1.Introduction
TNBC is breast cancer that expresses neither progesterone receptor (PR), estrogen receptor (ER), nor human epidermal growth factor receptor-2 (HER-2). One in six occurrences of breast cancer are caused by it, with a 5-year recurrence and metastasis rate of 15% and a survival rate of approximately 15% after 5 years. Compared to other subtypes, TNBC has a worse prognosis and a higher chance of recurrence.
In TNBC immunotherapy, the immune system's activation is critical. The immune system can resist foreign or internal bacteria, viruses, and other harmful ingredients, and it prevents tumor growth and reduces the possibility of metastasis by monitoring, recognizing and destroying cancer cells in real time. To protect healthy cells, the immune system uses immunological checkpoints to perform self-control [1]. This method is based on the recognition and binding of T cell receptors (TCRs) to antigens contained in major histocompatibility complexes (MHCs) on the surface of antigen presenting cells (APCs). CTLA-4, PD-1, and PD-L1 are all related to T cell immune response inhibition.
With 288 amino acids, PD-1 is a transmembrane protein. The protein’s structure contains extracellular IgV domains, a transmembrane region, and a intracellular tail region. PD-1 inhibits TCR signaling, as evidenced by the two phosphorylation sites found in the immunoreceptor tyrosine-based switch and inhibitory motif in the intracellular tail region. The PDCD1 gene encodes the human PD-1 protein. One of the ligands of PD-1, PD-L1, is a protein that the human CD274 gene encodes. This protein is also a transmembrane, weighing 40 kDa in molecular weight. T cell proliferation is inhibited by signals transmitted by the combination of PD-L1 and PD-1 on T cells. A protein receptor which functions as an immunological checkpoint and suppresses immune responses is called CTLA-4, or CD152. The human CTLA-4 gene encodes the CTLA-4 protein. This protein includes an extracellular V domain (VL), a transmembrane domain, and a cytoplasmic tail region.
Immunotherapy has reflected promising results in many diseases of gastric cancer, kidney cancer, melanoma and so on. In TNBC, monoclonal antibodies, alone or in conjunction with various treatments involving chemotherapy, radiation, neoadjuvant therapy, and targeted therapy, seem promising in both beginning and later stages of the disease.
2.The difficulty of treating TNBC in comparison to other types of breast cancer
TNBC is distinguished by early onset, high malignancy and invasiveness, poor prognosis, and high recurrence rate. ER, PR and HER-2 are important anti-cancer targets for other breast cancers. The negative expression of ER, PR and HER-2 means that these three receptors are not expressed on the surface of breast cancer cells, or their expression levels are low, which will limit endocrine therapy, targeted therapy, hormone therapy, etc. Common traditional treatments include chemotherapy and cytotoxic therapy. Although chemotherapy can reduce the risk of recurrence of TNBC, the overall survival rate has not significantly improved; cytotoxic therapy can cause severe cardiac toxicity and damage the gastrointestinal tract. Therefore, in recent years, with the continuous enrichment of treatment methods, immunotherapy, including combination with other treatments, has shown great potential to have relatively greater survival benefits for TNBC patients.
3.Overview of TNBC immunotherapy
According to current clinical trials and drug research, TNBC immunotherapy mainly includes the following: checkpoint inhibitor therapy, antibody-drug conjugate therapy, PARP inhibitor therapy, and some other novel immunotherapy methods (Figure 1).
Figure 1. Common therapies for TNBC immunotherapy.
3.1.Immune Therapy with Checkpoint Inhibitors
Currently, ongoing clinical studies with immune checkpoint inhibitors can be broadly categorized into two types: monotherapy and combination therapy with other drugs. The latter is frequently used for treating locally progressed or metastatic triple-negative breast cancer (mTNBC). In clinical practice, most immune drugs are monoclonal antibodies targeting PD-1 and PD-L1, as well as some commonly used monoclonal antibodies against CTLA-4, Lymphocyte activation gene-3 (LAG-3), etc. Chemotherapy combined with immune therapy has been proven to possess safety and tolerance in other malignancies. Pure chemotherapy may generate new antigens and disrupt tumor immune evasion mechanisms [2]. Some cancers also have a low response rate to single-dose immune checkpoint blocking, so chemoimmunotherapy will have a better effect in clinical treatment and even have a certain synergistic effect.
