1. Introduction
The gastric cancer is a kind of malignancy tumor, and many patients are diagnosed with terminal cancer due to atypical clinical symptoms [1]. For patients who discover the gastric cancer early without tumor metastasis, the clinical surgical resection is a common therapy strategy. But unfortunately, many patients experience recurrence after the surgery. For advanced gastric cancer, only patients with HER-2 positive can be treated with trastuzumab accompanied with adjuvant chemotherapy for the first-line treatment [2]. Thus, new target spots is in need to be discovered and new medicine targeting new spots is in emergency to be used for first line treatment. Immunotherapy is a hot spot recently in cancer treating, some immune checkpoint inhibitors (ICI, targeting at PD-1 and PD-L1 spots) are gradually used for advanced gastric cancer treatment [3]. But the immune tolerance also arises and the median survival for advanced patients is merely extended for approximately two months compared with chemotherapy alone [4].
Thus, new treatment plans are in great demand for curing advanced GC, aiming in prolonging survival time of the patients and enhancing their quality of life. There are two ways: one is developing more immune checkpoints inhibitors (such as the ones targeting at CTLA -4 and LAG-3), the other is improving the inhibitory state of tumor microenvironment to confront the reality of immunotherapy tolerance (such as the Sting agonists). In this review, some new immune checkpoint inhibitors and some Sting agonists will be introduced and some combined therapy (such as immunotherapy combined with targeting therapy and immunotherapy combined with chemotherapy) will be discussed aiming to establish a new treatment plan in advanced cancer.
2. Immune checkpoint in gastric cancer
2.1. PD-1/PD-L1
2.1.1. PD-1/PD-L1 structure. PD-1 (programmeddeath-1) is an important immunosuppressive molecule, which is a transmembrane protein, it contains an immunoglobulin variable region domain in extracellular parts, a transmembrane region, and within the cells, an immune receptor tyrosine-based inhibitor motif (known as ITIM) and an immune receptor tyrosine-based switch motif (known as ITSM). PD-1 belongs to the CD28/CTLA-4 family of co-receptors, with poor conservative gene sequence [5]. It plays a role in preventing excessive immune activation, however, the tumour cells can use its PD-L1 to unite PD-1 to avoid immune defences (Figure 1). PD-1 is mostly expressed in activated CD8+, CD4+ T cell, B cell and NK, DC, natural killer thymocyte cell [6].
PD-L1 (programmed Cell Death-Ligand 1) also named as CD274 and B7-H1 is a 40kDa transmembrane glycoprotein that is made up of extracellular segments, hydrophobic transmembrane segments, and short cytoplasmic regions. There is one immunoglobulin constant region (known as IgC) and one immunoglobulin variable region (known as IgV) in the extracellular domain. The cytoplasmic region in PD-L1 comprises a protein kinase C phosphorylation site [5]. PD-L1 is mainly expressed in T cells, B cells, dendritic cells (DC) , macrophages, mast cells, fibroblasts, and mesenchymal stem cells, while on many kinds of tumor cells it is also highly expressed to bind with PD-1 and escape from the attack of active T cells [6].
2.1.2. PD-1/PD-L1 medicine. Many inhibiting medicines are designed to prevent the PD-1/PD-L1 pathway (Figure 2). Pembrolizumab is an antibody attacking PD-1, which got US FDA approval for use in many cancers and also got FDA’s accelerated approval for curing the advanced gastric cancer [7,8]. And another approved inhibitor of PD-1 by FDA is Nivolumab. There are also other PD-1 medicine, such as dostarlimab, Xindiliximab injection, Treprizumab injection, Carolizumab injection, cemiplimab injection. While Atezolizumab, Avelumab, Durvalumab are some examples of inhibitors used for suppressing PD-L1. While these PD-1 and PD-L1 antibodies haven’t got the approval from FDA in treating gastric carcinoma.
