Introduction
Malignant tumor is the main cause of death of people today, and is an important global public health problem [1]. Traditional methods such as radiotherapy and surgical excision have great side effects on the body. Small molecule inhibitors are prone to off-target effects and drug resistance, while monoclonal antibodies are expensive to administer. Hence the need for a targeted treatment that is less resistant. Proteolytic targeting chimera(PROTAC) is a class of heterobifunctional molecules that selectively degrades target proteins through the ubiquitin-proteasome system. PROTAC contains a ligand targeting the target protein, which recruits the target protein to E3 ubiquitin ligase and then performs ubiquitination labeling, ultimately inducing ubiquitination degradation of the target protein. Since protein degradation targeting chimeras can remove some mutated and overexpressed pathogenic proteins, it has good targeting, drug resistance, and high degradability, compared with traditional antitumor drugs. Its research provides new ideas for the treatment of malignant tumors. In recent years, PROTAC technology has gradually matured in the research and development of anti-tumor drugs, and two PROTAC small molecule drugs have passed the phase I clinical trial. This paper introduces the research and development progress of PROTAC in anti-tumor via literature review, as well as the opportunities and challenges that will be faced in the future research. At the same time, the author also hope that this article can provide a reference for further research on PROTAC [2].
Degradation mechanism of PROTAC and its applications
Degradation mechanism
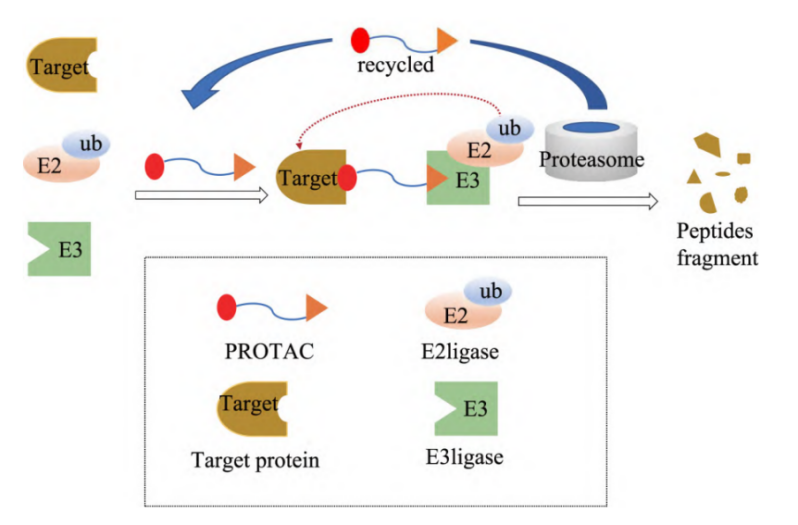
PROTAC is a class of heterofunctional molecule which selectively degrades the target protein by ubiquitin-proteasome system. PROTAC consists of 3 parts, with a Linger in the middle, one end of which is linked to the ligand bound to the target protein of interest (POI), and the other end to the ligand bound to E3 ligase. The basic principle is that PROTAC contains a ligand targeting the target protein, which recruits the target protein to the E3 ubiquitin ligase, then carries out ubiquitination labeling, and finally induces the ubiquitination degradation of the target protein. The degradation process can be divided into the following steps, first, POI and E3 ligase work together to form a ternary complex called POI-ProTAC-E3 ligase. E3 ligase then facilitates the transfer of ubiquitin (Ub) from E2 to lysine residues on POI. Multiple Ub chains are produced on the POI as a result of multiple rounds of ubiquitination. The ternary complex is broken up, the proteasome breaks down the POI, and the broken-up PROTAC starts up again.. (Figure 1)
Figure 1. Mechanism of action of PROTAC molecule.