3.1.1.PD-1 Inhibitors. Pembrolizumab refers to a humanized monoclonal immunoglobulin G4 antibody which connects to the PD-1 and suppresses its interaction with PD-L1 and PD-L2 [3,4]. The use of pembrolizumab is one of the methods for clinical treatment of mTNBC. In a study, conbined with pembrolizumab (immunotherapy) and eribulin (chemotherapy) was effective in treating mTNBC. The study included 167 individuals, with 7 in stage Ib and 160 in stage II. Group 1 had 66 patients that had never been treated before. The objective response rate and overall response rate (ORR) were both 25.8%; Group 2 included 101 patients who had already had therapy, and the ORR was counted as 21.8%. Patients with PD-L1+ tumors had a statistically greater ORR (combined positive score 1) than patients with PD-L1- tumors [5]. Another phase II clinical trial with pembrolizumab combination therapy, in comparison to a historical placebo group, patients received an intravenous injection of 200mg paclitaxel and oral therapy of 1000mg/m2 capecitabine every two days in the first two weeks of a twenty-one-day regimen. The main outcome was median progression-free survival (mPFS), while other outcomes were ORR, safety, and tolerability analysis, all of which showed significant improvement in the results. Compared with a historical control group treated with capecitabine alone, it was found that 80% of the subjects showed significant improvement in mPFS after adding paclitaxel for 2 months [6]. In the treatment of TNBC, the use of Pembrolizumab can improve event-free survival rate, but the prognosis still needs to be optimized to reduce the occurrence of adverse events (AEs) such as pneumonia, rash, and fatigue.
Nivolumab refers to a monoclonal antibody which targets the immunological checkpoint receptor PD-1 on the surface of T cells. Nab-paclitaxel has shown significant efficacy in other solid tumors, but clinical research on TNBC is still limited and currently in the in vitro research stage [7].
3.1.2.Inhibitors targeting PD-L1. Durvalumab is a human immunoglobulin G1κ (IgG1κ) monoclonal antibody which can inhibit the cooperation of PD-1 and PD-L1. In a clinical trial of durvalumab together with olaparib and paclitaxel, 73 participants randomly received standard paclitaxel neoadjuvant chemotherapy (DOP), and 299 participants received standard care (paclitaxel) control group. The combined efficacy of durvalumab and Olaparib raised the expected pathological complete response (pCR) rate from 20% to 37% (exceeding the control), from 27% to 47% in TNBC. AEs included thyroid dysfunction, fatigue, pneumonia, etc. [8].
Atezolizumab is an IgG1 monoclonal antibody which has been humanized and designed to be anti-PD-L1. In terms of efficacy, atezolizumab combined with albumin-bound paclitaxel can improve the treatment effect of TNBC, although this method significantly prolongs overall survival (OS) effect, this combination therapy also significantly alters PFS and ORR. However, compared with the use of albumin-bound paclitaxel alone, this combination produces greater toxicity [9]. There were 68 patients in a phase 2b clinical trial using atezolizumab coupled with anthracycline drugs for the treatment of mTNBC [10]. In the first group of 40 patients who got pegylated liposomal doxorubicin and cyclophosphamide at a low intensity, 18% experienced AEs, while the second group of 28 patients who received placebo, 7% experienced AEs [10]. In this study, the PFS of Group 1 improved after several months. After 15 months, the PFS of Group 1 further improved, with a rate of 14.7%, while the progression-free rate of Group 2 was 0% [10]. The combination therapy of atezolizumab monoclonal antibody had a higher incidence of AEs compared to monotherapy, mainly involving dermatology, gastrointestinal tract, and hematology, such as lymphadenopathy and subcutaneous nodules in the limbs [11].
Avelumab refers to an immune checkpoint inhibitor which blocks the PD-1 and PD-L1 pathway but doesn’t influence their relationship against human IgG1 monoclonal antibody which targets PD-L1. In a phase 1b study of JAVELIN solid tumor, there were 168 metastatic breast cancer (MBC) patients, comprising 58 TNBC patients, received avelumab treatment for 2-50 weeks. 13.7% of all experienced grade ≥3 treatment-related AEs, involving two cases of treatment-related deaths. The main AEs were fatigue, nausea, and hypothyroidism. The confirmed ORR for TNBC patients was 3.0% and 5.2%. PD-L1+ tumor-infiltrating immune cells demonstrated a better ORR than PD-L1- tumor-infiltrating immune cells in the total sample (16.70% vs 1.60%) and the TNBC subset (22.20% vs 2.60%) [12]. As a result, in a subgroup of TNBC patients, avelumab demonstrated adequate safety and clinical efficacy.
