1. Introduction
In the intricate tapestry of nature’s biochemical intricacies, there exists a clandestine world of chemical warfare, where certain fungi unleash potent toxins known as mycotoxins. These enigmatic compounds, produced by various fungal species, are hidden threats that have captivated the attention of scientists, biochemists, and toxicologists. Understanding the intricate biochemistry of mycotoxins and their toxic mechanisms is not only a scientific endeavor but also a crucial step in mitigating their impact on agriculture, food safety, and human health.
Mycotoxins represent a diverse group of secondary metabolites, intricately crafted by fungi belonging to genera such as Aspergillus, Penicillium, and Fusarium. These microorganisms, often found in soil, decaying organic matter, and a myriad of agricultural products, have evolved the production of mycotoxins as part of their biological warfare. This chemical arsenal serves to inhibit the growth of competing microorganisms and protect the fungi from predators, creating a remarkable example of nature’s biochemistry at work. The biochemistry of mycotoxins is a multifaceted realm, encompassing their chemical structures, modes of action, and intricate interactions with living organisms. Each mycotoxin possesses unique chemical properties and exerts its toxic effects through specific biochemical mechanisms. Some mycotoxins, such as aflatoxins, are potent hepatotoxins, while others, like ochratoxins, have nephrotoxic properties. Elucidating the biochemical intricacies of these compounds is pivotal for understanding their impact on human and animal health.
Mycotoxins enter the human and animal food chains through contaminated crops and agricultural products. When ingested, they can interact with biomolecules within the digestive system, setting off a cascade of biochemical events. These toxins may bind to proteins, DNA, and enzymes, disrupting vital cellular processes and potentially leading to acute or chronic health consequences [1]. The biochemistry of mycotoxin toxicity also extends to their metabolism and biotransformation within the body. The liver plays a crucial role in metabolizing mycotoxins, with enzymes facilitating detoxification processes [2]. Genetic variations in these enzymes can influence an individual’s susceptibility to mycotoxin-induced toxicity, highlighting the interplay between genetics and biochemistry in toxicology. Moreover, the biochemical pathways of mycotoxin exposure can vary depending on the route of exposure. Whether through ingestion, inhalation, or dermal contact, mycotoxins undergo distinct biochemical transformations within the body, affecting the toxicokinetics and toxicodynamics of these compounds.
This research will uncover the intricate molecular mechanisms that underlie their toxic effects. This exploration will shed light on the biochemical intricacies of nature’s fungal toxins, highlighting the importance of ongoing research and vigilance in safeguarding agriculture, food safety, and human health against these hidden threats.
2. Origins of mycotoxins
Mycotoxins are the metabolic by-products of various fungal species, with some of the most well-known producers being Aspergillus, Penicillium, and Fusarium. These fungi are found ubiquitously in the environment, thriving in diverse habitats such as soil, decaying plant matter, and stored grains. Different fungal species are associated with the production of specific mycotoxins, each with its own set of ecological niches and preferred environmental conditions. Understanding the specific fungal sources of mycotoxins is fundamental to addressing contamination in agriculture and food production. The development of mycotoxins is contingent upon a range of environmental factors. Temperature, humidity, and the availability of substrates, such as plant material, influence the growth and toxin production of these fungi. For instance, elevated moisture levels and warm temperatures provide favorable conditions for the proliferation of mycotoxin-producing fungi, making post-harvest storage of crops particularly susceptible to contamination. Therefore, comprehending the environmental conditions that foster mycotoxin development is pivotal in implementing preventive measures to mitigate their presence in agricultural and food supply chains.
Mycotoxins, despite their negative implications for human and animal health, may play pivotal roles in the ecology and survival of the fungi that produce them. These toxins can function as defense mechanisms against other microorganisms and predators, safeguarding the fungus from competition and predation. Mycotoxins may have signalling functions that help fungi adapt to their surroundings. Understanding the ecological roles and adaptive significance of mycotoxins provides insights into the intricate interplay between fungi, their toxins, and the ecosystems they inhabit. This knowledge can contribute to a more holistic approach to managing mycotoxin contamination while respecting the fungi’s ecological importance.
Mycotoxins encompass a diverse array of toxic compounds, classified into several major groups based on their chemical structures and biological effects. Notable among these groups are aflatoxins, produced primarily by Aspergillus species, and infamous for their carcinogenic properties. Ochratoxins, derived from Aspergillus and Penicillium species, are known for their nephrotoxic and carcinogenic effects [3]. Trichothecenes, produced by various Fusarium species, have garnered attention due to their ability to inhibit protein synthesis and disrupt cellular functions. These are just a few examples, as mycotoxins are categorized into multiple groups, each with its own set of health risks and regulatory limits.
