1. Introduction
Nasal congestion is generally defined as insufficient airflow to the nasal cavity sensed by the patient. Scientists define irreversible blockage of the nasal airway due to permanent structural changes as nasal obstruction. Still, the discrepancy is not considered in most research, so this research will not distinguish between these two terms. The most common symptoms are leaking mucus that results in an added effort to clean up due to rhinorrhoea, undermined quality of sleep which induces daytime sleepiness because of the stuffy nose at night, and mouth breathing leading to craniofacial growth disorders [1].
Nasal congestion inflicts sufferers and society in multiple ways, including direct costs involved in treating. Indirect costs arising from disturbed working and studying activities are also a significant component of the burden brought by nasal congestion [2]. For example, the constant sense of fullness in the nose brings substantial frustration. The stuffy nose inhibits patients’ ability to speak with clarity during socialisation. Disrupted sleep causes issues with combating fatigue and struggling to concentrate. In cases of severe congestion, decreased enjoyment of life also stems from losing smell. The consequent economic burden is never negligible. A 2020 study on chronic rhinosinusitis with nasal polyps (CRSwNP), whose main symptom is nasal congestion, found that the additional costs associated with CRSwNP per patient in Europe were approximately €1501, and the indirect costs associated with decreased productivity were €5659 per patient in a year [2].
There are already some feasible medical treatments available. Antihistamines reduce symptoms like sneezing and itching [3]. Intranasal corticosteroids greatly counter nasal mucosa inflammation. Leukotriene receptor antagonists (LTRAs), although not as effective as corticosteroids, mitigate allergic rhinitis. Numerous biologics, such as omalizumab, an anti-IgE antibody, and dupilumab, an antibody that binds to IL-4Rα, reduce inflammation by decreasing the amounts of their respective targets [4]. Allergen immunotherapy builds tolerance to allergens in allergic rhinitis [3]. Decongestants including alpha-adrenergic receptor agonists are short-term resorts for nasal congestion [5].
This research will comprehensively contrast the two main types of medical treatment for nasal congestion, alpha-adrenergic agonists and corticosteroids, in terms of mechanism of action, efficacy and possible side effects based on previous research and reviews, as a gap is found in focusing on comparing these two therapies, despite there are plenty of essays concentrating on only one of these drugs or giving a more thorough but less specific overview about nasal congestion. This research will facilitate the decision of use between alpha-adrenergic agonists and corticosteroids and provide prospective directions in future nasal congestion research.
2. The causes of nasal congestion
2.1. Allergic rhinitis
Immunoglobulin E (IgE), an antibody secreted by B cells in response to allergen exposure, binds to the surface of basophils and mast cells. During a subsequent invasion, the allergen attaches itself to these cells’ IgE antibodies, causing changes in gene transcription that result in the release of several chemokines and cytokines. These pro-inflammatory molecules include leukotrienes (IL) and histamine. The lining of the nasal cavity is infiltrated by inflammatory cells, such as mast cells, eosinophils, neutrophils, basophils, macrophages, and T lymphocytes [5]. The majority of those T cells are T helper 2 (Th2) cells, which release IL-3, 4, 5, and 13, which further encourage plasma cells to produce IgE. Arterioles dilate, vascular permeability increases, and rhinorrhoea and mucous secretion occur as a result [3], obstructing the nasal cavity.
2.2. Rhinosinusitis
Since rhinosinusitis is initially brought on by nasal inflammation, the term rhinosinusitis is now preferred over sinusitis. Rhinosinusitis is classified as acute or chronic based on whether it lasts less than 12 weeks or longer [5]. There are many similarities between the symptoms of allergic rhinitis (AR) and rhinosinusitis, including nasal discharge and nasal congestion. However, rhinosinusitis patients are more likely to experience facial pressure or pain, as well as anosmia or hyposmia (loss of scent or diminished sensitivity) [5].
