1. Introduction
Scorpions have existed on Earth for a long time. Currently there are more than 1500 known species of scorpions thriving on every continent on earth except Antarctica, with the most diverse and abundant existence in North Africa and parts of Asia especially the Middle East [1]. The global presence of scorpions in various geographic locations make them an intriguing subject for scientific exploration.
Scorpions’ venom serves as their primary defense mechanism and a tool for capturing prey. Typically, symptoms of scorpion stings vary depending on the composition of the venom. The main composition of scorpion venoms are various bioactive compounds, in which small peptides known as neurotoxins are responsible for the toxicity of scorpion venoms. When injected into the victims, these peptides target the voltage-gated channels for Na+, K+, Ca2+, and Cl- by binding tightly to the channels’ extracellular face and impeding passages for the ions [2]. Upon being stinged by scorpions, local pain response immediately sets in, with maximum pain severity being reached within 5 hours of envenomation. During this period, the neurotoxins released into the blood may cause nausea, vomiting, and sweating, with sometimes more severe symptoms such as restlessness and hypersalivation. Victims can develop symptoms including cardiovascular disturbances, paralysis, or even cardiac failure and even death in rare cases. Due to such a high level of toxicity, scorpion envenomation poses a considerable public health concern - each year over 1.2 million scorpion envenoming, leading to more than 3000 deaths, are recorded worldwide [3].
Despite its high toxicity, humans have been using scorpions and its toxins for medicinal use since ancient times. For example, Chinese people have been using dried whole bodies of scorpions in medical treatments since the Song Dynasty which is over a thousand years ago. The scorpions’ venom itself has also been used on other pathologies in European folk medicine [4]. These medical applications of scorpions indicate that scorpion or its venom contains components that can potentially be developed into medicines for various diseases.
Due to advances in biotechnology, a number of peptides form scorpion venoms has been identified and characterized, and the pharmacological potentials of the venom-derived peptides on various kinds of diseases have been more deeply investigated by scientists. The multifaced bioactive components found in these venoms have been shown to exhibit potent pharmacological activities, making them a valuable resource for drug development. Up to now, multiple therapeutic effects of scorpion venoms and their bioactive components, especially the neurotoxins, has been discovered. Such effects include anticancer, antibacterial, antifungal, antimalarial, antiviral etc. These properties present valuable potential for scorpion venom and its components to be developed into powerful medicines. This research will give a brief overview on the main therapeutic uses of scorpion venom-derived peptides and its potentials.
2. Scorpion venom composition
Scorpion venoms are mostly composed of amino acids, peptides, mucoproteins, nucleotides, lipids, amines, inorganic salts, and other contents depending on the species of the scorpions. Among these components, bioactive peptides known as neurotoxins are the main effective chemical responsible for most of the toxic effects and has been most deeply studied. These peptides can be divided into two groups based on its structure: disulfide-bridged peptides (DBPs) and non-disulfide-bridged peptides.
DBPs are relatively large peptides, usually containing 30 to 70 amino acids with 3 or 4 disulfide bridges, resulting in a significantly constrained structure. Two of the disulfide bridges are formed by 2 cysteines in the peptide [5]. Depending on different species, these DBPs may be sodium channel bound toxins (NaTxs), potassium channel bound toxins (KTxs), calcium channel bound toxins (CaTxs), chloride channel bound toxins (ClTxs). Compared to other peptides, DBPs can bind to its targets, especially voltage-dependent ionic channels, more tightly and more specifically. The disulfide bridges double or triple β-sheet in its secondary structure, next to an α-helix. The number of disulfide bridges correlates to the ion channel it interacts with, those with four disulfide bridges are smaller in size and functions on sodium or chloride channels, while those with only three bridges functions with potassium and calcium channels, due to a larger size. The DBPs mainly affect mammals, while some of them are reported to be interactive with insects or crustaceans. These features made DBPs extremely useful for investigating ion channels from structure and function, and potentially shed light on research in drug development for treatments on ion channel-related diseases.