3. Chemical properties and structures of common mycotoxins
Common mycotoxins exhibit distinct chemical structures that contribute to their toxic nature [4]. Aflatoxins, for instance, are characterized by a difuran ring structure, with B1 and B2 forms possessing a bifurcated lactone ring. Ochratoxins feature a chlorinated phenylalanine group, and trichothecenes are characterized by a sesquiterpene core structure. The diversity in chemical structures reflects the varied origins of mycotoxins and their interactions with biological molecules, which ultimately underpin their toxicological effects. Understanding these structures is essential for developing analytical methods and targeted interventions for mycotoxin control.
Mycotoxins owe their toxicity to their ability to interact with crucial biomolecules within living organisms. These interactions are highly specific and can lead to various biochemical disruptions. For instance, aflatoxins have a potent affinity for DNA, causing mutations and potentially leading to cancer [5]. Ochratoxins disrupt cellular metabolism by inhibiting enzymes involved in protein synthesis. Trichothecenes interfere with the ribosome’s function, impeding protein production. Mycotoxins can also generate reactive oxygen species, contributing to oxidative stress and cellular damage. These biochemical characteristics of mycotoxins underline their adverse health effects and necessitate a deeper understanding of their mechanisms to develop effective preventive strategies and therapies.
4. Biochemical mechanisms of mycotoxin toxicity
Mycotoxin toxicity primarily hinges on their intricate interactions with vital biomolecules within living organisms. These toxins can form covalent bonds with proteins, altering their structures and functions. Aflatoxins, for instance, attach themselves to DNA, leading to mutations, while ochratoxins interfere with enzymes involved in cellular processes [2]. Moreover, mycotoxins can bind to cellular membranes, impacting their integrity and disrupting signal transduction pathways. Understanding the specifics of these interactions is crucial in deciphering the biochemical basis of mycotoxin-induced toxicity and its repercussions on health.
Mycotoxins disrupt cellular processes through various mechanisms, which ultimately disturb the equilibrium of vital biochemical pathways. Protein synthesis can be impeded, leading to reduced production of essential cellular components. Additionally, mycotoxins can interfere with key metabolic pathways, such as those responsible for energy production or the synthesis of cellular components [6]. These disruptions contribute to a cascade of events that can lead to cell damage, dysfunction, and, in the long term, adverse health effects in exposed organisms.
Different mycotoxins exert unique biochemical effects that correspond to their chemical structures and biological activities. For instance, aflatoxins are potent carcinogens due to their ability to induce mutations by forming adducts with DNA bases [7]. Ochratoxins disrupt cellular metabolism, particularly affecting the kidneys, and have been linked to renal toxicity. Trichothecenes, on the other hand, are characterized by their ability to inhibit protein synthesis, leading to immune system suppression and gastrointestinal issues [8]. By understanding these specific biochemical effects, researchers can better grasp the intricacies of mycotoxin toxicity and develop targeted strategies for prevention and treatment in cases of exposure.
5. Mycotoxin exposure pathways
Exposure to mycotoxins can occur through various routes, each with distinct implications for health [8]. Ingestion is a common pathway, with contaminated food and feed being a primary source. Inhalation is relevant in occupational settings, particularly for individuals working in environments with high levels of airborne mycotoxin-containing dust. Additionally, dermal contact can lead to mycotoxin absorption through the skin. The choice of exposure route can significantly affect the extent of mycotoxin absorption and distribution within the body, as well as the potential health consequences associated with each pathway.
Mycotoxins can undergo biochemical transformations once they enter the body, including processes such as metabolism, distribution, and elimination. The liver plays a central role in detoxifying mycotoxins through enzymatic reactions, which aim to render these toxins less harmful and more easily excretable [9]. However, some mycotoxins and their metabolites can still exert toxic effects within the body. Understanding these metabolic processes is crucial, as they can influence the duration and intensity of mycotoxin exposure and thus the potential health risks.
The absorption, distribution, metabolism, and elimination of mycotoxins, collectively referred to as toxicokinetic [10], dictate the concentration of these compounds in the body over time. Toxicodynamics, on the other hand, encompasses the biochemical effects mycotoxins have on the body [11], as shown in Figure 1, including their interaction with cellular components and biological pathways. The interplay between toxicokinetics and toxicodynamics is central to assessing mycotoxin-related health risks. It determines how long mycotoxins persist in the body, where they accumulate, and how they exert their adverse effects. Knowledge of these processes is vital in designing effective strategies to reduce mycotoxin exposure and mitigate their harmful impacts on human and animal health [12, 13].