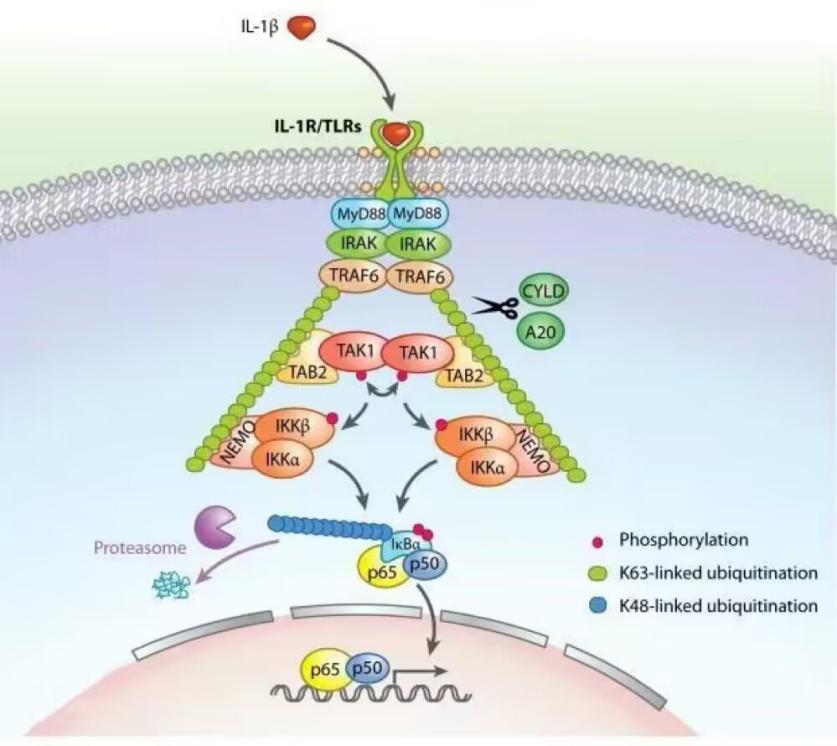
Under the control of a number of specialized enzymes, ubiquitin (a type of low molecular weight protein) molecules sort the proteins in the cell into categories, choose the target protein molecules from among them, and then modify the target protein in a specific way. The ubiquitin-activating enzyme, the binding enzyme, the ligase, and the degrading enzyme are some examples of these specialized enzymes. Protein location, metabolism, function, regulation, and destruction are all significantly influenced by ubiquitination. In addition, it controls practically every aspect of life, including the cell cycle, proliferation, apoptosis, differentiation, transfer, gene expression, transcriptional regulation, signaling, damage repair, inflammation, and immunology. A series of events involving the enzymes ubiquitin activator E1, ubiquitin binding E2, and ubiquitin ligase E3 are required for ubiquitin modification. First, the enzyme E1 binds to the Cys residues in the tail of ubiquitin molecules to activate it in the presence of ATP energy. Then, E1 gives the E2 enzyme the active ubiquitin molecules. The E2 enzyme and a few different E3 enzyme types then work together to recognize the target protein and perform ubiquitin modification. The target protein may be monoubiquitinated or polyubiquitinated, depending on the proportion of E3 to the target protein. The target protein is connected to a gap in the centre of the clamp-like E3 enzyme. The right domain of the enzyme locates the E2 enzyme to transfer the ubiquitin molecule, whereas the left domain of the enzyme determines the specific recognition of the target protein. Protein ubiquitination causes the tagged protein to be digested by proteases into more manageable peptides, amino acids, and ubiquitin that can be recycled.
Figure 2. Ubiquitination.
Application in tumor targets
The occurrence of malignant tumor is caused by the fact that, tumor-causing agents cause cells in local tissues to lose the normal control over their gene-level proliferation, and overexpression of nuclear receptor proteins, kinase proteins, cell cycle regulators and other functional proteins leads to abnormal hyperplasia and the formation of mass. PROTAC can degrade these proteins, inhibit the proliferation and migration of tumor cells, and promote their senescence and apoptosis.
Application in Depressant and Target
E3 ligase is a depressant that is required for proteasoma-mediated protein degradation. There are more than 600 known E3 ligases in the human body, but only a few are used in PROTACs. According to the E3 ligase targeted, PROTACs can be classified into CRBN-based inhibitors, VHL-based inhibitors and MDM2-based inhibitors. The CRBN-based depressants include BET targeting PROTACs, BTK targeting PROTACs, and ALK targeting PROTACs [3]. According to different targets, PROTAC can be divided into PROTAC molecules targeting nuclear receptors, PROTAC molecules targeting epigenetic proteins, PROTAC molecules targeting kinases, and PROTAC molecules targeting other proteins.
The important development history of PROTAC in anti-tumor drug research
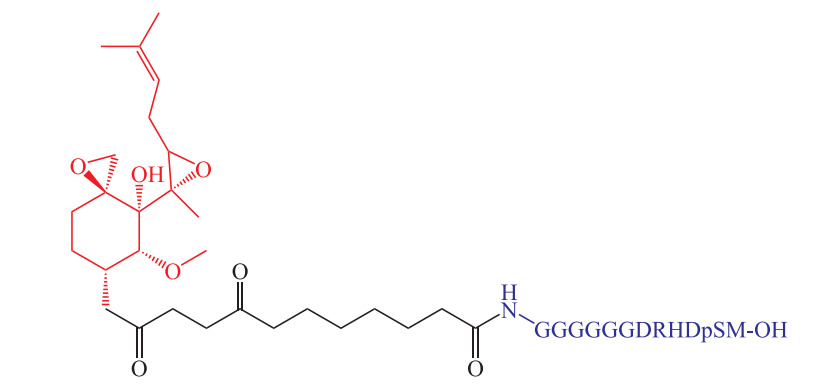
In 2001, the team of Prof. Craig M. Crews and Prof. Raymond J. Deshaies first proposed the concept of Protac and reported the first PROTAC molecule, ProTAC-1(Figure 3), which can target the degradation of MetAp-2, ER and AR [4].