Non-disulfide-bridged peptides are small peptides made up of 13 to 56 amino acids, having a partial α-helix structure. Unlike its disulfide-bridged relatives, only a few non-disulfide-bridged peptides were separated and studied from scorpion venoms [6], and they appear to have no known function-sequence relations.
Other toxic components include enzymes (larger proteins), adenosine, serotonin, benzoquinone derivatives, and various toxic elements. Enzymes have rarely been the center of interest until recently, as earlier research mainly focus on peptides. Phospholipases, discovered in Heterometrus laoticus and Opisthacanthus cayaporum, can possibly lead to symptoms like bleeding and tissue necrosis. Hyaluronidases, discovered in Heterometrus scaber, increase the toxicity of venoms by promoting distortion of other toxins from the sting site to other tissues and organs, leading to widespread permeation. Two 1,4-benzoquinone derivatives have also been extracted from the venom of Diplocentrus melici, which have possible antimicrobial effects. Apart from organic toxins, elements including magnesium, calcium, arsenic, magnesium, zinc, nickel, manganese, iron, calcium and copper were found in the venoms of Leiurus quinquestriatus, Androctonus crassicauda, and Androctonus bicolor [4].
3. Peptides with therapeutic uses and potentials
3.1. Anticancer peptides
According to estimates from the World Cancer Research Fund International, there were more than 18.1 million cancer cases (excluding non-melanoma skin cancer) around the world in 2020 (the latest year available), with 9.3 million of those cases in men and 8.8 million in women. The numbers were still rising year by year, mainly because of the growth of global population, especially population of the aged. However, most conventional treatments in use, such as surgery, chemotherapy, radiotherapy, still have defects. Poor tumoral selectivity may lead to low effectiveness and unwanted targeting of healthy cells which may cause severe side-effects. Therefore, current research on cancer mainly focuses on higher efficiency and selectivity.
Anticancer peptides present strong effects against multiple tumor cells. Its small size allows it to penetrate through tissue and reach the target region quickly with minimum interaction with healthy tissues. Scorpion venoms act as a valuable natural source of a number of peptides, with some of those having anticancer properties.