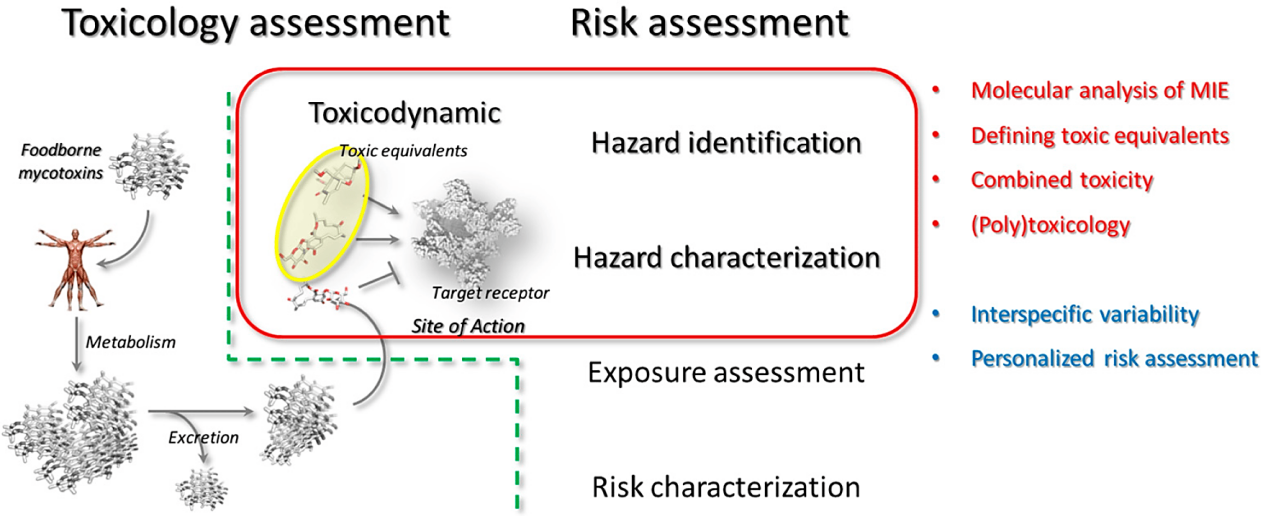
Figure 1. Interconnection between mycotoxins toxicology and risk assessment [11].
6. Metabolism and biotransformation of mycotoxins
The liver, a pivotal organ in the body’s detoxification machinery, plays a central role in processing mycotoxins once they enter the bloodstream. In response to mycotoxin exposure, the liver initiates a series of enzymatic reactions aimed at transforming these toxins into less harmful metabolites [12]. This detoxification process involves phase I and phase II reactions, which convert lipophilic mycotoxins into more hydrophilic forms, enhancing their excretion. While the liver’s detoxification efforts are generally protective, they can sometimes yield intermediary metabolites that retain toxicity. Understanding these processes is instrumental in comprehending mycotoxin elimination and the potential impact of detoxification on health.
Enzymes play a critical role in mycotoxin metabolism, catalyzing the various reactions involved in detoxification. Cytochrome P450 enzymes, a family of heme-containing proteins, are notably responsible for phase I reactions, which involve oxidation, reduction, and hydrolysis of mycotoxins. Phase II reactions, on the other hand, employ conjugation enzymes like glutathione-S-transferases, sulfotransferases, and UDP-glucuronosyltransferases to attach water-soluble moieties to mycotoxin metabolites, rendering them less toxic and facilitating excretion. The interplay of these enzymes in mycotoxin metabolism is central to the body’s defense against mycotoxin-induced harm.
Genetic factors play a significant role in determining an individual’s susceptibility to mycotoxin exposure. Polymorphisms in genes encoding detoxification enzymes can influence the efficiency of mycotoxin metabolism, affecting an individual’s ability to eliminate these toxins [12]. Genetic variations can lead to differences in mycotoxin clearance rates and the accumulation of toxic metabolites. Additionally, some individuals may possess genetic traits that make them more or less prone to the adverse health effects of mycotoxins. By understanding these genetic variations and their impact on metabolism and susceptibility, researchers can tailor prevention and intervention strategies to specific populations, ultimately enhancing the management of mycotoxin-related health risks.
7. Health effects of mycotoxins
Mycotoxins are notorious for their capacity to induce acute toxicity in individuals exposed to high concentrations of these toxic compounds. Such exposure can lead to a range of symptoms and clinical manifestations, including nausea, vomiting, diarrhea, abdominal pain, and in severe cases, organ failure or death. Aflatoxins, for example, can cause acute liver damage and may lead to acute aflatoxicosis, while the inhalation of mycotoxin-contaminated dust can result in respiratory distress [14]. Understanding the specific symptoms and pathways through which mycotoxins exert acute toxicity is crucial for prompt diagnosis and treatment in cases of poisoning.