Figure 3. Protac-1.
In 2004, VHL-based peptide PROTAC was first reported, which can effectively degrade AR, ERRα, kinase RIPK2, BCR-ABL fusion protein, etc.
PROTAC, based on MDM2, was first reported in 2008. Professor Craig M. Crews and his team synthesized PROTAC, a small molecule that can degrade BRD4 and stabilize p53, for the first time. It was the first PROTAC on the synergistic anti-proliferation effect of E3 ubiquitin ligand and target protein ligand.
In 2010, the first CIAP-based small molecule, PROTAC was reported. Ito et al. discovered Cereblon protein (CRBN), the main cause and molecular target of teratogen effects of thalidomide. Thalidomide and its derivatives have been approved for multiple myeloma.
Crews founded the first pharmaceutical company focused on PROTAC in 2013.
In 2019, the first PROTAC(ARV-110) entered clinical trials, with initial Phase I clinical data released in October of the same year.
Latest progress of PROTAC anti-tumor drug research and development
PROTAC in the treatment of non-small cell lung cancer(NSCLC)
Lung cancer is the most deadly cancer in the world and has a low 5-year survival rate. Nonsmall cell lung cancer (NSCLC), which accounts for the largest proportion of lung cancer and has a huge clinical need, epidermal growth factor receptor(EGFR), anaplastic lymphoma kinase(ALK), and kirsten rat sarcoma viral oncogene homolog (KRAS) is the most common mutation, and small molecule PROTAC is a highly effective treatment for lung cancer that targetes mutated genes [4].
Targeted EGFR degradation in NSCLC . EGFR is a class of tyrosine kinase-type transmembrane receptor. Its overexpression is closely associated with the proliferation and proliferation of tumor cells and the inhibition of cell apoptosis. According to statistics, about half of NSCLC patients have EGFR-activated mutations or overexpression, so EGFR is a key target for NSCLC treatment [5].
The first generation EGFR-Tkis showed a good therapeutic effect on patients with EGFR 19del and EGFR L858R mutations, but the patients would show acquired drug resistance and could not be effectively treated later. The second generation of EGFR-TKIs enhanced inhibition by introducing Michael receptors, but produced T790M mutations. The third generation of EGFR-TKIs can overcome the resistance caused by T790M mutations to a certain extent and effectively treat BMS in NSCLC, where patients will develop new mutations, such as C797S. In 2021, Li's team designed a PROTAC molecule that targets both PARP and EGFR for degradation. In the same year, Zhang's team introduced the leaving group HALG into PROTAC and designed oxygen-depleted activated PROTAC molecules that could be activated in tumor tissues and release active depressants. Many tumor cells have the characteristics of hypoxia, and PROTAC partially degrades EGFR 19del under hypoxia conditions, thus improving the selectivity of the drug for tumor cells.
At present, many research teams are also using PROTAC technology to carry out research on targeted EGFR degradation, hoping to find an effective strategy to overcome EGFR resistance mutations. Many teams are synthesizing TCLIPTACs, hoping to solve the problem of PROTACs' high molecular weight and thus low cellular permeability. With the solution of these problems, EGFR-PROTACs will play an important role in the treatment of NSCLC.
Targeted ALK degradation in NSCLC . In 2020, Wei's team reported a PROTAC molecule activated by ultraviolet light. The photosensitive group of the molecule is removed after ultraviolet irradiation, which can target the degradation of the ALK protein. Light is a spatiotemporal and non-invasive exogenous stimulus that has attracted much attention in the field of antitumor drugs in recent years [6].
In 2021, Jiang's team developed the ALK-PROTAC molecule that can be taken orally, with oral bioavailability of 16%.
Targeted KRAS degradation in NSCLC. In 2020, Crews reported the first PROTAC molecule capable of degrading KRAS G12C in the NCI-H2030 cell line, as well as mutated KRAS. This is an important breakthrough in the field of non-patent proteins as a target.
PROTAC in targeted therapy of leukemia
Leukemia accounts for a high proportion of malignant tumors in children.
