1. Introduction
Cyanide, an inorganic salt or organic substance with a cyano group, is a deadly xenobiotic. Cyanide can be obtained from a variety of sources, including industrial, medical, and even common household products. In addition, any material containing carbon and nitrogen may release cyanide during pyrolysis, such as many synthetic polymers or certain natural materials. Cyanide is known for its powerful toxic effects and transient onset. This highly effective and deadly poison is often used as the first choice for suicide, poisoning, assassination and terrorism. Cyanide can actually be seen everywhere in our lives. And also, cyanide is widely present in nature, and cyanide in plants is found in seeds such as bitter almonds, loquat kernels and apple kernels, as well as cassava, corn, beans and a large number of plants. Amygdalin, found in plum seeds and pits, is the most well-known food source of cyanide. Amygdalin is a cyanogenic glucoside, a sugar compound with a cyanogenic group attached, and it is also a purified form of the natural cyanide compound, amygdalin. The significant problem with intravenous amygdalin is not cyanide toxicity, but its constant excretion in the urine. Amygdalin can be metabolized to hydrogen cyanide by the enzyme glucosidase in the gastrointestinal tract.
Sources of cyanide include not only a variety of natural plants as well as industrial synthesis, but can also be released from certain compounds through chemical reactions [1]. For example, amygdalin cyanide poisoning, amygdalin is usually different concentrations of amygdalin, amygdalin obtained from certain nuts or stone kernels enters the human body with food ingestion, and cyanide can be released in the emulsifying enzyme complex when it is catabolized by B-glucosidase. In this case, amygdalin is ingested with emulsified foods, and small doses can lead to severe poisoning or death. If amygdalin ingested from plant sources has a delayed onset of symptoms and a slower progression, it provides more time to diagnosis and intervention clinically, and is more likely to obtain favorable outcomes.
Cyanide is both widely available and readily available in various forms. One form of cyanide is highly active salts, volatile, water-soluble liquid forms of hydrogen cyanide or cyanide chloride, which can be used in many industrial applications, including chemical synthesis, electroplating, metallurgy, and as pesticides. Another common form is a chemical group called nitrile, such as acetonitrile and propionitrile. As parent compounds, they do not produce significant toxicity, but are metabolized and decomposed into cyanide under the action of the corresponding enzymes in the liver. People exposed to these organic cyanides experience delayed signs and symptoms because an incubation period of several hours passes, during which the toxin turns to form and is released. When the toxin forms and is released after an incubation period of several hours, the patient will experience delayed signs and symptoms. In addition, hydrogen cyanide gas is produced when these salts are mixed with strong acids, posing significant risks to both industrial accidents and intentional exposure. There is the form of insoluble salts such as mercury cyanide and copper cyanide, which can be found in industrial settings, especially in waste generated during mining. Although they are significantly less toxic than their salt counterparts, they can still cause considerable impact and harm to the environment by accumulating them.
Until chemicals were isolated and identified, cyanide was used as a poison. Cyanide is actually an important chemical raw material, mainly used for gold refining, electroplating, metal treatment and basic chemical synthesis, such as organic synthesis of medicine, pesticides and food additives. Cyanide can enter the human body through skin contact, respiratory inhalation, oral administration, injection and other ways. Its ingestion can lead to a high body burden of cyanide, severe symptoms, and unique toxicodynamics [2]. Cyanide exposure has the ability to cause significant social disruption, such as causing severe social unrest and public panic, a threat that requires focused public health attention and preparedness. This research will discuss the toxicity, symptoms, and treatment methods of cyanide.
2. Toxicity of cyanide
Cyanide is highly toxic and deadly, mainly due to its rapidity of action. The form of cyanide significantly affects toxic doses. Cyanide poisoning is still a major threat to human health and safety, commonly known as potassium cyanide (KCN), hydrocyanic acid (hydrogen cyanide), and sodium cyanide (NaCN).
The main effect of cyanide poisoning is caused by inducing non-competitive inhibition of the activity of chromosomal cytochrome oxidase. The mechanism of action of cyanide is that the precipitated cyanide ions combine with the trivalent iron of oxidized cytochrome oxidase in mitochondria, preventing its reduction, resulting in blockage of the cellular respiratory chain, disorders of the cellular respiratory system, and a series of reaction consequences caused by tissue cell hypoxia, such as oxygen oxidative metabolism, phosphorylation, cellular hypoxia and lactic acidosis [3]. This binding of cyanogenic ions enables almost complete inhibition of cytochrome oxidase activity, resulting in anaerobic metabolism, severe reduction in ATP production and lactic acid accumulation [1].