2.3. Nasal polyposis
The internal mucosa has a thin layer of loose areolar connective tissue called the lamina propria. Due to the invasion of pathogens, a Th-2 response occurs, resulting in the secretion of Th2 cytokines and subsequently the induced accumulation of IgE and eosinophils. Nasal polyps are eventually formed when the sub-epithelial stroma is remodelled. It is again linked to inflammatory conditions that cause nasal congestion, such as chronic rhinosinusitis, asthma, and aspirin-exacerbated respiratory disease (AERD) [6].
2.4. Rhinitis medicamentosa
‘Rhinitis medicamentosa’ (RM) or ‘rebound congestion’ belongs to drug-induced rhinitis, and is caused by overusing topical nasal decongestants (usually more than 7 days) [5]. There are various intranasal decongestants, including ephedrine, phenylephrine, naphazoline, oxymetazoline, xylometazoline and so forth [7]. It accounts for one percent to nine percent of visits to allergy or otolaryngology clinics [8]. There has not been a definitive explanation of its pathophysiology, however, several reasonable hypotheses have been developed. There is a hypothesis that chronic vasoconstriction results in ischemia of the nasal mucosa and nasal oedema. The second hypothesis describes that congestion and reactive hyperaemia occur when the constrictor mechanisms are exploited, and sensitivity to decongestant molecules decreases. Higher doses are required for consistent use due to enhanced tolerance. Another theory is that greater vascular permeability and oedema stem from alterations in the vasomotor tone [7]. After being informed about rhinitis medicamentosa, the continued use of internasal decongestants must be stopped [7].
3. Mechanisms of action
3.1. Alpha-adrenergic agonists
G-protein coupled receptors (GPCR) for alpha-1 and -2 adrenergic receptors are found at the postsynaptic membrane of vascular smooth muscles [9]. Alpha-1 receptors are Gq type receptors. Following these receptors’ activation, phospholipase C (PLC) is activated. PLC catalyses the production of IP3 and DAG which have roles in signalling. IP3 induces the endoplasmic reticulum to release calcium ions upon entering the cytoplasm, and the resultant elevated intracellular calcium concentration causes smooth muscle to contract [10]. The stimulation of alpha-2 receptors has also been proven to relieve nasal congestion via contraction of nasal mucosa due to its effects on the vasoconstriction of capacitance vessels in different species [11].
3.2. Corticosteroids
The adrenal cortex produces the hormones known as corticosteroids (CSs). Glucocorticoids (GCs) and mineralocorticoids are included in CS. GCs mainly exhibit anti-inflammatory and immunosuppressive properties. In order to prevent inflammation or the influx of inflammatory cells into the airway, GCs have an effect on the cells that line the airway, controlling gene transcription through glucocorticoid receptors (GRs), a subclass of nuclear receptor. Pro-inflammatory cytokines like TNF-α, IL-1β, and IL-6, as well as their receptors, have their expression inhibited [12]. Hence the reduced level of inflammation mitigates nasal congestion.
3.3. Comparison
Alpha-adrenergic receptor agonist sprays can cause contraction of smooth muscles after binding to their receptors to lower the extent of nasal congestion rapidly. They affect the eventual manifestation of the congestion-causing disease. Meanwhile, the prolonged use of intranasal corticosteroids allows them to present anti-inflammatory effects which ultimately suppress nasal cavity inflammation. They alter the fundamental mechanism that causes nasal congestion.
4. Efficacy
Imidazolines oxymetazoline and xylometazoline are major α-adrenergic receptor agonists as nasal decongestants. However, they have slight differences in mode of action and therefore efficacy due to discrepancies in affinity and potency at the α-adrenergic receptor subtypes [13]. 0.1% xylometazoline has a great decongestant effect for up to 10 hours in contrast to placebo sprays. It is an effective resort for treating nasal congestion and does not present rebound congestion within 10 days [14], as shown in Figure 1. The efficacy of oxymetazoline (0.05%) spray for the first time was examined to relieve nasal congestion for a maximum of 12 hours in one dosage [15].