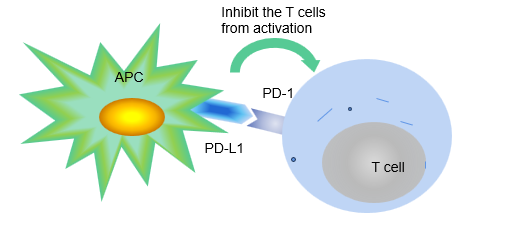
Figure 1. In naïve ways, PD-L1 on APK can bind with PD-1 on T cells thus to suppress the T cells from inordinate activation in case they attack the normal tissues.
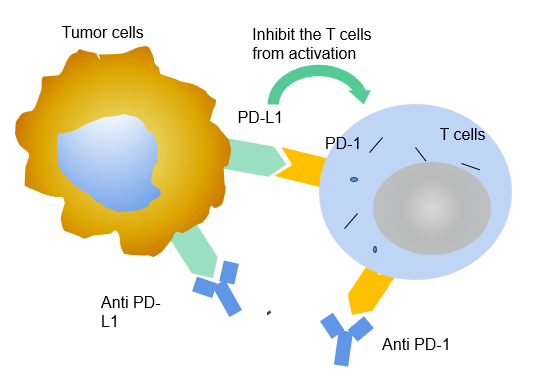
Figure 2. Some tumour cells also have PD-L1, which can lead to the misidentification for T cells. The T cells are deactivated owing to the bind between PD-1 and PD-L1, thus cease to assault the tumour cells.
2.2. CTLA-4
CTLA-4 (Cytotoxic T-lymphocyte antigen 4) is also expressed on the activated T cells, when CTLA-4 (CD152) binds to B7 (include B7-1 ligand and B7-2 ligand) on the surface of antigen presenting cells (APK) with a higher affinity than CD28 on T cells, it can competitively inhibit the binding of B7 to CD28 on the surface of T cells and prevent the T cell to be activated (Figure 3) [9]. There are some CTLA-4 inhibitors that are still on clinical tests for gastric cancer and especially the unite of CTLA-4 and PD-1 inhibitors is a focus these years (Figure 4). The AK104, which is an CTlA-4/PD-1 antibody, is in phase II to cure the patients with advanced gastric carcinoma locally by Peking University, which is highly expected [10]. Also, AI-061 which is also a co-formulation drug product for 1:1 ratio PD-1and CTLA-4 is recruiting volunteers for Phase one test for gastric cancer treatment [11]. Another clinical test is on Phase IA/IB/II to recruit people to test the ONC-392, which is a humanized anti-CTLA4 IgG1 monoclonal antibody for gastric cancer, also combined with Pembrolizumab to see it’s effect [12].
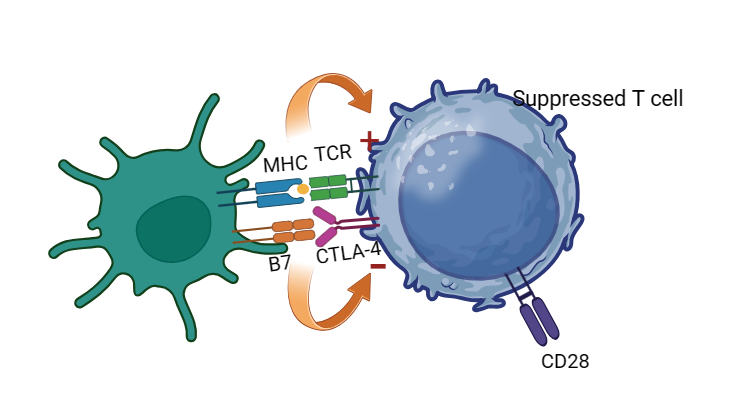
Figure 3. The TCR (T cell receptor) can bind with the MHC (major histocompatibility complex) and can activate the T cells, while with the aid of bind between B7 and CD28. The CTLA-4 has a higher affinity than CD28 with B7, thus the binding between CTLA-4 and B7 can suppress the T cells.