Iatrogenic sources are also one of the causes of cyanide poisoning. Each molecule of potent antihypertensive nitroprusside releases up to five cyanide groups, so toxicity may develop as cyanide accumulates in the body with long-term drug use. The toxicity of a substance is inextricably linked to its dose. There are certain safe limits to cyanide intake, and its lethal dose is related to a person’s age, weight, physical strength, and even the amount of food left in the stomach at that time. In general, the average lethal amount of sodium cyanide is 150 mg, potassium cyanide 200 mg, hydrogen cyanide 100 mg, that is, adults oral 150-250 mg cyanide can cause sudden death. Cyanide dissociates cyano-based ions in the human body, which cause a series of biochemical reaction barriers within cells. Cyanide ions can bind to ferric iron in oxidized cytochrome oxidase in the mitochondria, hinder the reduction of ferric iron in oxidase, thereby affecting normal cell respiration, so that tissue cells cannot use oxygen in the blood, resulting in tissue hypoxia, and the body falls into a state of internal asphyxia. The central nervous system will rapidly lose function, and then the human body will have respiratory muscle paralysis, cardiac arrest, multiple organ failure and other symptoms and rapid death.
It is estimated that the lethal dose for adults is 50-200 mg. In fact, the lethal dose of cyanide is actually relatively high compared to other potential chemical weapons such as botulinum toxin. Its mechanism of action is the rapid diffusion of cyanide into tissues and irreversible binding to the target site. Symptoms of intravenous and inhaled cyanide occur most rapidly, while apparent toxicity from ingestion is delayed by several minutes until a certain concentration has accumulated in the blood. Because acute cyanide toxicity lacks characteristic symptoms and lacks a reasonable index of suspicion, it is often easily confused with other poisoning syndromes or missed. Victims may have bitter taste of almonds, but only about 40-60% of the population has the genes needed to detect this smell, which is not a reliable marker. A quick indicator of cyanide is to visualize retinal arteries and veins in red. This is due to an abnormal increase in poor oxygen extraction oxygen in the intravenous supply and more pronounced red. Lactic acid concentration can be used as a marker for the severity of cyanide poisoning. Cyanide concentrations are used only to confirm exposure, and patient care should be based on a suspicion index and clinical presentation.
The cyanide poisoning is a metabolic acidosis, due to the transformation of aerobic metabolism to anaerobic metabolism, resulting in a significant increase in lactic acid concentration, and severe hyperanion gap acidosis follows. Cyanide induces cellular hypoxia, leading to oxidative stress and lipid membrane peroxidation. The long-term effects of cyanide exposure include significant complications such as neurological disorders.
3. Symptoms of cyanide poisoning
Some clinical signs and symptoms may be associated with cyanide poisoning [1]. Clinical manifestations are usually initially hyperpnea and central nervous system irritation. Thus, there are different late symptoms such as vomiting and bradycardia. Long-term consumption of cyanated substances can also indirectly cause some related syndromes, such as optic nerve atrophy, nutritional ataxia neuropathy and other neurological diseases. The symptoms and onset time of cyanide poisoning are closely related to the physicochemical structure of cyanide. The onset time of cyanide in different phases varies by several times, with gaseous hydrocyanic acid being a few seconds, while solid or liquid cyanide is a few minutes, and cyanide-producing compounds taking several hours. Therefore, effective treatment of cyanide poisoning requires a race against the clock.
4. Analysis of treatment methods
The pathway of endogenous cyanide metabolism occurs mainly in the liver, and sulfur and cyanocobalamin’s reaction metabolism is normally encountered in low concentrations of cyanide, and some enzymes act to catalyze the conversion of cyanide to thiocyanate. These enzymatic pathways, while very effective, are not sufficient to work effectively in cases of poisoning due to sulfur donor depletion. In cases of acute intoxication, endogenous. The pathways of cyanide metabolism are rapidly overwhelmed and the depletion of conjugated substrates allows cyanide accumulation and progression of toxic effects.
Diagnosing cyanide poisoning is difficult, especially for emergency physician or rescue worker at the scene, which remains a challenge on their capability of the fast and ‘on the scene’ suspect or diagnosis of cyanide poisoning. The difficulty of cyanide poisoning clinical diagnosis is related to the poisoning and the history of aetiology. For example, in fire victims, carboxyhemoglobin, the identifier of carbon monoxide poisoning is easy to measure and durable, while cyanide toxin identification is difficult due to its short half-life and poor stability [3]. However, asphyxia syndrome can be induced by carbon monoxide or cyanide, suggesting that clinicians cannot confirm the diagnosis by examination of the exposed site. Therefore, cyanide poisoning caused by smoke inhalation, and the patient’s signs and symptoms related to cyanide exposure or carbon monoxide exposure remain to be distinguished and clarified [3]. Cyanide poisoning can cause adverse effects in addition to inhalation or ingestion of adequate doses of cyanide. Because there are many sources of potential cyanide exposure and there is no specific cyanide assay suitable for acutely poisoned patients, the diagnosis of cyanide is based on a history of cyanide exposure. Supportive measures can be used for the treatment of cyanide, as well as specific and effective antidotes available.