The primary cause of chronic myeloid leukemia (CML) is the oncogenic fusion protein BCR-ABL1, which is an active tyrosine protein kinase that causes the proliferation of white blood cells in the bone marrow. BCR-ABL1 kinase specific inhibitors can block BCR-ABL1 kinase activity without damaging normal cells and improve the prognosis of leukemia patients, but the recurrence rate is high after drug withdrawal. Burslem et al. developed a PROTAC molecule targeting BCR-ABL1, namely GMB-475. The molecule inhibited human leukemia cells K562, and when combined with other drugs, the IC50 value was significantly reduced. The results of this study may ameliorate dose-dependent side effects and drug resistance, but the leukemia stem cells can survive in the presence of BCR-ABL1, which is not a cure for leukemia [7].
The development of BTK inhibitors is also a hot topic. When stimulated by antigens, BTK phosphorylates and activates, thereby driving a variety of cell signal transduction pathways that promote survival and proliferation, and affecting the transcription of corresponding proteins. At the same time, BTK can also activate factors located in the nucleus and induce the transcription of anti-apoptotic proteins and growth factors, thus promoting proliferation and increasing cell survival. However, most patients with CLL develop gene mutations that lead to drug resistance and relapse. Buhimschi et al. developed a PROTAC molecule targeting BTK, namely MT-802. It can rapidly target the degradation of wild type and C481S mutant BTK.
BRD4 PROTAC nanomaterials in the treatment of glioma
Glioma is a primary invasive brain tumor with a high recurrence rate. In the treatment of anti-glioma tumors, the efficiency of chemotherapy drugs to cross the blood-brain barrier (BBB) is low, and the large number of infiltrating tumor-associated macrophages (TAMs) in glioma also hinders the efficacy of drugs. Using BRD4 as a novel anti-glioma target, a tumor-targeting amphiphilic micelle was developed to treat glioma. Subsequently, we further investigated the effects of drug-loaded micelles on the proliferation and apoptosis of glioma cells and the polarization of M2 macrophages and their mechanisms [8].
Yang's team constructed a brain-targeting BRD4 depressant micelle. According to the experimental findings, SPPARV-825 micelles can effectively treat glioma by lowering M2 macrophage polarization, causing apoptosis in glioma cells, and suppressing the growth of glioma cells.
Opportunities and challenges for PROTAC anti-tumor drug developm
Current development opportunities for PROTAC include the progressively overcoming drug resistance, continuously improving targeting selectivity, affecting non-kinase-dependent function by degrading proteins, potentially degrading "non-patent" targets, and providing novel and rapidly reversible chemical protein knockout methods.
The development direction of PROTAC is to break the rule of "five rules of drug class", and conquering the target of "non-patent drug". In addition, it also needs to establish a scientific evaluation system, improve rational drug design, and further expand the E3 ubiquitin ligand. In recent years, PROTAC anti-tumor research and development has entered a critical period, and more and more PROTAC anti-tumor drugs will enter the preclinical and clinical studies to test the therapeutic effect of PROTAC. The problems faced by PROTAC are being solved one by one, and it is expected to become the latest blockbuster anti-tumor means after small molecule inhibitors and monoclonal antibodies.
Conclusion
The development process of PROTAC is time-consuming and complex, which may lead to an off-target effect, and the higher the concentration, the better the effect. However, PROTAC molecules have been developed for many important targets in the treatment of malignant tumors, and have been confirmed to have activity in vivo and in vitro. With the further understanding of PROTAC and the ubiquitin-proteasome system, the efficiency of finding effective PROTAC will be greatly improved, and more and more drugs will enter the clinical research stage, so that PROTAC can truly play the potential of targeted treatment of malignant tumors.
This paper tries to summarize the current progress of PROTAC anti-tumor drugs, but due to space limitations, it cannot include all the latest studies and just started studies, and as time goes by, there will be more updated results to be summarized.
Reference
References
[1]. Zhan Ying, Wang Tao, Liu Changbai. Proteolytic targeted chimeric applied to experimental research progress of malignant tumor therapy [J]. Chinese journal of new drugs and clinical, 2022, 9 (7) : 393-398. The DOI: 10.14109 / j.carol carroll nki xyylc. 2022.07.02.
[2]. Duanmu Yantao, Xu Qian, Xia Yu, Cao Jianzhong, Lu Maofang, Wang Fudong, Peng Dongming. Research progress of PROTAC protein targeting chimera in the development of antitumor drugs [J]. Chinese medicine, 2022, 25 (8) : 1424-1430. The DOI: 10.19962 / j.carol carroll nki issn1008-049 - x. 2022.08.024.