3.1.3.Inhibitors targeting CTLA-4. One of the earliest immune checkpoint medications to target the CTLA-4 pathway was tremelimumab. This IgG2 monoclonal antibody inhibits the interactions of the antigen-presenting cell ligands B7-1 (CD80), B7-2 (CD86), and CTLA-4 receptor generated on activated T cells, leading to an anti-tumor effect. There were 18 evaluable patients in a single-arm pilot study, 11 ER+ and 7 TNBC, and only three patients have the response (ORR=17%), hence the project didn’t progress to Phase 2. It can be seen that pertuzumab has a better clinical benefit rate in TNBC, but attention should also be paid to the occurrence of AEs such as rash and hepatitis [13].
Ipilimumab is a CTLA-4 pathway antagonist. It is a human IgG1 monoclonal antibody which blocks to the CTLA-4 receptor on activated T cells. Despite multiple clinical trials for the therapyt of tumors that are solid, there has been minimal research on the efficacy and toxicity of TNBC. A total of 19 individuals were included in a clinical trial testing ipilimumab in breast cancer patients, with 12 receiving ipilimumab treatment. Among these 12 patients, 6 received ipilimumab monotherapy, and the remaining 6 patients received cryoablation treatment in addition to ipilimumab monotherapy. Following combination immunotherapy, there was a rise in T helper type 1 cytokines in the tumor. Therefore, the combined use of ipilimumab and cryoablation may be beneficial for the therapy of breast cancer. This medication may cause diarrhea, constipation, and other side effects [14].
3.1.4.Other monoclonal antibody drugs and other targets. Dostarlimab is a PD-1 receptor antagonist presently being tested in clinical trials for the therapy of many different malignancies. Cemiplimab is a monoclonal IgG1 antibody which inhibits the PD-1 pathway. LAG-3 is a novel immunological therapy target that, in conjunction with PD-1, suppresses T cell activity [15]. Fianlimab (REGN3767) is another LAG-3 targeting antibody being tested as a neoadjuvant therapy with cemiplimab. Other immunotherapy techniques targeting checkpoint inhibitors are being studied, including VISTA [16], TIGIT [17], 4-1BB, and OX40 [18,19].
3.2.Antibody-Drug Conjugates (ADCs)
ADC is a targeted biopharmaceutical composed of monoclonal antibodies, linkers, and drugs, which uses monoclonal antibodies as carriers to transport small molecule drugs to tumor cells for action. According to the most recent research findings, Trophoblast cell-surface antigen 2 (Trop-2) is a viable target for anti-tumor therapy due to its high level of expression on the surface of feeding layer cells. Trop-2 is a transmembrane calcium signal transduction factor which is represented in humans by the TACSTD2 gene. It is commonly overexpressed in several forms of malignant epithelial cancers, including breast cancer [20]. Trop-2 actively interacts with multiple critical molecular signaling pathways that have historically been linked to cancer formation and progression, hence contributing to tumor progression.
Sacituzumab-govitecan-hziy (SG) refers to an anti-Trop-2 antibody-drug combination that has showed significant therapeutic results in patients with mTNBC when compared to chemotherapy. PFS and OS were both superior to monotherapy [21]. Patients with moderate or high Trop-2 expression showed the best efficacy. In a clinical trial including 468 patients, 235 were given SG and 233 were given chemotherapy (median age 54 years, all previously treated with paclitaxel). The mPFS with SG treatment was 5.6 months, 3.9 months higher than chemotherapy. Three patients in each group died following AEs, but none of the deaths were considered related to SG treatment [21]. AEs associated with SG in the treatment of mTNBC include neutropenia, vomiting, nausea, and dyspnea [22].
3.3.PARP inhibitors
PARP inhibitors are pharmacological inhibitors of poly ADP-ribose polymerase (PARP) that have been shown to have a significant effect in BRCA-related MBC and to enhance PD-L1 on the tumor cells, blocking T cell-mediated tumor killing [23]. Although PARP inhibitors are targeted drugs, combining them with checkpoint inhibitors can restore immune recognition in PARP inhibitor-treated breast cancer patients. Two regularly used monotherapy PARP inhibitors are olaparib and talazoparib. Table 1 displays the clinical data for both of them.