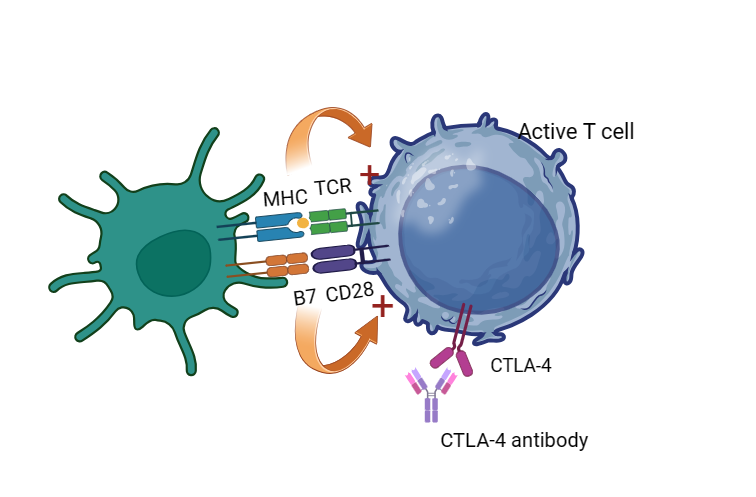
Figure 4. With the help of CTLA-4 antibody that has a higher affinity with CTLA-4 than B7, the CD28 can unite B7 and keep T cells active.
2.3. LAG-3
LAG-3 is a transmembrane protein that is characteristically expressed at T cells in activation, B cells, NK cells and also in plasmacytoid dendritic cells [13]. Four ligands of LAG-3 are reported in the past research which includes Galectin-3, major histocompatibility complex II (known as MHC II), along with fibrinogen-like protein 1 (known as FGL1), and also hepatic sinusoid endothelial cell lectin (known as LSECtin), with MHC II the major ligand [14]. Though it manifested that the inhibitory sound effect of LAG-3 on immune system is not aroused by the competitive binding between MHC II and CD4, it’s sure that it helps tumor cells to escape from the immune system.LAG-3 is a important potential checkpoint in fighting against tumours and cancers, with many clinical researches and pre-clinical researches. A clinical phase 2-3 research uses relatlimab (known as a LAG-3 antibody) combined with Nivolumab (known as a PD-1 antibody ) shows that with both relatlimab and nivolumab the median progression-free survival (PFS) was around 10.1 months (confidence level:95%; confidence interval (CI) :6.4 - 15.7) comparing with 4.6 months (confidence level:95%; CI:3.4 - 5.6) with nivolumab, meanwhile the rate of progression-free survival at 12 months was 47.7% (confidence level:95%; CI:41.8 - 53.2) with combination of relatlimab and nivolumab comparing with 36.0% (confidence level: 95%; CI:30.5 - 41.6) with nivolumab [15], which manifests a prosperous prospects of LAG-3 antibody unites with other immune checkpoint inhibitors. A clinical research testing the safety, efficacy and tolerability of a LAG-3 antibody (BMS-986016) combined or not combined with Nivolumab (PD-1 antibody) in treating the solid tumour (include gastric cancer) is on Phase 2 [16]. As we can see that there is still a big potential of designing Lag-3 inhibitors in treating gastric cancer, as LAG-3 is still a new discovered checkpoint even with not much medicine shooting on the market, with only Opdualag which is suitable for treating melanoma.
3. Treatment of Gastric cancer
For individuals with early-stage gastric cancer, the primary curative approach involves resection surgery, often accompanied by adjuvant chemotherapy (radiotherapy typically yields minimal benefits), resulting in a commendable five-year survival rate of over 90% [17]. However, the challenge lies in the atypical symptoms associated with gastric cancer, rendering early detection and diagnosis a formidable task. Consequently, the majority of patients are diagnosed at an advanced stage of the disease.