Cyanide poisoning also has specific symptoms that aid in its presence and diagnosis. For example, hydrocyanic acid smells bitter almonds, and when this taste is present, this finding has high diagnostic value. However, there is a high probability that no one noticed the bitter almond smell, or was hindered by other odors such as a strong smoke smell. Although the symptoms of cyanide or cyanide compound poisoning have been described in detail, they remain difficult to diagnose due to their complexity and diversity. There are also symptoms that can only be made after a post-mortem and are proven by prior post-mortem attempts. In patients without an overt cyanide poisoning syndrome, acid lactic acid acidosis with normoglycemia, convulsions, and tachypnea with cyanosis at the time of death is likely to be caused by cyanide poisoning. Blood cyanide concentration cannot be measured on the spot and results can be obtained, so it is still not very useful and helpful in emergency situations. In addition, the determination and diagnosis of cyanide concentration in the blood is still correspondingly difficult. Due to the instability of cyanide in the blood, it will quickly disappear and decompose, so the sooner the better when determining and analyzing, otherwise its accurate concentration cannot be obtained, and it is easy to underestimate its severity. The lethal dose of human cyanide and its corresponding blood cyanide concentration vary according to the human constitution, so it cannot be accurately defined [3]. Because the clinical manifestations, diagnosis, and prognostic factors of cyanide poisoning are unclear, emergency management and optimal treatment options for cyanide poisoning remain challenging and subject to discussion. The survival prognosis of cyanide poisoning is related to the form, route, quantity, type of poisoning, and speed of treatment.
Although cyanide has many possible sources of exposure and exposure to ingestion routes in everyday life, life-threatening acute cyanide poisoning is rare [1]. Cyanide is one of the few toxic drugs with a specific antidote. In fact, cyanide in life will not have too much impact on the human body as long as it is properly handled. Heating food containing cyanide can effectively remove the cyanide and evaporate it. In addition, the common sodium cyanide and potassium cyanide are a solid crystal or powder in the normal state, which is more stable and easier to control, and will not have harmful effects on the environment or the human body in this case. Cyanide is not a cumulative poison, it is easily degraded or eliminated, and the relevant technology has been perfected. The microorganisms in the natural water body have a strong purification effect on low concentrations of cyanide, which can be converted into non-toxic compounds, and the human body also has a certain degradation ability to the cyanide, and there is also a corresponding detoxification mechanism for a small amount of cyanide that has not reached the toxic dose into the body. High concentrations of cyanide can be artificially degraded to safe concentrations with chlorine or hydrogen peroxide. Therefore, the conditions for cyanide poisoning are more limited and harsher, and countries have specific qualified requirements for cyanide content in food and water. If unfortunately cyanide poisoning does not mean death, there are now a variety of first aid methods, such as skin contact can be washed, mistakenly taken can induce vomiting for medical treatment, and cyanide also exists specific drugs to treat.
Among all the anions cyanide is extremely poisonous to mankind and affects many physiological functions [4], and different detection methods have been used to detect cyanide, as shown in Figure 1. Cyanide poisoning targets the neurological, cardiovascular, and respiratory systems, while inducing deep metabolic disorders leading to permanent neurological dysfunction ranging from various extrapyramidal syndromes to stopping hypoxic vegetative states [3]. There are several treatment options for cyanide poisoning. The goal of supportive care for cyanide-induced life-threatening conditions is to reverse organ failure, such as coma, seizures and cardiovascular failure. However, it is effective only in the early stages of cyanide poisoning, and purification depending on the assumed absorption pathway is also an effective method.