INCS administration is fast and simple. CSs are superior to most agents and are a first-line therapy of AR [5, 16]. One medication that has been shown to be useful in treating nasal congestion brought on by seasonal allergic rhinitis is mometasone furoate (MF). The symptoms can be relieved with MF nasal spray (MFNS) administrated once a day and further improved with continuous use for over 14 days. With a duration of 15 days of MFNS administration, 18% more patients reported a >30% improvement in nasal congestion than the placebo group, and there are 6% more patients who received MFNS responded with a >50% relief than the control group [17].
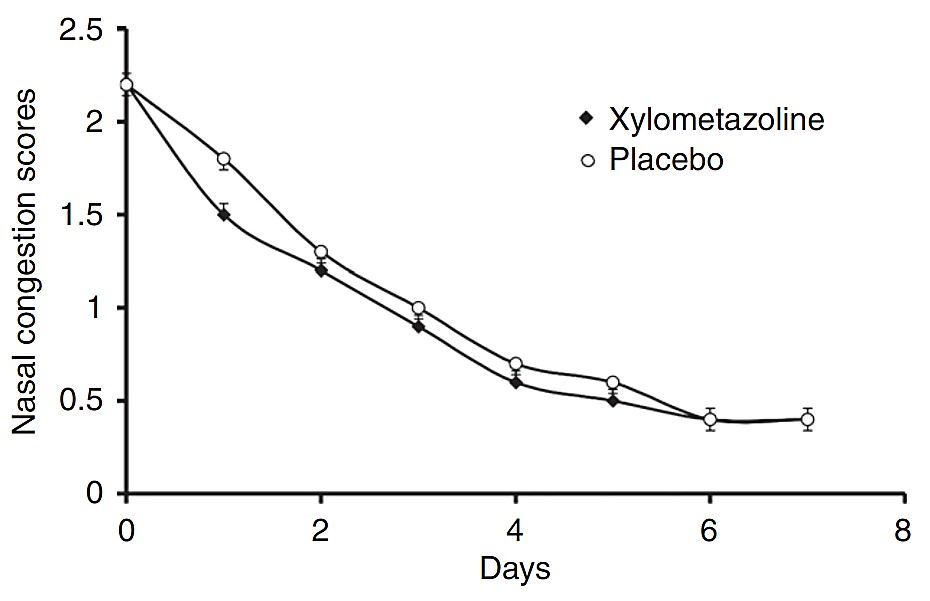
Figure 1. The nasal congestion symptom score for intranasal treatment with xylometazoline and placebo [14].
Alpha-adrenergic receptor agonist nasal decongestants including oxymetazoline and xylometazoline present excellent ability in nasal congestion relief, for example, Barnes et al. verified the effect of intranasal xylometazoline within 15 minutes after the spray was greater than the daily administration of INMF after 28 days [5, 18]. However, α-adrenergic agonists provide quick relief by stimulating the contraction of the smooth muscles, without directly targeting the underlying inflammation. Therefore, they are recommended for a temporary alleviation of the stuffy nose, for example, in a period of having a cold. In contrast, as drugs aiming to suppress inflammation which causes nasal congestion in the first place, corticosteroids can gradually mitigate the symptoms in the long term fundamentally.
It is worth noting the potential of the combination therapy of intranasal alpha-adrenergic receptor agonists and corticosteroids. Neighbors et al. analysed the enhanced effect of the combined use of corticosteroid and oxymetazoline sprays against rhinitis symptoms compared with separate ones with no sign of induced rhinitis medicamentosa [19]. However, this strategy is seldom investigated and carried out. Further elucidation of its efficacy and possible side effects is still required [5].
5. Safety
Rhinitis medicamentosa is a result of overusing topical nasal decongestants, usually for more than a week, which in turn causes nasal congestion again [7]. Along with dryness, epistaxis, burning, and such moderate symptoms [5, 14], some other side effects may also occur as the dose goes high or in specific predispositions. Alpha-1 agonists may induce hypertension, dysrhythmias including tachycardia and so forth. Sleepiness and drowsiness are two side effects of alpha-2 receptor agonists frequently observed. [9].