For patients diagnosed with advanced gastric cancer, treatment typically involves a combination of chemotherapy, targeted therapy, and immunotherapy, with a focus on inhibiting PD-1. In cases where chemotherapy alone is employed, using a platinum and fluoropyrimidine doublet regimen, the median survival period is less than one year [18]. Targeted therapy with HER2 antibodies is limited to individuals with positive HER2 antigen tests. Notably, in a recent clinical phase 2 trial which involve advanced patients with HER-2 positive tumours, treatment with Trastuzumab Deruxtecan, an HER2 antibody, had a result of the median survival to be 12.5 months, as opposed to 8.4 months for those receiving chemotherapy [19]. Although this demonstrates a modest improvement in survival, it still falls short of reaching the two-year mark. In cases where PD-1 inhibitors are used as standalone therapy, they are generally well-tolerated, but their effectiveness tends to diminish in less than a year, largely due to the intricate tumor microenvironment [20].
Consequently, there is an urgent need for the development of new agents that target novel sites, particularly in the realm of immunotherapy, to extend a lifeline to patients battling advanced and metastatic gastric cancer. Furthermore, the demand for combination therapies, instead of relying solely on a single approach, is substantial. This approach is vital in countering the ability of tumor to develop tolerance, ultimately leading to an enhancement in the median survival rate.
4. Immunotherapy combined with other treatments
A clinical research for phase II used a designed TMEscore R package to provide tumor microenvironment statistics aiding the immunotherapy, and with a positive result [21]. In another Phase II research, patients who used zolbetuximab + EOX had a significant enhance in PFS with a hazard ratio (HR) to be 0.44 and the 95% CI from 0.29 to 0.67 (P < 0.0005) and also an ascend in OS with HR to be 0.55 and 95% CI from 0.39 to 0.77 (P < 0.0005) comparing with the patients who used EXO alone, which shows a prospect of combined therapy of first-line EOX with zolbetuximab, which is an immune checkpoint inhibitors [22]. A research showed the feasibility of a neoadjuvant unity of Nivolumab and ipilimumab neoadjuvant with ideal toxicity as well with a super high Polymerase Chain Reaction (PCR) rate (an indicator that manifests the growth speed of a tumour) in treating a dMMR/MSI-H gastric carcinoma/GEJ adenocarcinoma patient [23]. Another research combined camrelizumab (an immune checkpoint inhibitor), apatinib (an anti-angiogenesis agent) with S-1 ± oxaliplatin (a chemotherapy adjuvant drug) in treating cT4a/bN+ gastric carcinoma and resulted in a CPR of 15.8%, MPR of 26.3%, with highly satisfying safety and relatively high feasibility, which showed a good prospect of treating the MSI-H along with PD-L1 positive patients [24]. For first line therapy, patients with HER2-negative gastric/gastroesophageal junction cancer are faced a median overall survival for less than one years. Thus, a Chinese team is combining nivolumab and chemotherapy to treat patients in that situation. Compared with chemotherapy only, the united therapy of nivolumab together with chemotherapy had a successful increase in median overall survival (OS) (together 14.3 vs alone 10.2 months) , median PFS (together 8.3 vs alone 5.6 months) , ORR (together 66% vs alone 45%) and median DOR (together 12.2 vs alone 5.6 months) ,while the safety of such treatment was qualified [25].Agent SHR-1701 is an antibody against PD-1 and TGF- β, which was in the first phase clinical test for advanced solid tumor (especially gastric cancer) .The research tested the best dose for the second phase research (30mg/kg every three weeks, which with the highest ORR) ,with an ORR of 20% and 12-month overall survival rate of 54.5%) [26]. It shows an positive efficacy and a standard toxicity and with a considerable prospect. Another research looked into a recent highlight pathway-STING pathway. Different from inhibiting the immune checkpoints to keep the T cells active, this pathway is in charge of innate immune pathway which can initiating the antitumor immunity [27]. A STING agonist (MSA-2) was made into low polarity pro-drug and was encapsulated by liposomes, which was intravenous injected into the tumor mice. The unity of PD-1 inhibitor with this STING agonist eradicated completely the tumors of mice compared with treating with each agent separately [28]. It shows a broad prospect in combining STING and immune checkpoint pathway together in the future clinical research and also the first line research, which can mobilize more immune cells and also more activated T cells in the tumor microenvironment.