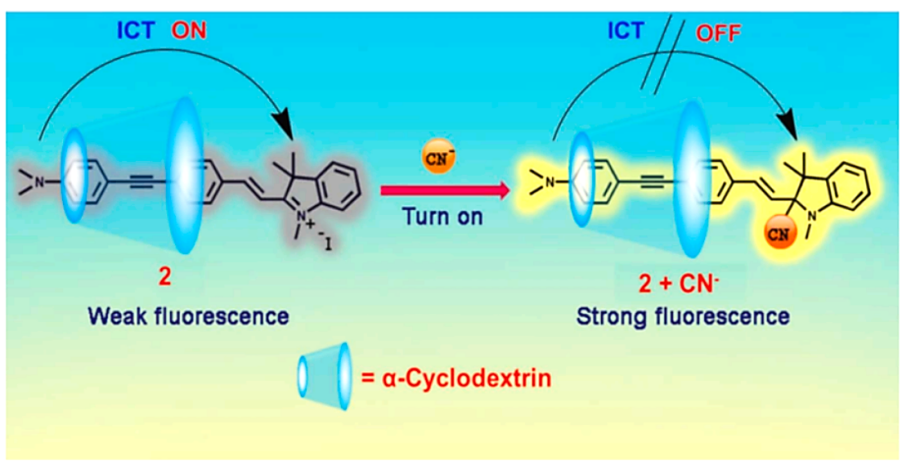
Figure 1. The cyanide sensing mechanism [4].
There are also specific treatments, such as management of the antidote cyanide. There are different types of cyanide antidote-moglobin inducers, including amyl nitrite, sodium nitrite, and 4-dimethylaminophenol, sodium thiosulfate, dicobalamin, and hydroxoxocobalamin [3]. Besides, the only cyanide antidote available in the United States is a three-component cyanide ampero supplement containing sodium thiosulfate, amyl nitrite, and sodium nitrite [5].
The mechanism of action of methemoglobin inducers is mainly by converting ferrous hemoglobin to ferric iron, subject globin formation may then compete with cytochrome oxidases for cyanide binding and experimental data suggest that mechanisms other than methemoglobin induction are also important antitoxic effects of these drugs [3]. Rhodanese and sulfur transferase are able to convert cyanide to less toxic thiocyanate in the presence of its natural substrate---thiosulfate, which can be eliminated in tea urine. Because it is an effective but slow treatment, it cannot be used as a first-line cyanide antidote [3]. Cobalt has a high affinity for cyanide, and each molecule can bind two cyanides. Hydrocobalamin, both experimental and clinical, is generally a potent cyanide antidote. Hydrocobalamin binds to cyanide and the form cyanocobalamin is non-toxic compounds, which are then excreted in the urine. Its negative inotropic effect on cyanide is rapid. However, this methemoglobulin inducer may induce serious side effects, including cardiovascular collapse, which may be more severe in the absence of cyanide and need to be used with caution [3]. Sodium nitrite can cause methemoglobinemia and severe hypotension, leading to potentially fatal reductions in blood oxygen-carrying capacity, as well as shock and death, and must be monitored at all times in a hospital management setting [3]. In addition, thiocyanates have antithyroid activity, and dicobalamin EDTA often has harmful side effects on the cardiovascular aspect and it is also poorly tolerated. The mainstay of treatment is a 100% oxygen and cyanide-specific antidote, which aims to reduce the amount of cyanide available for binding to respiratory enzymes in cells [6].
The mechanism of action of nitrite is mainly the induction of methemoglobinemia. Since methemoglobin has a greater affinity for cyanide than the trivalent iron portion of cytochrome oxidase, it can be used to exchange cyanide from respiratory enzymes to enable the enzymes to perform their normal functions [1]. In the absence of methemoglobinemia, sodium nitrite can be detoxified by local vasodilation or other mechanisms. Sodium thiosulfate acts as a sulfhydryl donor, reversibly binding to cyanide in the extracellular space to form thiocyanate that is minimally toxic and excreted by the kidneys, thereby enhancing cyanide clearance. The only significant adverse effects of sodium thiosulfate were rare hypersensitivity reactions and infusion rate-dependent hypotension. Long-term exposure to thiocyanates can cause toxicity due to the equilibrium between thiocyanate and cyanide. For patients presenting within 1 hour of cyanide ingestion, oral and gastric lavage and administration of activated charcoal may be considered to attempt cyanide recovery. Despite the low level of binding of activated charcoal to cyanide, its administration has been shown to be effective due to the relatively small lethal dose of cyanide.
Ventilation with 100% oxygen increases tissue oxygen supply, an important component of supportive care and synergistic with antidotes. Oxygen may contribute to increased respiratory excretion of cyanide, restore cytochrome oxidase activity by replacing cyanide, stimulate activation of other oxidizing systems, and may indirectly promote rhodinase metabolism by catalyzing cyanide. Hemodialysis is advocated in certain cases of cyanide poisoning; This will be especially beneficial in the face of worsening acidosis and kidney failure while leading to thiosulfate buildup and inducing additional toxicity of its own. Due to various human activities and informal waste disposal, the generation and legacy of cyanide waste has become an increasingly common problem today, mainly including: coke plants, automobile manufacturing, oil refining and mining [7]. The intense toxicity of cyanide and the environmental concerns and exposure risks associated with its industrial use continue to generate interest in rapid, safe and effective cyanide disposal methods [8].