Olaparib, with the chemical formula C24H23FN4O3 and the chemical structure depicted in FIG.2, is an enzyme involved in DNA repair that is used to block PARP. In the PETREMACII experiment, patients with primary TNBC more than 2 cm were given olapani alone for 10 weeks before chemotherapy. 18 of 32 patients (56.3%) exhibited an objective response to olapani. Aside from hereditary HR mutations, olapani had a high clinical response rate, fewer adverse events, and a low likelihood of fatigue [24]. Another combination of vastatin with olapani and paclitaxel therapy has been described above.
Table 1. The clinical data of Olaparib and Talazoparib.
|
Drug |
clinical research |
therapeutic regimen |
mPFS (mth) |
ORR (%) |
|
Olaparib |
OlympiAD (49.6% TNBC) |
Olaparib vs chemo-therapy |
7 vs 4.2 |
59.90% vs 28.80% |
|
Talazoparib |
EMBRACA (46%TNBC) |
talazoparib vs che-motherapy |
8.6vs5.6 |
62.60%vs 27.20% |
Talazoparib, with the chemical formula C19H14F2N6O and the chemical structure shown in FIG.2, is an oral PARP inhibitor for treating advanced breast cancer with germline BRCA mutations. In a TNBC-containing test using aviote monoclonal antibodies and tarazopanil, the ORR was 18.2% and the median duration of remission (MDOR) was 11.1 (3.4-20.4) months; The ORR in the BRCA mutation and DNA damage repair (DDR) positive breast cancer cohort was 34.8%, and eight of nineteen patients got relief with DDR positive tumors, including six patients with BRCA1/2 mutant tumors, with an MDOR of 15.7 months. When the data was shut off, the response of three patients with BRCA mutant malignancies was nevertheless underway [24]. Anemia, thrombocytopenia, and neutropenia are the main AEs. The combination of Avelumab and Talazoparib has a good promise for treating TNBC, according to this experiment [25].
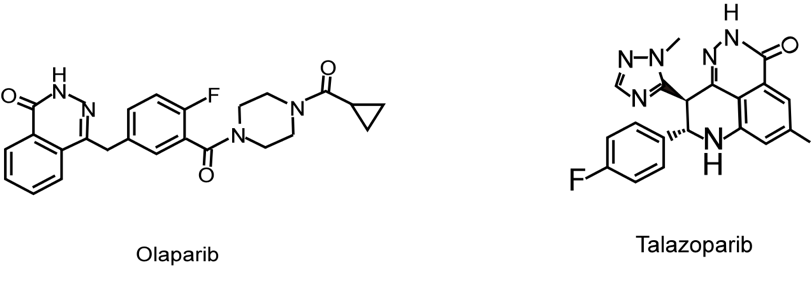
Figure 2. The chemical structure of Olaparib and Talazoparib.
4.Other novel immunotherapies
Other novel immunotherapeutic methods, such as cancer-testis antigens (CTA), chimeric antigen receptor-modified T cells (CAR-T), RNA vaccines, as well as novel antigen vaccines, are still under investigation and some clinical trials have revealed promising therapeutic prospects in.
4.1.Targeting cancer-testis antigens for immunotherapy
CTA is a tumor-associated antigen that has attracted widespread attention as a potential clinical biomarker in targeted immunotherapy due to its cancer-restricted expression and strong immunogenicity. Immunohistochemistry was used to evaluate the expression of NY-ESO-1, MAGEA1, MAGE-A4, KK-LC-1, and PRAME in 51 TNBC patients. KK-LC-1, MAGE-A4, and NY-ESO-1 expression was substantially greater in TNBC than in non-TNBC; 76.47% of TNBC had at least any one of the five CTAs. Patients who have MAGE-A4 or PRAME expression demonstrated substantially longer disease-free survival [26].
4.2.CAR-T therapy
CAR-T therapy refers to a more precise and efficient immunotherapy. The key barriers to treatment progress include molecular heterogeneity, low immunogenicity, and a lack of effective medicines, which the CAR-T therapy method effectively overcomes. This method combines resonant stimulation with T cell effector function. Although advances in the therapy of resistant hematologic malignancies have been made, there are still significant hurdles in the application of CAR-T therapy in solid tumors. The following are the primary barriers to CAR-T therapy in the treatment of solid tumors: 1. Only a subset of tumor cells contacted by CAR-T express the CAR-T-redirected target antigen; The immune-suppressive features of the tumor microenvironment limit CAR-T antitumor efficacy; 3. The extracellular matrix is an extremely crucial physical barriers linking tumor cells and CAR-T in solid tumor CAR-T treatment [27]. Many antigens have been discovered as possible targets for CAR-T cell treatment in TNBC through preclinical models and early clinical trials.
