1. Introduction
Neurological disorders have a profound impact on an individual’s quality of life and overall health. However, the emergence of implantable neural electrodes as neuro-engineering devices has revolutionized the treatment of these diseases. By surgically implanting electrodes in the nervous system, neural signals can be simulated, suppressed, or modulated, allowing precise control and intervention of neural activity. This innovative treatment offers new hope and unprecedented opportunities for patients with neurological diseases.
The development of implantable neural electrodes has a long history, which can be traced back to the 1960s. At the time, scientists began to realize that electrical stimulation of the nervous system could alter the body’s physiological and behavioral responses. This finding has stimulated interest in implantable neural electrodes to explore their potential in nervous system regulation and therapy. The earliest studies on implantable neural electrodes mainly focused on animal experiments. Scientists implant electrodes into animals’ brains or other areas of the nervous system to electrically stimulate them and study their effects on behavior and physiological functions. These studies have laid the foundation for subsequent clinical applications and provided scientists with valuable data and insights. With the progress of science and technology, the research of implantable neural electrodes has gradually expanded to the field of human clinical application. Currently, the market primarily provides mature implantable nerve stimulators such as deep brain stimulation (DBS), vague nerve stimulation (VNS), and spinal cord stimulation (SCS). These devices have undergone significant advancements and are widely utilized in clinical practice. This article aims to comprehensively review the significance and application of implantable nerve electrodes in the field of neural engineering, discuss various types of nerve electrode treatment options, and explore their potential in treating nervous system diseases. A deeper understanding of these techniques by delving into the complexity of implantable neural electrodes and their effects on neural activity could pave new paths for discovering novel treatments for neurological diseases while contributing to the development of more effective therapies. This paper will contribute to advancing neuro-engineering research and improving patient outcomes affected by neurological diseases [1].
Implantable neural electrodes are neuro-engineering devices that are crucial in treating various neurological diseases. Electrodes that are surgically implanted into the nervous system can mimic or inhibit neural signals for therapeutic purposes. This innovative approach holds great promise in the field of neurology, showing great potential in improving the lives of patients with neurological disorders.
As the basic unit of the nervous system, neurons have the ability to generate electrical signals, which are the main way of information transmission in neural networks. Implantable neural electrodes are able to intercept and record these electrical signals, providing valuable information to understand the function of the nervous system. In addition, these electrodes can deliver electrical pulses to neurons, achieving precise control of neural activity.
Treatment options related to the level of implantable nerve electrical stimulation generally fall into 3 categories: stimulation, inhibition, and modulation.
Stimulation involves the application of electric impulses to stimulate or activate nerve activity. The method has been widely investigated and has demonstrated great success in many neurodegenerative disorders, including Parkinson. Perestelo et al. found that deep brain stimulation (a form of neural electrode-based stimulation) significantly improved motor symptoms and improved quality of life in Parkinson’s patients [2].
Inhibition is to suppress or suppress abnormal nerve activity by providing accurate electric pulses with specified parameters. The technique has been demonstrated to ease the symptoms of illnesses like epilepsy. In a Closed Circuit Deep Brain Stimulation in Intractable Epilepsy Research, Nathaniel et al. demonstrated that a closed circuit DBS with an implanted nerve electrode can enhance seizure control and decrease adverse reactions [3].
In addition, regulation refers to the ability to modulate neural activity to restore balance and optimize function. Micera et al. proposed that electrical stimulation (ES) can partially restore motor and sensory functions. ES can be delivered through different interfaces and the invasive/selective ratio can be modified by changing the structure of the electrode [4].
In summary, implantable neural electrodes have revolutionized the field of neural engineering through their ability to mimic or inhibit neural signals. By harnessing the electrical activity of neurons, these electrodes provide innovative treatment options for a variety of neurological diseases. The treatment options for implantable nerve electrical levels typically fall into three categories
DBS: Electrical stimulation regulates neural activity by surgically implanting electrodes into specific brain regions, thereby alleviating symptoms. This technique is widely employed to treat Parkinson’s disease, tremors, anxiety disorders, depression, and various conditions. Tourette syndrome (TS) is a complex neurological disorder characterized by motor and vocal tics. Although symptoms typically attenuate during adolescence and adulthood, some persistently experience severe manifestations. While the effectiveness of traditional treatments is limited, DBS has shown promising results in the management of the disease [5].