5. Conclusion
Indeed, as indicated above, there are currently only a limited number of FDA-approved immune checkpoint inhibitors for gastric cancer, with only two agents targeting PD-1 having received approval. However, there are numerous inhibitors in various phases of clinical testing. Some hospitals are actively investigating combinations of immunotherapy assisted with chemotherapy for the first-line treatment in dealing with the advanced gastric carcinoma. It is anticipated that more novel immune checkpoint inhibitors will be introduced to provide additional treatment options, particularly benefiting HER-2 negative advanced gastric carcinoma patients.
Moreover, the combination of various immune checkpoint inhibitors that target different immune checkpoints, along with the unite of immune checkpoint inhibitors with anti-angiogenic agents, chemotherapy, targeted antibody therapies, and even STING agonists, holds substantial promise as a therapeutic approach for advanced gastric cancers. These strategies aim to enhance the median survival rate of patients and represent significant avenues of research and development in the field.
References
[1]. Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond) 2021;41(10):1037–1048. doi: 10.1002/ cac2.12197.
[2]. Wagner AD, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064.
[3]. Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol (2018) 4:e180013. doi: 10.1001/jamaoncol.2018.0013
[4]. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (Checkmate 649): A randomised, open-label, phase 3 trial. Lancet (2021) 398(10294):27–30.doi: 10.1016/S0140-6736(21)00797-2
[5]. Sandra Schöniger, Bharat Jasani. The PD-1/PD-L1 Pathway: A Perspective on Comparative Immuno-Oncology. Animals.2022;12(19);2661-2667.
[6]. Krzysztof M Zak, et al. Structural biology of the immune checkpoint receptor PD-1 and its ligands PD-L1/PD-L2 Structure 25 (8), 1163-1165, 2017.
[7]. Keytruda(pembrolizumab) prescribing information. www.accessdata.fda.gov/drugsatfda_docs/ label/2016/125514s012lbl.pdf
[8]. FDA grants accelerated approval to pembrolizumab for advanced gastric cancer. www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm577093.htm
[9]. Richard V. Parry et al. CTLA-4 and PD-1 receptors Inhibit T-Cell Activation by Distinct mechanisms. Molecular and Cellular Biology. 2005 Nov;25(21):9543-53.
[10]. Jiafu Ji, Peking University (Responsible Party). AK104 in Locally Advanced MSI-H/dMMR Gastric Carcinoma and Colorectal Cancer. ClinicalTrials. NCT04556253
[11]. OncoC4, Inc. (Responsible Party).Safety, PK and Efficacy of AI-061 in Advanced Solid Tumors(PRESERVE-009).ClinicalTrials.NCT05858736
[12]. OncoC4, Inc. (Responsible Party) Safety, PK and Efficacy of ONC-392 in Monotherapy and in Combination of Anti-PD-1 in Advanced Solid Tumours and NSCLC (PRESERVE-001). Clinical trials.NCT04140526
[13]. Monica V Goldberg et al. LAG-3 in Cancer Immunotherapy. Curr Top Microbiol Immunol. 2011;344;269-78.
[14]. Jin-Ling Huo et al. The promising immune checkpoint LAG-3 in cancer immunotherapy: from basic research to clinical application. Front Immunol. 2022.13.956090.