Many efforts have been made to develop safe and effective detoxification and utilization techniques to reduce the harmful effects of cyanide on public health and the environment [9]. The study of potential antidotes is complicated by the fact that the pharmacokinetics of individual drugs can change due to cyanide toxicity, making it difficult to predict the efficacy and toxicity of antidotes. Because cyanide inhibits many enzyme systems, many potential detoxification mechanisms remain untapped. Exogenous administration of enzymes or substrates commonly involved in cyanide metabolism and detoxification may prove beneficial. Some studies have reported treatments such as carrier erythrocytes containing rhodinase and thiosulfate to reverse cyanide toxicity. When assessing the usefulness of antidotes, determining whether they reduce or even eliminate the need for incremental supportive care is one of the main points [3]. Studies have shown that antidotes with first-line therapeutic potential are being developed in terms of fewer side effects and are safer. Future research should move towards finding faster, safer, more effective, and better tolerated cyanide toxicity treatments and antidotes [10].
5. Conclusion
This research systematically analyzed the toxicity and mechanism of action of cyanide, as well as its symptoms and treatment methods. Although cyanide is a highly toxic and fast-acting poison, its toxicity is closely related to the dose, and toxicity will only occur when a certain dose is reached. Even if cyanide has multiple exposure routes and ways to enter the human body, within a safe dose, cyanide can be metabolized by our own detoxification mechanism, and there are multiple rescue measures for misconduct. Even if it is unfortunate to be poisoned, there are now a variety of effective treatment channels and drugs, and its mortality rate has been reduced year by year. However, there are still great difficulties in the diagnosis of cyanide poisoning, and its antidote still has great side effects and limited use conditions, so the research of rapid and effective diagnosis methods and efficient and safe antidotes is the future development trend.
References
[1]. Hall AH and Rumack BH 1986 Annals of Emergency Medicine 15(9) 1067-1074
[2]. Hendry-Hofer T B, Ng P C, Witeof A E, et al. 2019 Journal of medical toxicology 15 128-133
[3]. Baud F J. 2007 Human & experimental toxicology 26(3) 191-201
[4]. Chakraborty S, Paul S, Roy P, et al. 2021 Inorganic Chemistry Communications 128 108562
[5]. Fortin J L, Giocanti J P, Ruttimann M, et al. 2006 Clinical Toxicology 44(sup1) 37-44
[6]. Holland M A, Kozlowski L M. 1986 Clinical pharmacy 5(9) 737-741
[7]. Alvillo-Rivera A, Garrido-Hoyos S, Buitron G, et al. 2021 Journal of Materials Research and Technology 12 1418-1433
[8]. Ma J, Dasgupta P K. 2010 Analytica chimica acta 673(2) 117-125
[9]. Dong K, Xie F, Wang W, et al. 2021 Journal of Cleaner Production 302 126946
[10]. Gracia R, Shepherd G. 2004 Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 24(10) 1358-1365
Cite this article
Jiang,Y. (2023). Analysis of the toxicity and treatment methods of cyanide. Theoretical and Natural Science,27,235-240.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Hall AH and Rumack BH 1986 Annals of Emergency Medicine 15(9) 1067-1074
[2]. Hendry-Hofer T B, Ng P C, Witeof A E, et al. 2019 Journal of medical toxicology 15 128-133
[3]. Baud F J. 2007 Human & experimental toxicology 26(3) 191-201
[4]. Chakraborty S, Paul S, Roy P, et al. 2021 Inorganic Chemistry Communications 128 108562
[5]. Fortin J L, Giocanti J P, Ruttimann M, et al. 2006 Clinical Toxicology 44(sup1) 37-44
[6]. Holland M A, Kozlowski L M. 1986 Clinical pharmacy 5(9) 737-741
[7]. Alvillo-Rivera A, Garrido-Hoyos S, Buitron G, et al. 2021 Journal of Materials Research and Technology 12 1418-1433
[8]. Ma J, Dasgupta P K. 2010 Analytica chimica acta 673(2) 117-125
[9]. Dong K, Xie F, Wang W, et al. 2021 Journal of Cleaner Production 302 126946
[10]. Gracia R, Shepherd G. 2004 Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 24(10) 1358-1365