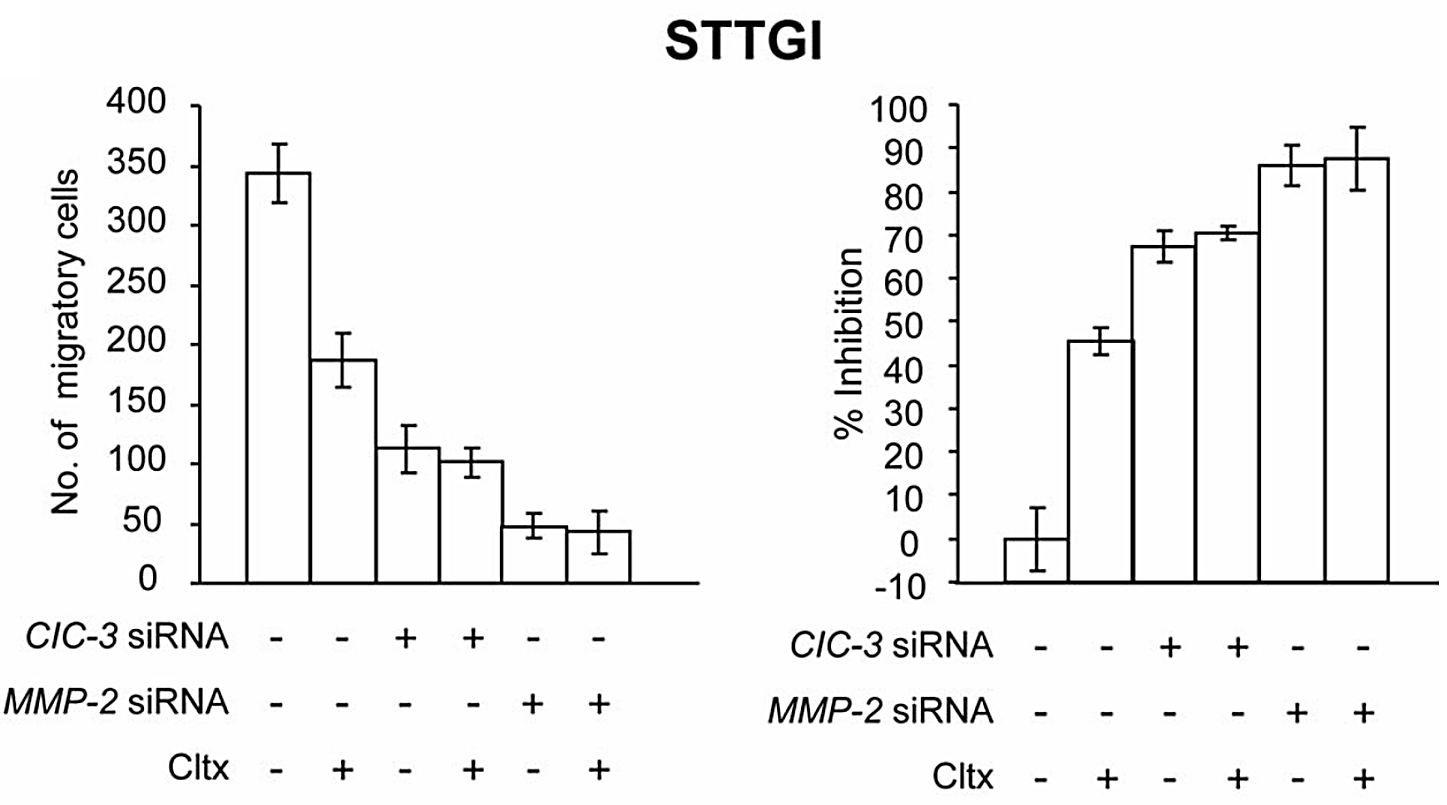
Figure 1. The number of migrated cells per well and percentage inhibition of invasion under different treatments [7].
Chlorotoxin (Cltx), first discovered in the venom of Leiurus quinquestriatus, is a disulfide-bridged peptide made up of 36 amino acids bridged by 4 disulfide bridges with a high activity. This specific type of neurotoxin binds exclusively to glioma cells, with no known major interaction with other cells, and inhibits the chloride flow of the target cell. Chlorotoxin inhibits the chloride ion channels in cells by binding to metalloprotease-2 (MMP-2), causing the glioma cell membrane to lose its gelatinase activity, and results in depletion of the whole membrane through endocytosis of MMP-2 with chloride channel ClC-3 [7]. As shown in Figure 1, compared with siRNA treatment alone, adding Cltx to siRNA treated STTGI cells only slightly increased the inhibition of invasion. This interaction only exists on neuroectodermal glioma cells, not on normal human tissue including neurons, which presents valuable potential in developing glioma-specific treatments. More recently, chlorotoxin has been found to be effective on other cancers cells including breast cancer and pancreatic cancer [4]. It can also be used to improve the anti-tumoral effect of drugs used in chemotherapy, such as doxorubicin [8]. A similar short-chained neurotoxin named BmKCT separated also from Leiurus quinquestriatus has a 68% sequence identity to chlorotoxins, resulting in similar chloride channel-inhibiting properties [4].
Iberiotoxin, found in the venom of Mesobuthus tumulus, is a disulfide-bridged peptide consisting of 37 amino acids bridged by 4 disulfide bonds. Its genetic sequence presents 68% identity to charybdotoxin, but presents a relatively highly selectivity as a blocker of calcium-activated potassium channels, unlike charybdotoxin which has poor selectivity. Iberiotoxins inhibits the potassium ion channels and affects glioma cells in the S phase, inducing cell death. It also inhibits the growth of hormone insensitive prostate cancer cell line of prostate carcinoma to exhibit functional overexpression of the gene KCNMA1 [9].