4.3.RNA vaccines
RNA vaccines are synthetic vaccines that are relatively safer compared to traditional vaccines. Their role is to use RNA to educate cells to generate antigens.Researchers created nanoparticles (NPs) to deliver mRNA vaccines expressing the tumor antigen MUC1 to dendritic cells in the lymph nodes in order to activate and increase tumour-specific T lymphocytes in an RNA vaccination investigation. CTLA-4 monoclonal antibodies are being combined associated with mRNA vaccines to increase anti-tumor efficacy. When in comparison with either vaccinations or monoclonal antibodies alone, combining vaccines with anti-CTLA-4 monoclonal antibodies can remarkably boost anti-tumor immune responses [28]. This shows that NP-based mRNA vaccines associated with CTLA-4 inhibitors used to treat TNBC could be a promising combination immunotherapy.
4.4.Multi-epitope peptide vaccine.
In cancer immunotherapy, multi-epitope peptide vaccines are receiving widespread attention. Currently, multiple multi-epitope peptide vaccines targeting TNBC are being designed, such as those based on Semaphorin 4A (Sema4A), myeloid zinc finger protein 1 (MZF1), and SOX9. Sema4A is a signaling protein recently identified as a major regulatory factor in TNBC progression; MZF1 is a transcription factor and a carcinogenic inducer of TNBC metastasis; SOX9 is also considered a key regulatory factor in TNBC metastasis. Using appropriate adjuvants and epitope combinations, these research teams identified and evaluated the potential of antigen peptide-induced immune responses against Sema4A, MZF1, and SOX9, and conducted immune simulation studies and computer cloning on the constructed vaccines, indicating that in the target organisms, the designed chimeric vaccines can elicit robust humoral and cellular immunological responses [29-31]. Based on this evidence, these types of vaccines may become a promising therapy for TNBC in the future.
5.Combination therapy
When compared to monotherapy, combination therapy has a greater effect in the therapy of TNBC. Current first-line treatment options typically include making use of immune checkpoint inhibitors in conjunction with chemotherapy, as well as the combination therapy of immune checkpoint inhibitors and PARP inhibitors, as discussed in the preceding section. There are, of course, several different combination regimens.
5.1.Combination Immunotherapy Based on Ferroptosis
Ferroptosis is a type of iron-dependent controlled death of cells characterized by the generation of lipid peroxides that differs genetically and biochemically from other kinds of regulated cell death such as apoptosis. The study team proposed and experimentally verified the combination therapy of GPX4 inhibitor, which inhibits glutathione peroxidase 4 (GPX4), and immune checkpoint inhibition for LAR tumors based on ferroptosis research in the context of breast cancer. A ferroptosis map was created by integrating multi-omics data from a large cohort of TNBC samples (n=465), indicating diverse phenotypes in iron death-related metabolites and pathways in TNBC. It has been demonstrated that inhibiting GPX4 not only promotes tumor iron death but also boosts anti-tumor immunity. Combination therapy with anti-PD1 antibodies and GPX4 inhibitors outperformed monotherapy [32].
5.2.Combination immunotherapy of angiogenesis inhibitors, PD-1 immune checkpoint inhibitors, and chemotherapy
The FUTURE-C-Plus phase II trial was designed to assess the possibility of combining famitinib (an angiogenesis inhibitor), camrelizumab (a PD-1 monoclonal antibody), and chemotherapy in patients who have advanced immunomodulatory TNBC. The objective response rate was 81.3%, and the mPFS was 13.6 months, with no treatment-related fatalities recorded [33]. This study demonstrates the triple combination regimen's effectiveness, safety, and therapeutic potential in immunomodulatory TNBC.
6.Conclusion
This article mostly provides a synopsis of the medicines and treatments available for TNBC immunotherapy. Although TNBC is the toughest type of breast cancer to fight against, and developing an optimum treatment plan for TNBC patients is a substantial unmet need, it is indisputable that immunotherapy has made significant advances in tumor treatment, providing promise for transforming outcomes for some patients. Significant progress has been achieved in TNBC checkpoint inhibitors and therapeutic medicines in recent years, resulting to the identification of various novel immunotherapies. This investigation and advancement of immunotherapy options holds promise for the future treatment of TNBC. Immunotherapy is still in its infancy, and it faces a number of hurdles that must be overcome and investigated further. Basic and translational research have the potential to create new opportunities for the treatment of TNBC and other cancers that now lack viable pharmacological treatments.