In addition to acute toxicity, mycotoxin exposure is associated with a spectrum of chronic health conditions. Prolonged exposure to low levels of mycotoxins, often found in contaminated food and environments, can lead to insidious health effects. These include immune suppression, growth stunting, carcinogenesis, and a range of chronic diseases. Ochratoxins, for instance, are linked to nephrotoxicity and kidney disease. Mycotoxins can also contribute to immunosuppression, leaving individuals more susceptible to infections. Understanding the biochemical mechanisms that underlie these chronic health conditions is paramount for assessing long-term risks and implementing preventive measures.
Biochemical biomarkers play a crucial role in assessing mycotoxin exposure and associated health effects. By measuring specific molecules or indicators in biological samples, such as blood or urine, researchers and clinicians can identify the extent of exposure and assess the potential risks. Biomarkers can reveal the presence of mycotoxin metabolites, indicate the level of toxin exposure, and provide insights into the biochemical processes triggered by mycotoxins within the body. These biomarkers not only aid in diagnosis but also facilitate the monitoring of mycotoxin-related health conditions, enabling timely interventions and targeted strategies for individuals at risk.
8. Synergistic effects and co-exposure
Mycotoxin exposure often occurs alongside exposure to other toxic substances, resulting in intricate interactions. These interactions can amplify the adverse effects of mycotoxins and other toxins, potentially leading to more severe health consequences. Understanding how mycotoxins interact with these co-exposures is crucial for a comprehensive evaluation of health risks.
Synergistic toxicity involves the biochemical mechanisms by which mycotoxins and other toxic substances intensify each other’s harmful effects. This may result from cumulative damage to cellular components or interference with essential biochemical pathways. The understanding of these mechanisms aids in discerning how co-exposure impacts health and can inform the development of more effective risk assessments.
The recognition of synergistic effects and co-exposure has profound implications for risk assessment and public health policy. It necessitates a broader perspective on chemical exposures and emphasizes the need for comprehensive strategies that account for the combined impact of multiple toxic substances. Such insights are instrumental in shaping regulations and interventions aimed at safeguarding public health and reducing the risks associated with mycotoxin and co-exposure.
9. Analytical techniques for studying mycotoxins’ biochemistry
Accurate and sensitive methods for detecting and quantifying mycotoxins in biological samples are pivotal for understanding the extent of exposure and assessing health risks. Various analytical techniques, such as liquid chromatography-mass spectrometry (LC-MS) and enzyme-linked immunosorbent assays (ELISA), are employed for this purpose. These methods play a critical role in research, regulation, and public health, enabling the precise measurement of mycotoxin concentrations.
The field of mycotoxin analysis continually evolves with the emergence of advanced analytical tools and technologies. Innovative approaches, including high-resolution mass spectrometry and genomics-based methods, offer enhanced accuracy and efficiency in mycotoxin detection and quantification. Staying abreast of these technological advances is vital for maintaining the highest standards in mycotoxin research and regulation.
Accurate mycotoxin analysis is a cornerstone of both research and regulation. In research, it provides the basis for understanding exposure patterns, biochemical mechanisms, and health effects. In regulation, it informs safety standards and quality control measures for food and feed production. The reliability of analytical techniques directly influences the assessment and management of mycotoxin-related health risks, making precision and quality in analysis imperative.
10. Conclusion
Current research in mycotoxin biochemistry is characterized by a dynamic and evolving landscape. Scientists worldwide are actively investigating the latest developments in mycotoxin detection, exposure assessment, and health effects. This ongoing research expands our knowledge of mycotoxins and contributes to the development of more effective strategies for prevention and intervention. Despite significant progress, emerging issues and challenges continue to shape the field of mycotoxin biochemistry. Factors like climate change, altered agricultural practices, and evolving fungal strains pose new challenges in managing mycotoxin contamination. Additionally, the interplay between mycotoxins and human health remains a subject of constant scrutiny, underscoring the need for vigilance in addressing these challenges.
There are some potential directions for improving mycotoxin safety and mitigation. The future of mycotoxin safety and mitigation hinges on the development of innovative strategies and technologies. Potential directions include precision agriculture to reduce mycotoxin contamination at the source, advanced mycotoxin control measures, and the use of predictive modelling to anticipate mycotoxin outbreaks. These approaches hold the promise of enhancing food and feed safety and reducing the health risks associated with mycotoxin exposure. Throughout this exploration of mycotoxins, we have delved into their origins, classification, modes of action, exposure pathways, metabolism, health effects, synergistic interactions, analytical techniques, and the current state of research. We have unraveled the biochemical intricacies that underlie mycotoxin toxicity, from acute effects to chronic health conditions, considering the role of biochemical biomarkers and co-exposure with other toxic substances.