[15]. Bristol-Myers Squibb (Responsible Party). Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. ClinicalTrials. NCT03470922
[16]. Bristol-Myers Squibb (Responsible Party). An Investigational Immuno-therapy Study to Assess the Safety, Tolerability and Effectiveness of Anti-LAG-3 With and Without Anti-PD-1 in the Treatment of Solid Tumours. ClinicalTrials.NCT01968109
[17]. Zhaoyang Tan et al. Recent Advances in the Surgical Treatment of Advanced Gastric Cancer: A Review. Med Sci Monit.2019:25:3537-3541.
[18]. Elizabeth C Smyth et al. Gastric cancer. Lancet. 2020;396(10251):635-648.
[19]. Kohei Shitara et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. ClinicalTrials.2020;382(25):2419-2430.
[20]. Yuanda Liu et al. Tumour microenvironment-mediated immune tolerance in development and treatment of gastric cancer. Front Immunol. 2022:13:1016817.
[21]. Dongqiang Zeng et al. Tumour microenvironment evaluation promotes precise checkpoint immunotherapy of advanced gastric cancer. J Immunother Cancer.2021.9(8).e002467
[22]. U Sahin et al. FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal Adenocarcinoma. Ann Oncol. 2021;32(5):609-619.
[23]. Thierry André et al. Neoadjuvant Nivolumab Plus Ipilimumab and Adjuvant Nivolumab in Localized Deficient Mismatch Repair/Microsatellite Instability–High Gastric or Esophagogastric Junction Adenocarcinoma: The GERCOR NEONIPIGA Phase II Study. J Clin Oncol. 2023;41(2):255-26.
[24]. Song Li et al. Neoadjuvant therapy with immune checkpoint blockade, antiangiogenesis, and chemotherapy for locally advanced gastric cancer, Nature Communications.2023;14(1):8.
[25]. Tianshu Liu et al. First-line nivolumab plus chemotherapy vs chemotherapy in patients with advanced gastric, gastroesophageal junction and esophageal adenocarcinoma: CheckMate 649 Chinese subgroup analysis. International Journal of Cancer. 2023;152(4):749-760.
[26]. Dan Liu et al. Bifunctional anti-PD-L1/TGF-βRII agent SHR-1701 in advanced solid tumors: a dose-escalation, dose-expansion, and clinical-expansion phase 1 trial. BMC medicine. 2022; 20; 408.
[27]. Ruilei Huang et al. Targeting STING for cancer immunotherapy: From mechanisms to translation. International immunopharmocology. 2022;113(Pt A):109304.
[28]. Xiaona Chen et al. Chemically programmed STING-activating nano-liposomal vesicles improve anticancer immunity. Nature Communications.2023;14;4584
Cite this article
Ji,R. (2023). Immunotherapy and combined treatment in gastric cancer. Theoretical and Natural Science,27,141-147.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond) 2021;41(10):1037–1048. doi: 10.1002/ cac2.12197.
[2]. Wagner AD, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064.
[3]. Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol (2018) 4:e180013. doi: 10.1001/jamaoncol.2018.0013
[4]. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (Checkmate 649): A randomised, open-label, phase 3 trial. Lancet (2021) 398(10294):27–30.doi: 10.1016/S0140-6736(21)00797-2
[5]. Sandra Schöniger, Bharat Jasani. The PD-1/PD-L1 Pathway: A Perspective on Comparative Immuno-Oncology. Animals.2022;12(19);2661-2667.
[6]. Krzysztof M Zak, et al. Structural biology of the immune checkpoint receptor PD-1 and its ligands PD-L1/PD-L2 Structure 25 (8), 1163-1165, 2017.
[7]. Keytruda(pembrolizumab) prescribing information. www.accessdata.fda.gov/drugsatfda_docs/ label/2016/125514s012lbl.pdf
[8]. FDA grants accelerated approval to pembrolizumab for advanced gastric cancer. www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm577093.htm
[9]. Richard V. Parry et al. CTLA-4 and PD-1 receptors Inhibit T-Cell Activation by Distinct mechanisms. Molecular and Cellular Biology. 2005 Nov;25(21):9543-53.