Authors Contribution
All the authors contributed equally and their names were listed in alphabetical order.
References
[1]. EITanbouly M A and Noelle R J Rethinking peripheral T cell tolerance: checkpoints across a T cell's journey 2021 Nat. Rev. Immunol 21 257-67
[2]. Emens L A and Middleton G The interplay of immunotherapy and chemotherapy: harnessing potential synergies 2015 Cancer. Immunol. Res 3 436-43
[3]. Amir E and Cescon D W Pembrolizumab monotherapy in metastatic triple-negative breast cancer 2021 Lancet. Oncol 22 415-17
[4]. Heeke A L and Tan A R Checkpoint inhibitor therapy for metastatic triple-negative breast cancer 2021 Cancer. Metastasis. Rev 40 537-47
[5]. Tolaney S M et al. Eribulin plus pembrolizumab in patients with metastatic triple-negative breast cancer (ENHANCE 1): a phase Ib/II study 2021 Clin. Cancer. Res 27 3061-68
[6]. Shah A N et al. Phase II study of pembrolizumab and capecitabine for triple negative and hormone receptor-positive, HER2-negative endocrine-refractory metastatic breast cancer 2020 J. Immunother. Cancer 8
[7]. Tentler J J et al. RX-5902, a novel beta-catenin modulator, potentiates the efficacy of immune checkpoint inhibitors in preclinical models of triple-negative breast cancer 2020 BMC. Cancer 20 1063
[8]. Pusztai L et al. Durvalumab with olaparib and paclitaxel for high-risk HER2-negative stage II/III breast cancer: results from the adaptively randomized I-SPY2 trial 2021 Cancer. Cell 39 989-98 e5
[9]. Sharmni Vishnu K, Win T T, Aye S N and Basavaraj A K Combined atezolizumab and nab-paclitaxel in the treatment of triple negative breast cancer: a meta-analysis on their efficacy and safety 2022 BMC. Cancer 22 1139
[10]. Rossevold A H et al. Atezolizumab plus anthracycline-based chemotherapy in metastatic triple-negative breast cancer: the randomized, double-blind phase 2b ALICE trial 2022 Nat. Med 28 2573-83
[11]. Tsunoda A et al. Atezolizumab-induced sarcoidosis-like reaction in a patient with metastatic breast cancer 2022 Case. Rep. Oncol. Med 2022 2709062
[12]. Dirix L Y et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer 2018 Breast. Cancer. Res. Treat 167 671-86
[13]. Santa-Maria C A et al. A pilot study of durvalumab and tremelimumab and immunogenomic dynamics in metastatic breast cancer 2018 Oncotarget 9 18985-96
[14]. McArthur H L et al. A pilot study of preoperative single-dose ipilimumab and/or cryoablation in women with early-stage breast cancer with comprehensive immune profiling 2016 Clin. Cancer. Res 22 5729-37
[15]. Andrews L P, Marciscano A E, Drake C G and Vignali D A LAG3(CD223) as a cancer immunotherapy target 2017 Immunol. Rev 276 80-96
[16]. Zong L, Mo s, Yu s, Zhou Y, Zhang M, Chen J and Xiang Y Expression of the immune checkpoint VISTA in breast cancer 2020 Cancer. Immunol. Immunother 69 1437-46
[17]. Boissiere-Michot F, Chateau M C, Thezenas S, Guiu S, Bobrie A and Jacot W Correlation of the TIGIT-PVR immune checkpoint axis with clinicopathological features in triple-negative breast cancer 2022 Front. Immunol 13 1058424
[18]. Morris A, Vetto J T, Ramstad T, Funatake C J, Choolun E, Entwisle C and Weinberg A D Induction of anti-mammary cancer immunity by engaging the OX-40 receptor in vivo 2001 Breast. Cancer. Res. Treat 67 71-80
[19]. Melake M J et al. OX40 and 4-1BB delineate distinct immune profiles in sarcoma 2022 Oncoimmunology 11 2066050
[20]. Kwapisz D Sacituzumab govitecan-hziy in breast cancer 2022 Am. J. Clin. Oncol 45 279-85
[21]. Bardia A et al. Sacituzumab govitecan in metastatic triple-negative breast cancer 2021 N. Engl. J. Med 384 1529-41
[22]. Spring L M, Nakajima E, Hutchinson J, Viscosi E, Blouin G, Weekes C, Rugo H, Moy B and Bardia A Sacituzumab govitecan for metastatic triple-negative breast cancer: clinical overview and management of potential toxicities 2021 Oncologist 26 827-34