Hitherto, intranasal corticosteroids (INCSs) have always been a relatively safe drug. Discomfort or burning, epistaxis, and dryness are symptoms similar to alpha agonists. Crusting, headache, sore throat and foul taste are mild symptoms but are of little prevalence. Septal perforation was reported in several isolated cases, and there was anecdotal data on temporary increased intraocular pressure [5]. The intranasal corticosteroid mometasone furoate, for instance, possesses excellent safety. Pregnant women and growing children can safely use it. The populace has verified its excellence in efficacy and safety since it has been on the market for about 24 years [16].
The nature of these drugs being intranasal sprays indicates that the nasal mucosa might be irritated due to improper ways of administration. These include mild symptoms such as discomfort resulting from burning, epistaxis, dryness, crusting, and sore throat. Hypertension, dysrhythmias, sedation and fatigue are probable adverse effects of alpha-adrenergic receptor agonists, but rhinitis medicamentosa should draw more attention since a vicious circle of nasal congestion may be generated by overusing topical nasal decongestants indiscriminately. INCSs, however, have no harms other than the ones mentioned before and are preferred for long-term use.
6. Conclusions
Nasal congestion, inflicting inconvenience on both individuals and societies, stems from swelling from inflamed nasal mucosa. Intranasal alpha-adrenergic receptor agonists and corticosteroids as prevalent medical therapies, can be utilised in different situations due to the discrepancies in modes of action, effectiveness and potential undesirable effects. Alpha-adrenergic receptor agonists have potent effects on the amelioration of nasal congestion but imply the risk of rhinitis medicamentosa from long-term use. Corticosteroid sprays provide gradual alleviation of nasal inflammation thus improving nasal ventilation. This research further clarifies and emphasises their application scenarios and underlying reasons, facilitating the understanding of the links and differences between these two drugs. With improved explanation, patients suffering from nasal congestion may opt for the most suitable medication while the misuse of intranasal decongestants leading to the vicious cycle of rebound congestion, which is a vital issue, can be addressed. Unfortunately, according to current literature, there are still several aspects of the mechanisms of action that need clarification for better application of the sprays in the future. At the same time, investigations into their combined use provide promising prospects in nasal congestion therapy.
References
[1]. Grippaudo C, Paolantonio EG, Antonini G, Saulle R, La Torre G, Deli R. 2016 Acta. Otorhinolaryngol. Ital. 36(5) 386-394
[2]. Lourijsen E S, Fokkens W J, Reitsma S. 2020 Rhinology 58(3) 213-217
[3]. Small P, Keith P K, Kim H. 2018 Allergy, asthma & clinical immunology 14(2) 1-11
[4]. Laidlaw TM and Buchheit KM. 2020 Ann Allergy Asthma Immunol 124(4) 326-332
[5]. Wise S K, Damask C, Roland L T, et al. 2023 International forum of allergy & rhinology 13(4) 293-859
[6]. Goulioumis AK, Kourelis K, Gkorpa M, Danielides V. 2023 Indian J. Otolaryngol. Head Neck Surg. 75(Suppl 1) 733-741
[7]. Wahid NWB, Shermetaro C. Rhinitis Medicamentosa. StatPearls, Treasure Island (FL): StatPearls Publishing; 2023.
[8]. Lockey R F. 2006 Journal of allergy and clinical immunology 118(5) 1017-1018
[9]. Taylor BN, Cassagnol M. Alpha-Adrenergic Receptors. StatPearls, Treasure Island (FL): StatPearls Publishing; 2023.