SCS: By placing electrodes surgically inside the spine, the technique is effective in blocking the delivery of pain signals and relieving chronic pain. It serves as an effective method for managing lasting pain. EMG is an electrical stimulus that is injected into the spine, which is used to stimulate the spinal cord with electrical impulses. Millions of people around the world have seizures that are linked to irreversible brain or spine injuries that can result in long-term disability and lower quality of life. There are a number of therapies for the management of convulsions, but they all have their disadvantages. SCS can be regarded as an ongoing trial [6].
VNS: Electrodes are implanted in the neck region to modulate electrical activity within the brain by stimulating the vagus nerve. This therapeutic intervention is specifically indicated for certain types of epilepsy and depression management. VNS is an effective neuromodulation therapy for epilepsy. VNS is performed by implanting an electrode lead into the vagus nerve in the patient’s neck to modulate brain electrical activity and reduce seizures’ frequency and severity. The implantation of VNS is relatively simple and reversible, which can be adjusted according to the needs of the patient. Once implanted, the electrode leads undergo regular electrical stimulation through an external device and battery. The electric stimulus travels through the vagal nerve to the brain, which stops the abnormal electric activity, thus decreasing the probability of a seizure. A number of studies have shown that VNS is effective in non-responders to anti-epileptics [7].
2. Generation Epilepsy Management System
Kremen Vaclav et al. present an epilepsy management system (Figure 1) offers real-time, personalized treatment and monitoring for patients with epilepsy and physicians through the collaborative integration of brain implant devices, handheld devices, and cloud computing resources [8]. Their latest clinical studies have shown the effectiveness of seizure prediction, seizure detection, and therapeutic electric stimulation. The system builds a wireless connection between the handset and the cloud, enabling the user to connect seamlessly with the patient. Doctors have real-time access to the EEG information of the brain (waking/sleeping, pre-attack and epileptic fits), administration of electric stimulation and monitoring of the condition of the patient.
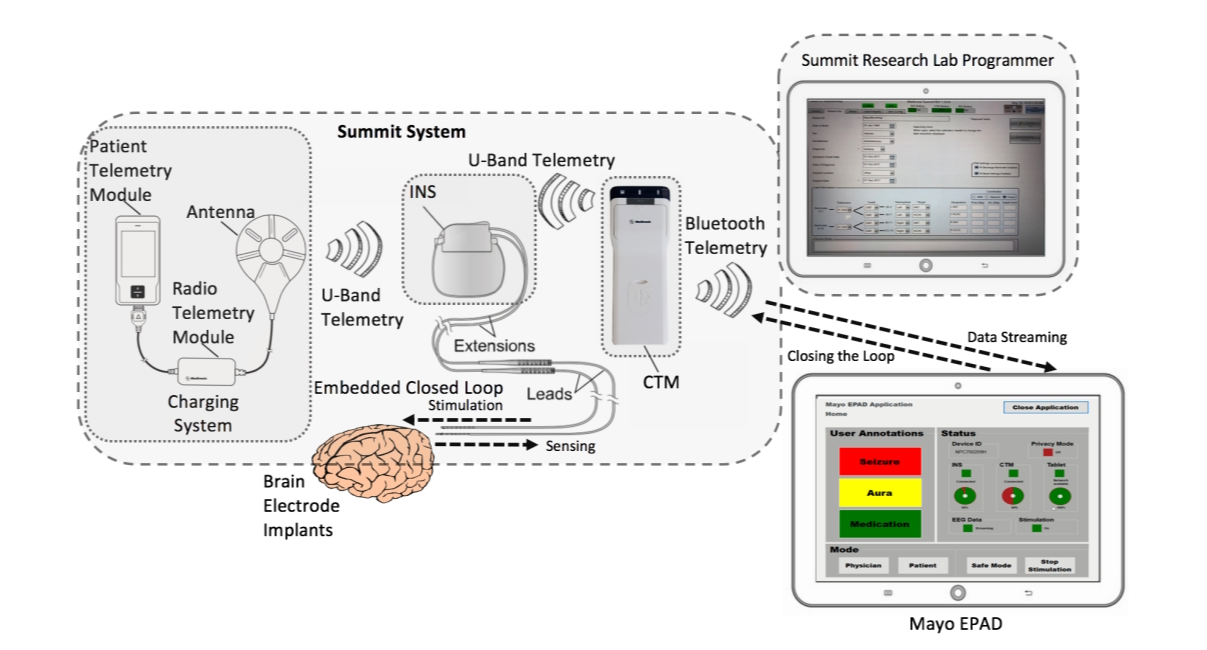
Figure 1. The Medtronic Investigational Summit RC + S System comprises an Implanted Nerve Stimulator (INS), Patient Telemetering Unit (PTM), Wireless Telemetering Module (RTM), Epileptic Assistance Apparatus (EPAD) and Research Laboratory Developer (RLP) [8].
