticulum stress-related diseases.
References
[1]. Krishna , P. m, sundhar, mohandas, & kunka , ramkumar Mohanram. (2022). Role of ER Stress Inhibitors in the Management of Diabetes. Sciencedirect, 50-55.
[2]. Hu, G., & Pan, W. (n.d.). (2022). Endoplasmic Reticulum Physiology and Pathology. ResearchGate, 77-78.
[3]. Yang, L., Huang, H., Wang, T., Zhou, L. D., Chen, Q., Li, D., Chen, Z. S., & Lin, P. (2023). Endoplasmic Reticulum-Targetable Selenium-Doped Carbon Nanodots with Redox-Responsive Fluorescence for in Situ Free-Radical Scavenging in Cells and Mice. ResearchGate,23-25.
[4]. Wan S , Jiang L . (2016). Endoplasmic reticulum (ER) stress and the unfolded protein response (UPR) in plants. Protoplasma, 253(3):753-764.
[5]. YANG Zheng-ting, LIU Jian-xiang. (2016). Endoplasmic Reticulum Stress Response in Plants. Biotechnology Bulletin. National Library of Medicine, 32(10): 84-96
[6]. Martyna , Ś., Francesca , F., Francesco , fazi, & Silvia , masciarelli. (2022). Understanding ER Homeostasis and the UPR to Enhance Treatment Efficacy of Acute Myeloid Leukemia. Sciencedirect, 80-88.
[7]. Stefan J. Marciniak, Joseph E. Chambers, David Ron. (2021). Pharmacological targeting ofendoplasmic reticulum stress in disease. ResearchGate, 33-34.
[8]. Joachim Krebs, Jody Groenendyk, Marek Michalak. (2011). Ca2+-Signaling, Alternative Splicing and Endoplasmic Reticulum Stress Responses. ResearchGate, 120-125.
[9]. Imai Y, Takahashi R. (2004). How do Parkin mutations result in neurode generation? CurrOpin N eurobiol,14 (3): 9-384 .
[10]. Zhang, B. Z., & Xia, L. Z. (2003). ER Stress and Disease. Foreign medical hygiene fascicles, (3) 44-45.
[11]. Qin, J., & Zhao, H. J. (2019). Progress in the Study of the Mechanism of ER Stress in the Development of Metabolic Related Diseases and Its Therapeutic Effect. National Library of Medicine, 201-202.
[12]. Zhang K, Shen X, Wu J, et al. (2006). Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell, ,124 (3):587-599.
[13]. Hu, C. Q., Du, H. Y., he, K., Fu, G. W., & Xia, M. X. (2021). The Role of Endoplasmic Reticulum Stress in Liver Cance. Nature Reviews Cancer, (2) 33-34.
Cite this article
Liu,R. (2023). Review of ER stress and unfolded protein response. Theoretical and Natural Science,23,112-116.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Krishna , P. m, sundhar, mohandas, & kunka , ramkumar Mohanram. (2022). Role of ER Stress Inhibitors in the Management of Diabetes. Sciencedirect, 50-55.
[2]. Hu, G., & Pan, W. (n.d.). (2022). Endoplasmic Reticulum Physiology and Pathology. ResearchGate, 77-78.
[3]. Yang, L., Huang, H., Wang, T., Zhou, L. D., Chen, Q., Li, D., Chen, Z. S., & Lin, P. (2023). Endoplasmic Reticulum-Targetable Selenium-Doped Carbon Nanodots with Redox-Responsive Fluorescence for in Situ Free-Radical Scavenging in Cells and Mice. ResearchGate,23-25.
[4]. Wan S , Jiang L . (2016). Endoplasmic reticulum (ER) stress and the unfolded protein response (UPR) in plants. Protoplasma, 253(3):753-764.
[5]. YANG Zheng-ting, LIU Jian-xiang. (2016). Endoplasmic Reticulum Stress Response in Plants. Biotechnology Bulletin. National Library of Medicine, 32(10): 84-96
[6]. Martyna , Ś., Francesca , F., Francesco , fazi, & Silvia , masciarelli. (2022). Understanding ER Homeostasis and the UPR to Enhance Treatment Efficacy of Acute Myeloid Leukemia. Sciencedirect, 80-88.
[7]. Stefan J. Marciniak, Joseph E. Chambers, David Ron. (2021). Pharmacological targeting ofendoplasmic reticulum stress in disease. ResearchGate, 33-34.
[8]. Joachim Krebs, Jody Groenendyk, Marek Michalak. (2011). Ca2+-Signaling, Alternative Splicing and Endoplasmic Reticulum Stress Responses. ResearchGate, 120-125.
[9]. Imai Y, Takahashi R. (2004). How do Parkin mutations result in neurode generation? CurrOpin N eurobiol,14 (3): 9-384 .
[10]. Zhang, B. Z., & Xia, L. Z. (2003). ER Stress and Disease. Foreign medical hygiene fascicles, (3) 44-45.
[11]. Qin, J., & Zhao, H. J. (2019). Progress in the Study of the Mechanism of ER Stress in the Development of Metabolic Related Diseases and Its Therapeutic Effect. National Library of Medicine, 201-202.
[12]. Zhang K, Shen X, Wu J, et al. (2006). Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell, ,124 (3):587-599.
[13]. Hu, C. Q., Du, H. Y., he, K., Fu, G. W., & Xia, M. X. (2021). The Role of Endoplasmic Reticulum Stress in Liver Cance. Nature Reviews Cancer, (2) 33-34.