[3]. He Jinmei, You Lin, Rong Shujun, Zhang Liang, Wang Xin. Targeting the EGFR PROTAC drug research progress [J]. Chinese journal of medicinal chemistry, 2022, 32 (11) : 881-891. The DOI: 10.14142 / j.carol carroll nki cn21-1313 / r. 2022.11.010.
[4]. Jiang Lin, Zhang Jingbo, Hu Jiaqi, Qi Haixiang, Xu Heng. Research progress of protein degradation targeting chimera in the treatment of non-small cell lung cancer [J]. Chinese Journal of Lung Cancer,202,25(07):477-481. (in Chinese)
[5]. Xie Miaohong, Gao Mingming, Ling Jiannan, Du Wenting. Research progress of targeted small molecule protein degradation chimeras [J]. Chinese modern applied pharmacy, 2021, 38 (22) : 2891-2899. The DOI: 10.13748 / j.carol carroll nki issn1007-7693.2021.22.024.
[6]. Zeng Shenxin, Huang Wenhai, Shen Zhenrong. Opportunities and challenges of targeting chimeras for protein degradation in the development of small molecule drugs [J]. Pharmaceutical Advances,20,44(11):801-816.
[7]. Xin Benkai, Guo Lei, Xiao Yechen, Zhang Jizhou. Research progress of proteolytic targeted chimerism in targeted therapy of leukemia [J]. International Journal of Gerontology, 201,42(06):376-380.
[8]. Tingting Yang,Yuzhu Hu,Junming Miao,Jing Chen,Jiagang Liu,Yongzhong Cheng,Xiang Gao.A BRD4 PROTAC nanodrug for glioma therapy via the intervention of tumor cells proliferation,apoptosis and M2 macrophages polarization[J].Acta Pharmaceutica Sinica B,2022,12(06):2658-2671.
Cite this article
Liu,C. (2023). Progress and Challenges of PROTAC in the Development of Anti-tumor Drugs. Theoretical and Natural Science,4,743-748.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Biological Engineering and Medical Science (ICBioMed 2022), Part II
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Zhan Ying, Wang Tao, Liu Changbai. Proteolytic targeted chimeric applied to experimental research progress of malignant tumor therapy [J]. Chinese journal of new drugs and clinical, 2022, 9 (7) : 393-398. The DOI: 10.14109 / j.carol carroll nki xyylc. 2022.07.02.
[2]. Duanmu Yantao, Xu Qian, Xia Yu, Cao Jianzhong, Lu Maofang, Wang Fudong, Peng Dongming. Research progress of PROTAC protein targeting chimera in the development of antitumor drugs [J]. Chinese medicine, 2022, 25 (8) : 1424-1430. The DOI: 10.19962 / j.carol carroll nki issn1008-049 - x. 2022.08.024.
[3]. He Jinmei, You Lin, Rong Shujun, Zhang Liang, Wang Xin. Targeting the EGFR PROTAC drug research progress [J]. Chinese journal of medicinal chemistry, 2022, 32 (11) : 881-891. The DOI: 10.14142 / j.carol carroll nki cn21-1313 / r. 2022.11.010.
[4]. Jiang Lin, Zhang Jingbo, Hu Jiaqi, Qi Haixiang, Xu Heng. Research progress of protein degradation targeting chimera in the treatment of non-small cell lung cancer [J]. Chinese Journal of Lung Cancer,202,25(07):477-481. (in Chinese)
[5]. Xie Miaohong, Gao Mingming, Ling Jiannan, Du Wenting. Research progress of targeted small molecule protein degradation chimeras [J]. Chinese modern applied pharmacy, 2021, 38 (22) : 2891-2899. The DOI: 10.13748 / j.carol carroll nki issn1007-7693.2021.22.024.
[6]. Zeng Shenxin, Huang Wenhai, Shen Zhenrong. Opportunities and challenges of targeting chimeras for protein degradation in the development of small molecule drugs [J]. Pharmaceutical Advances,20,44(11):801-816.
[7]. Xin Benkai, Guo Lei, Xiao Yechen, Zhang Jizhou. Research progress of proteolytic targeted chimerism in targeted therapy of leukemia [J]. International Journal of Gerontology, 201,42(06):376-380.
[8]. Tingting Yang,Yuzhu Hu,Junming Miao,Jing Chen,Jiagang Liu,Yongzhong Cheng,Xiang Gao.A BRD4 PROTAC nanodrug for glioma therapy via the intervention of tumor cells proliferation,apoptosis and M2 macrophages polarization[J].Acta Pharmaceutica Sinica B,2022,12(06):2658-2671.