[23]. Jiao S et al. PARP Inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression 2017 Clin. Cancer. Res 23 3711-20
[24]. Eikesdal H P et al. Olaparib monotherapy as primary treatment in unselected triple negative breast cancer 2021 Ann. Oncol 32 240-49
[25]. Yap T A et al. Avelumab plus talazoparib in patients with advanced solid tumors: the JAVELIN PARP medley nonrandomized controlled trial 2023 JAMA. Onco 9 40-50
[26]. Xiao J et al. Expression of four cancer-testis antigens in TNBC indicating potential universal immunotherapeutic targets 2023 J. Cancer. Res. Clin. Oncol
[27]. Nasiri F et al. CAR-T cell therapy in triple-negative breast cancer: hunting the invisible devil 2022 Front. Immunol 13 1018786
[28]. Liu L, Wang Y, Miao L, Liu Q, Musetti S, Li J and Huang L Combination immunotherapy of MUC1 mRNA nano-vaccine and CTLA-4 blockade effectively inhibits growth of triple negative breast cancer 2018 Mol. Ther 26 45-55
[29]. Paranthaman P and Veerappapillai S Design of a potential Sema4A-based multi-epitope vaccine to combat triple-negative breast cancer: an immunoinformatic approach 2023 Med. Oncol 40 105
[30]. Krishnamoorthy H R and Karuppasamy R Design and in silico validation of a novel MZF-1-based multi-epitope vaccine to combat metastatic triple negative breast cancer 2023 Vaccines (Basel) 11
[31]. Rajendran Krishnamoorthy H and Karuppasamy R Designing a novel SOX9 based multi-epitope vaccine to combat metastatic triple-negative breast cancer using immunoinformatics approach 2023 Mol. Divers 27 1829-42
[32]. Yang F et al. Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy 2023 Cell. Metab 35 84-100 e8
[33]. Wu S Y et al. Combined angiogenesis and PD-1 inhibition for immunomodulatory TNBC: concept exploration and biomarker analysis in the FUTURE-C-Plus trial 2022 Mol. Cancer 21 84
Cite this article
Yang,C.;Bao,J. (2023). Progress of related drugs and new drugs for immunotherapy of triple-negative breast cancer. Theoretical and Natural Science,27,158-166.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. EITanbouly M A and Noelle R J Rethinking peripheral T cell tolerance: checkpoints across a T cell's journey 2021 Nat. Rev. Immunol 21 257-67
[2]. Emens L A and Middleton G The interplay of immunotherapy and chemotherapy: harnessing potential synergies 2015 Cancer. Immunol. Res 3 436-43
[3]. Amir E and Cescon D W Pembrolizumab monotherapy in metastatic triple-negative breast cancer 2021 Lancet. Oncol 22 415-17
[4]. Heeke A L and Tan A R Checkpoint inhibitor therapy for metastatic triple-negative breast cancer 2021 Cancer. Metastasis. Rev 40 537-47
[5]. Tolaney S M et al. Eribulin plus pembrolizumab in patients with metastatic triple-negative breast cancer (ENHANCE 1): a phase Ib/II study 2021 Clin. Cancer. Res 27 3061-68
[6]. Shah A N et al. Phase II study of pembrolizumab and capecitabine for triple negative and hormone receptor-positive, HER2-negative endocrine-refractory metastatic breast cancer 2020 J. Immunother. Cancer 8
[7]. Tentler J J et al. RX-5902, a novel beta-catenin modulator, potentiates the efficacy of immune checkpoint inhibitors in preclinical models of triple-negative breast cancer 2020 BMC. Cancer 20 1063
[8]. Pusztai L et al. Durvalumab with olaparib and paclitaxel for high-risk HER2-negative stage II/III breast cancer: results from the adaptively randomized I-SPY2 trial 2021 Cancer. Cell 39 989-98 e5
[9]. Sharmni Vishnu K, Win T T, Aye S N and Basavaraj A K Combined atezolizumab and nab-paclitaxel in the treatment of triple negative breast cancer: a meta-analysis on their efficacy and safety 2022 BMC. Cancer 22 1139
[10]. Rossevold A H et al. Atezolizumab plus anthracycline-based chemotherapy in metastatic triple-negative breast cancer: the randomized, double-blind phase 2b ALICE trial 2022 Nat. Med 28 2573-83