3. Tuning Electrical Stimulation for Thalamic Visual Prosthesis
Amr Jawwad, et al. proposed a method for adjusting thalamic visual prostheses. This approach involves estimating an electrical stimulus that is equivalent to a given natural visual stimulus, resulting in a similar response when introduced into the lateral brain nucleus [9]. Furthermore, they employed artificial neural networks and autoencoders to control the firing of neurons in the LGN model (Figure 2) during the adjustment of electrical stimuli. The method consists of two stages: Firstly, when a visual stimulus is provided as input, the system predicts the neuronal response through the visual coding stage; the electrical stimulus decoder then utilizes these responses to estimate the required electrical stimulation for triggering such responses. This study introduces a novel approach utilizing machine learning techniques to optimize electrical stimulation and enhance simulated natural vision within lateral brain nucleus vision prostheses. Consequently, it improves visual function in patients with retinal degenerative diseases and provides new directions for future development of visual prostheses.
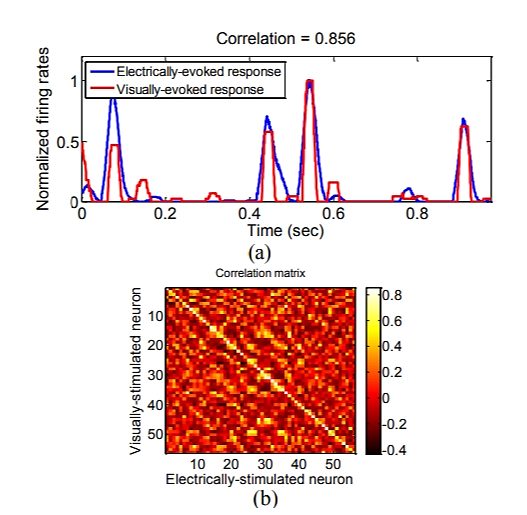
Figure 2. (a) The firing rates of a representative neuron in response to electrical stimulation are depicted in blue, while the firing rates elicited by visual stimulation are represented in red. (b) A correlation matrix illustrates the associations between the responses evoked [9].
4. Implantable Graphene-based Neural Electrode Interfaces
In their research, Ta Chuang Liu et al have presented an implanted multichannel nerve probe that can detect nerve chemistry and nerve electric signals in a multichannel way via a nonenzyme neurochemistry interface. A single step cycle voltammogram (CV) electrochemistry was used to synthesize the improved electrode on nerve tip. Gold was oxidized and GO was reduced at the same time, so that they could be tightly bonded by the electrostatic action of chloride (Cl-). The RGO/Au2O3 modified electrode exhibited significant improvement in electrocatalysis at a low deposition rate of 10 mVs-1. Optical images are show in Fg3. Nerve probe with or without rGO/Au2O3 electrode. The results showed that the H2O2 reaction was fast under 5 s, and the detecting limit was below 0. 63 μM. The H2O2 concentration of rGO/Au2O3 electrode was 100.48 μm + 4.5 μm for 1 h in an acute cerebral infarction model, which is significantly greater than that of the non-coated electrode. At the same time, SSEPs (SEP) were used to confirm the function of nerve activity. The new type of implanted probe with localized rGO/Au2O3 could serve as a rapid and reliable sensor for H2O2 or other neurochemistry [10, 11].