Mycotoxins continue to pose a formidable threat to agriculture, food safety, and human health. The knowledge we gain from unravelling their biochemical mysteries directly informs our ability to assess risks, develop preventive measures, and safeguard the well-being of both humans and animals. The agricultural sector relies on advancements in mycotoxin research to secure food supplies and prevent economic losses. As a cornerstone of public health, understanding mycotoxins’ biochemical complexities is paramount in ensuring the safety of the global food chain. In conclusion, our exploration of mycotoxins underscores the imperative of continued research and unwavering vigilance in addressing the biochemical complexities of these fungal toxins. The ever-evolving challenges presented by mycotoxins demand ongoing efforts to refine our understanding, detection methods, and risk management strategies. This necessitates a multidisciplinary approach, bringing together scientists, regulators, and industries to collaboratively mitigate the adverse effects of mycotoxin exposure. As we look ahead, the biochemical mysteries of mycotoxins will continue to unravel, paving the way for a safer, more secure future in agriculture, food safety, and public health.
References
[1]. Benkerroum N. 2020 International journal of environmental research and public health 17(2) 423
[2]. Li P, Su R, Yin R, et al. 2020 Toxins 12(2) 121
[3]. Malir F, Ostry V, Pfohl-Leszkowicz A, et al. 2016 Toxins 8(7) 191
[4]. Bräse S, Encinas A, Keck J, et al. 2009 Chemical reviews 109(9) 3903-3990
[5]. Wild C P, Turner P C. 2002 Mutagenesis 17(6) 471-481
[6]. Rocha O, Ansari K, Doohan F M. 2005 Food additives and contaminants 22(4) 369-378
[7]. Verma R J. 2004 International Journal of Human Genetics 4(4) 231-236
[8]. Wu Q, Wang X, Nepovimova E, et al. 2017 Archives of toxicology 91 3737-3785
[9]. Ellis W O, Smith J P, Simpson B K, et al. 1991 Critical Reviews in Food Science & Nutrition 30(4) 403-439
[10]. Lorenz N, Dänicke S, Edler L, et al. 2019 Mycotoxin research 35 27-46
[11]. Dellafiora L, Dall’Asta C, Galaverna G. 2018 Toxins 10(2) 52
[12]. Janik E, Niemcewicz M, Podogrocki M, et al. 2021 Molecules 26(22) 6868
[13]. Reverberi M, Ricelli A, Zjalic S, et al. 2010 Applied microbiology and biotechnology 87 899-911
[14]. Simon P. 1996 Journal of Toxicology: Toxin Reviews 15(3) 239-249
Cite this article
Qian,R. (2023). Unravelling the biochemical mysteries of mycotoxins: Nature's fungal toxins. Theoretical and Natural Science,27,216-222.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Benkerroum N. 2020 International journal of environmental research and public health 17(2) 423
[2]. Li P, Su R, Yin R, et al. 2020 Toxins 12(2) 121
[3]. Malir F, Ostry V, Pfohl-Leszkowicz A, et al. 2016 Toxins 8(7) 191
[4]. Bräse S, Encinas A, Keck J, et al. 2009 Chemical reviews 109(9) 3903-3990
[5]. Wild C P, Turner P C. 2002 Mutagenesis 17(6) 471-481
[6]. Rocha O, Ansari K, Doohan F M. 2005 Food additives and contaminants 22(4) 369-378
[7]. Verma R J. 2004 International Journal of Human Genetics 4(4) 231-236
[8]. Wu Q, Wang X, Nepovimova E, et al. 2017 Archives of toxicology 91 3737-3785
[9]. Ellis W O, Smith J P, Simpson B K, et al. 1991 Critical Reviews in Food Science & Nutrition 30(4) 403-439
[10]. Lorenz N, Dänicke S, Edler L, et al. 2019 Mycotoxin research 35 27-46
[11]. Dellafiora L, Dall’Asta C, Galaverna G. 2018 Toxins 10(2) 52
[12]. Janik E, Niemcewicz M, Podogrocki M, et al. 2021 Molecules 26(22) 6868
[13]. Reverberi M, Ricelli A, Zjalic S, et al. 2010 Applied microbiology and biotechnology 87 899-911
[14]. Simon P. 1996 Journal of Toxicology: Toxin Reviews 15(3) 239-249