[10]. Jiafu Ji, Peking University (Responsible Party). AK104 in Locally Advanced MSI-H/dMMR Gastric Carcinoma and Colorectal Cancer. ClinicalTrials. NCT04556253
[11]. OncoC4, Inc. (Responsible Party).Safety, PK and Efficacy of AI-061 in Advanced Solid Tumors(PRESERVE-009).ClinicalTrials.NCT05858736
[12]. OncoC4, Inc. (Responsible Party) Safety, PK and Efficacy of ONC-392 in Monotherapy and in Combination of Anti-PD-1 in Advanced Solid Tumours and NSCLC (PRESERVE-001). Clinical trials.NCT04140526
[13]. Monica V Goldberg et al. LAG-3 in Cancer Immunotherapy. Curr Top Microbiol Immunol. 2011;344;269-78.
[14]. Jin-Ling Huo et al. The promising immune checkpoint LAG-3 in cancer immunotherapy: from basic research to clinical application. Front Immunol. 2022.13.956090.
[15]. Bristol-Myers Squibb (Responsible Party). Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. ClinicalTrials. NCT03470922
[16]. Bristol-Myers Squibb (Responsible Party). An Investigational Immuno-therapy Study to Assess the Safety, Tolerability and Effectiveness of Anti-LAG-3 With and Without Anti-PD-1 in the Treatment of Solid Tumours. ClinicalTrials.NCT01968109
[17]. Zhaoyang Tan et al. Recent Advances in the Surgical Treatment of Advanced Gastric Cancer: A Review. Med Sci Monit.2019:25:3537-3541.
[18]. Elizabeth C Smyth et al. Gastric cancer. Lancet. 2020;396(10251):635-648.
[19]. Kohei Shitara et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. ClinicalTrials.2020;382(25):2419-2430.
[20]. Yuanda Liu et al. Tumour microenvironment-mediated immune tolerance in development and treatment of gastric cancer. Front Immunol. 2022:13:1016817.
[21]. Dongqiang Zeng et al. Tumour microenvironment evaluation promotes precise checkpoint immunotherapy of advanced gastric cancer. J Immunother Cancer.2021.9(8).e002467
[22]. U Sahin et al. FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal Adenocarcinoma. Ann Oncol. 2021;32(5):609-619.
[23]. Thierry André et al. Neoadjuvant Nivolumab Plus Ipilimumab and Adjuvant Nivolumab in Localized Deficient Mismatch Repair/Microsatellite Instability–High Gastric or Esophagogastric Junction Adenocarcinoma: The GERCOR NEONIPIGA Phase II Study. J Clin Oncol. 2023;41(2):255-26.
[24]. Song Li et al. Neoadjuvant therapy with immune checkpoint blockade, antiangiogenesis, and chemotherapy for locally advanced gastric cancer, Nature Communications.2023;14(1):8.
[25]. Tianshu Liu et al. First-line nivolumab plus chemotherapy vs chemotherapy in patients with advanced gastric, gastroesophageal junction and esophageal adenocarcinoma: CheckMate 649 Chinese subgroup analysis. International Journal of Cancer. 2023;152(4):749-760.
[26]. Dan Liu et al. Bifunctional anti-PD-L1/TGF-βRII agent SHR-1701 in advanced solid tumors: a dose-escalation, dose-expansion, and clinical-expansion phase 1 trial. BMC medicine. 2022; 20; 408.
[27]. Ruilei Huang et al. Targeting STING for cancer immunotherapy: From mechanisms to translation. International immunopharmocology. 2022;113(Pt A):109304.
[28]. Xiaona Chen et al. Chemically programmed STING-activating nano-liposomal vesicles improve anticancer immunity. Nature Communications.2023;14;4584