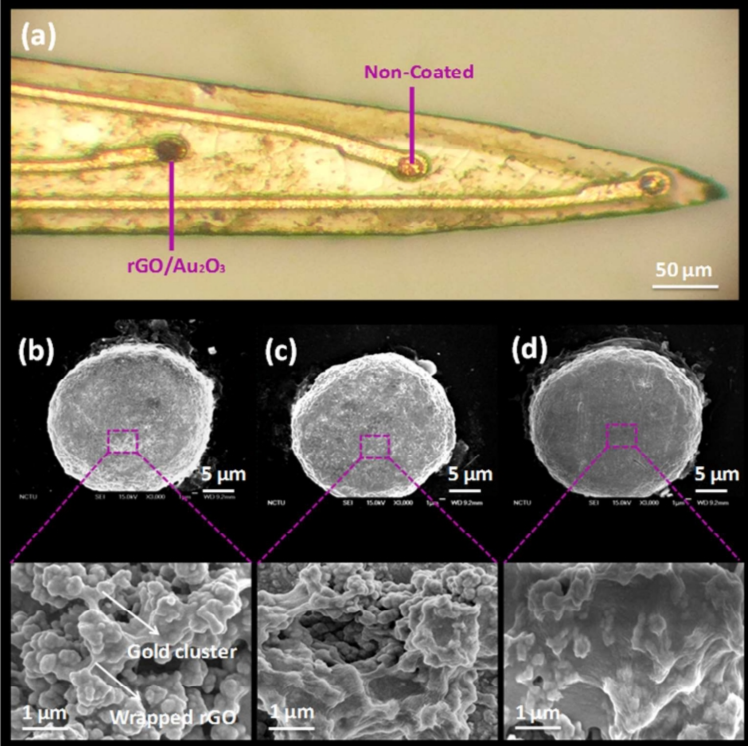
Figure 3. (a) Optical image of the neural probe coated with and without rGO/ Au2O3 electrodes. SEM images of the rGO/Au2O3 electrodes. (b) 10 mVs-1, (c) 50 mV s-1, and (d) 250 mV s-1 [10].
5. Conclusion
In conclusion, implantable neural electrodes have revolutionized the field of neural engineering and hold tremendous promise in the treatment of various neurological diseases. These electrodes not only have transformed therapy but also significantly contributed to a deeper understanding of intricate workings of the nervous system. By intercepting and recording electrical signals, these electrodes provide invaluable insights into neural function and aid researchers in ending the complexity of the nervous system.
The field of implantable neurostimulators has made significant advancements in recent years, enabling real-time monitoring and personalized treatment for patients with epilepsy through the integration of brain-implanted devices, handheld devices, and cloud computing resources. Real-time data analysis allows for the adjustment of treatment parameters, resulting in improved seizure control and enhanced management of epilepsy. In the field of prostheses, researchers have achieved notable progress in refining electrical stimulation techniques for thalamic visual prostheses. This optimization of the electrical stimulation mode delivered to the thalamus has significantly improved patients’ visual perception. Another major breakthrough is the development of an implantable graphene neural electrode interface which offers high electrical conductivity, biocompatibility, and precise recording and monitoring capabilities when implanted into the brain. This innovation provides new possibilities for understanding brain function and diagnosing nervous system diseases. Furthermore, integrating implantable neural electrodes with wireless communication technology and miniaturized electronics enables closed-loop systems that dynamically adjust stimulation parameters to optimize therapeutic effects. This closed-loop approach enhances both efficacy and efficiency in nerve stimulation therapy summary, recent advancements in the field of implantable neurostimulators have expanded possibilities for more precise, efficient, and personalized neurostimulation therapies. Implantable neural electrodes have had a on the field of neural engineering while offering a novel approach to treating disorders within the nervous system. With continued progress in research and development, they possess significant potential to further advance our comprehension surrounding its intricacies while providing patients with more effective and personalized treatments in future endeavors.