[11]. Tsunoda A et al. Atezolizumab-induced sarcoidosis-like reaction in a patient with metastatic breast cancer 2022 Case. Rep. Oncol. Med 2022 2709062
[12]. Dirix L Y et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer 2018 Breast. Cancer. Res. Treat 167 671-86
[13]. Santa-Maria C A et al. A pilot study of durvalumab and tremelimumab and immunogenomic dynamics in metastatic breast cancer 2018 Oncotarget 9 18985-96
[14]. McArthur H L et al. A pilot study of preoperative single-dose ipilimumab and/or cryoablation in women with early-stage breast cancer with comprehensive immune profiling 2016 Clin. Cancer. Res 22 5729-37
[15]. Andrews L P, Marciscano A E, Drake C G and Vignali D A LAG3(CD223) as a cancer immunotherapy target 2017 Immunol. Rev 276 80-96
[16]. Zong L, Mo s, Yu s, Zhou Y, Zhang M, Chen J and Xiang Y Expression of the immune checkpoint VISTA in breast cancer 2020 Cancer. Immunol. Immunother 69 1437-46
[17]. Boissiere-Michot F, Chateau M C, Thezenas S, Guiu S, Bobrie A and Jacot W Correlation of the TIGIT-PVR immune checkpoint axis with clinicopathological features in triple-negative breast cancer 2022 Front. Immunol 13 1058424
[18]. Morris A, Vetto J T, Ramstad T, Funatake C J, Choolun E, Entwisle C and Weinberg A D Induction of anti-mammary cancer immunity by engaging the OX-40 receptor in vivo 2001 Breast. Cancer. Res. Treat 67 71-80
[19]. Melake M J et al. OX40 and 4-1BB delineate distinct immune profiles in sarcoma 2022 Oncoimmunology 11 2066050
[20]. Kwapisz D Sacituzumab govitecan-hziy in breast cancer 2022 Am. J. Clin. Oncol 45 279-85
[21]. Bardia A et al. Sacituzumab govitecan in metastatic triple-negative breast cancer 2021 N. Engl. J. Med 384 1529-41
[22]. Spring L M, Nakajima E, Hutchinson J, Viscosi E, Blouin G, Weekes C, Rugo H, Moy B and Bardia A Sacituzumab govitecan for metastatic triple-negative breast cancer: clinical overview and management of potential toxicities 2021 Oncologist 26 827-34
[23]. Jiao S et al. PARP Inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression 2017 Clin. Cancer. Res 23 3711-20
[24]. Eikesdal H P et al. Olaparib monotherapy as primary treatment in unselected triple negative breast cancer 2021 Ann. Oncol 32 240-49
[25]. Yap T A et al. Avelumab plus talazoparib in patients with advanced solid tumors: the JAVELIN PARP medley nonrandomized controlled trial 2023 JAMA. Onco 9 40-50
[26]. Xiao J et al. Expression of four cancer-testis antigens in TNBC indicating potential universal immunotherapeutic targets 2023 J. Cancer. Res. Clin. Oncol
[27]. Nasiri F et al. CAR-T cell therapy in triple-negative breast cancer: hunting the invisible devil 2022 Front. Immunol 13 1018786
[28]. Liu L, Wang Y, Miao L, Liu Q, Musetti S, Li J and Huang L Combination immunotherapy of MUC1 mRNA nano-vaccine and CTLA-4 blockade effectively inhibits growth of triple negative breast cancer 2018 Mol. Ther 26 45-55
[29]. Paranthaman P and Veerappapillai S Design of a potential Sema4A-based multi-epitope vaccine to combat triple-negative breast cancer: an immunoinformatic approach 2023 Med. Oncol 40 105
[30]. Krishnamoorthy H R and Karuppasamy R Design and in silico validation of a novel MZF-1-based multi-epitope vaccine to combat metastatic triple negative breast cancer 2023 Vaccines (Basel) 11
[31]. Rajendran Krishnamoorthy H and Karuppasamy R Designing a novel SOX9 based multi-epitope vaccine to combat metastatic triple-negative breast cancer using immunoinformatics approach 2023 Mol. Divers 27 1829-42
[32]. Yang F et al. Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy 2023 Cell. Metab 35 84-100 e8
[33]. Wu S Y et al. Combined angiogenesis and PD-1 inhibition for immunomodulatory TNBC: concept exploration and biomarker analysis in the FUTURE-C-Plus trial 2022 Mol. Cancer 21 84