[10]. Biazi GR, Frasson IG, Miksza DR, de Morais H, de Fatima Silva F, Bertolini GL, et al. 2018 J. Cell Biochem. 119(9) 7300-7309
[11]. Corboz M R, Rivelli M A, Mingo G G, et al. 2008 Pulmonary pharmacology & therapeutics 21(3) 449-454
[12]. Derwich M, Mitus-Kenig M and Pawlowska E. 2021 Int. J. Mol. Sci. 22(14) 7405
[13]. Haenisch B, Walstab J, Herberhold S, Bootz F, Tschaikin M, Ramseger R, et al. 2010 Fundam. Clin. Pharmacol. 24(6) 729-739
[14]. Eccles R, Martensson K, Chen S C. 2010 Current medical research and opinion 26(4) 889-899
[15]. Druce H M, Ramsey D L, Karnati S, et al. 2018 Rhinology 56(4) 343-350
[16]. Passali D, Spinosi M C, Crisanti A, et al. 2016 Multidisciplinary Respiratory Medicine 11(1) 1-5.
[17]. Urdaneta E, Tunceli K, Gates D. 2019 Allergy & Asthma Proceedings 40(3) 173-179
[18]. Barnes M L, Biallosterski B T, Gray R D, et al. 2005 Rhinology 43(4) 291
[19]. Neighbors C L, Salvador C F, Zhu B, et al. 2022 The Journal of Laryngology & Otology 136(1) 8-16.
Cite this article
Dai,Y. (2023). Intranasal alpha-adrenergic receptor agonists and corticosteroids as medical treatments for nasal congestion. Theoretical and Natural Science,27,230-234.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Grippaudo C, Paolantonio EG, Antonini G, Saulle R, La Torre G, Deli R. 2016 Acta. Otorhinolaryngol. Ital. 36(5) 386-394
[2]. Lourijsen E S, Fokkens W J, Reitsma S. 2020 Rhinology 58(3) 213-217
[3]. Small P, Keith P K, Kim H. 2018 Allergy, asthma & clinical immunology 14(2) 1-11
[4]. Laidlaw TM and Buchheit KM. 2020 Ann Allergy Asthma Immunol 124(4) 326-332
[5]. Wise S K, Damask C, Roland L T, et al. 2023 International forum of allergy & rhinology 13(4) 293-859
[6]. Goulioumis AK, Kourelis K, Gkorpa M, Danielides V. 2023 Indian J. Otolaryngol. Head Neck Surg. 75(Suppl 1) 733-741
[7]. Wahid NWB, Shermetaro C. Rhinitis Medicamentosa. StatPearls, Treasure Island (FL): StatPearls Publishing; 2023.
[8]. Lockey R F. 2006 Journal of allergy and clinical immunology 118(5) 1017-1018
[9]. Taylor BN, Cassagnol M. Alpha-Adrenergic Receptors. StatPearls, Treasure Island (FL): StatPearls Publishing; 2023.
[10]. Biazi GR, Frasson IG, Miksza DR, de Morais H, de Fatima Silva F, Bertolini GL, et al. 2018 J. Cell Biochem. 119(9) 7300-7309
[11]. Corboz M R, Rivelli M A, Mingo G G, et al. 2008 Pulmonary pharmacology & therapeutics 21(3) 449-454
[12]. Derwich M, Mitus-Kenig M and Pawlowska E. 2021 Int. J. Mol. Sci. 22(14) 7405
[13]. Haenisch B, Walstab J, Herberhold S, Bootz F, Tschaikin M, Ramseger R, et al. 2010 Fundam. Clin. Pharmacol. 24(6) 729-739
[14]. Eccles R, Martensson K, Chen S C. 2010 Current medical research and opinion 26(4) 889-899
[15]. Druce H M, Ramsey D L, Karnati S, et al. 2018 Rhinology 56(4) 343-350
[16]. Passali D, Spinosi M C, Crisanti A, et al. 2016 Multidisciplinary Respiratory Medicine 11(1) 1-5.
[17]. Urdaneta E, Tunceli K, Gates D. 2019 Allergy & Asthma Proceedings 40(3) 173-179
[18]. Barnes M L, Biallosterski B T, Gray R D, et al. 2005 Rhinology 43(4) 291
[19]. Neighbors C L, Salvador C F, Zhu B, et al. 2022 The Journal of Laryngology & Otology 136(1) 8-16.