References
[1]. Sun F T and Morrell M J 2016 Neurotherapeutics 9(2) 296-304
[2]. Perestelo, L, Rivero-Santana, A, Pérez-Ramos, J, Serrano-Pérez, P, Panetta, J, & Hilarion, P 2014 Journal of Neurology 261(11) 2051-2060
[3]. Sisterson, N D, Wozny, T A, Kokkinos, V, Constantino, A, & Richardson, R M 2019 Neurotherapeutics 16(1) 119-127
[4]. Micera, S, Keller, T, Lawrence, M, Morari, M, & Popović, D B 2010 IEEE Engineering in Medicine and Biology Magazine 29(3) 64-69
[5]. Baldermann J C, Schüller T, Huys D, Becker I, Timmermann L, Jessen F, Visser-Vandewalle V, Kuhn J. Brain Stimul. 2016 Mar-Apr;9(2):296-304.
[6]. Nagel S J, Wilson S, Johnson M D, Machado A, Frizon L, Chardon M K, Reddy C G, Gillies G T, & Howard M A (2017) Neuromodulation 20(4) 307-321
[7]. Pérez L, Faulkner H, Higgins S, Koutroumanidis M, & Leschziner G 2020 Practical Neurology 20(3) 189-198
[8]. Kremen V, Brinkmann B H, Kim I, Guragain H, Nasseri M, Magee A L, Attia T P, Nejedly P, et al. 2018 IEEE Journal of Translational Engineering in Health and Medicine 6 1-10
[9]. Liu C, Chuang M C, Chu C Y, Huang W C, Lai H Y, Wang C T, Chu W L, Chen S Y, & Chen Y Y 2016 ACS Applied Materials & Interfaces 8(1) 187-196
[10]. Jawwad A, Abolfotuh H H, Abdullah B, Mahdi H M, & Eldawlatly S 2016 Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society 8 5431-5434
[11]. V Kremen et al 2018 IEEE Journal of Translational Engineering in Health and Medicine, 9(2) 296-304
Cite this article
Li,A. (2023). Development and application of implantable electrical neural electrodes. Theoretical and Natural Science,27,125-130.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Sun F T and Morrell M J 2016 Neurotherapeutics 9(2) 296-304
[2]. Perestelo, L, Rivero-Santana, A, Pérez-Ramos, J, Serrano-Pérez, P, Panetta, J, & Hilarion, P 2014 Journal of Neurology 261(11) 2051-2060
[3]. Sisterson, N D, Wozny, T A, Kokkinos, V, Constantino, A, & Richardson, R M 2019 Neurotherapeutics 16(1) 119-127
[4]. Micera, S, Keller, T, Lawrence, M, Morari, M, & Popović, D B 2010 IEEE Engineering in Medicine and Biology Magazine 29(3) 64-69
[5]. Baldermann J C, Schüller T, Huys D, Becker I, Timmermann L, Jessen F, Visser-Vandewalle V, Kuhn J. Brain Stimul. 2016 Mar-Apr;9(2):296-304.
[6]. Nagel S J, Wilson S, Johnson M D, Machado A, Frizon L, Chardon M K, Reddy C G, Gillies G T, & Howard M A (2017) Neuromodulation 20(4) 307-321
[7]. Pérez L, Faulkner H, Higgins S, Koutroumanidis M, & Leschziner G 2020 Practical Neurology 20(3) 189-198
[8]. Kremen V, Brinkmann B H, Kim I, Guragain H, Nasseri M, Magee A L, Attia T P, Nejedly P, et al. 2018 IEEE Journal of Translational Engineering in Health and Medicine 6 1-10
[9]. Liu C, Chuang M C, Chu C Y, Huang W C, Lai H Y, Wang C T, Chu W L, Chen S Y, & Chen Y Y 2016 ACS Applied Materials & Interfaces 8(1) 187-196
[10]. Jawwad A, Abolfotuh H H, Abdullah B, Mahdi H M, & Eldawlatly S 2016 Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society 8 5431-5434
[11]. V Kremen et al 2018 IEEE Journal of Translational Engineering in Health and Medicine, 9(2) 296-304