TSAP-2 is a kind of non-disulfide-bridged peptide found in Tityus serrulatus, which showed anticancer effects on multiple different cancer cells including those of prostate carcinoma, lung adenocarcinoma, oral squamous carcinoma, breast carcinoma, and glioblastoma [10].
Other anticancer peptides include neopladine-1 and neopladine-2 from Tityus discrepans, maurocalcine from Maurus palmatus. They all present apoptotic activity on cancer cells through either activating or deactivating related genes [4].
Through in silico, in vivo and in vitro experiments, scorpion venom-derived peptides presents anticancer effects through directly acting as therapeutic agents or auxiliary component for other drugs, and presents large potential for further research and development.
3.2. Antibacterial peptides
Bacteria related health problems has always been a major threat to humans, and with an increasing number of microbial pathogens developing resistance to common conventional antibacterial drugs, there is currently an urgent need to develop and discover new treatments to bacterial infections.
Antimicrobial peptides (AMPs) have been found in different species, it is an effective method against microbial pathogens due to its high selectivity of bacterial membranes and membrane-disrupting effects. Recently research have been focusing on developing new antibiotics from these peptides [4]. Several non-disulfide-bridged peptides extracted from scorpion venoms have antibacterial properties and can be categorized as antimicrobial peptides [6]. The reason they appear in scorpion venoms is still unclear, possible usages include having similar functions as neurotoxins or simply as the component for antibacterial response inside the scorpion’s venom gland itself.
Stigmurin, a peptide from the venom of Tityus stigmurus, is a non-disulfide-bridged peptide having 73 amino acids. This peptide presents antibacterial properties against Methicillin-resistant Staphylococcus aureus (MRSA), with little hemolytic activity even at 139.5 μmol/L, but it showed no effect on Escherichia coli even at maximum dose. Opistoporin 1, a non-disulfide-bridged peptide found in the venom of Opistophtalmus carinatus, and parabutoporin from Parabuthus schlechteri, both showed a 50% growth of inhibitory effect on Saccharomyces cerevisiae at a dose of 2 μmol/L [11]. Hadruin, a non-disulfide-bridged peptide containing 41 amino acid without cysteine, found in Hadrurus aztecus, showed a strong inhibiting effect against the growth of various bacteria including Escherichia coli, Enterococcus cloacae, Salmonella thyphi, Pseudomonas aeruginosa, Serratia marscences, and Klebsiella pneumonia.
However, a main defection of AMPs is their cytotoxicity against all eukaryotic cells, including healthy human cells, which can result in numerous serious side effects in clinical use. This problem also exists on most scorpion venom-derived AMPs, limiting the development of related drugs still in lab and not on human [5]. Therefore, scorpion venom-derived AMPs are full of potential and particularly valuable in the development of new antibiotics.
Despite the problem of hemolysis, there is still potential in the scorpion venom-derived AMPs. Studies have shown that the toxicity of AMPs against humans can be manual reduced, and they can be used together with classic antibiotics to receive an additive or sometimes even synergistic effect [12]. These solutions can be possibly used on scorpion venom-derived AMPs as well. The high effectiveness of scorpion venom-derived AMPs on common antibiotic-resistant pathogens means that it still presents huge potential as a next-generation antibiotic.
3.3. Antifungal peptides
Fungal infection has been a major threat to public health for centuries, with its mortality still on the rise. It is especially threatening to people who are immunocompromised or immunosuppressed. Current treatments for fungal infection still face the problem of easily developed drug-resistance as fungi can actively transport the chemicals out of the body through efflux pumps. Therefore, there is a urgent need for new drug candidates that can cope with this resistance mechanism of fungi.
Opistoporin-1 and parabutoporin is tested to show a 50% inhibition on the growth of yeast Saccharomyces cerevisiae, pandinin-2 also showed inhibition on the growth of Candida albicans, mucin-18 was effective against Aspergillus fumigatus, Candida albicans, and Saccharomyces cerevisiae [4]. These effects prove that some non-disulfide-bridged peptides derived from scorpion toxins can indeed be effective against drug-resistant fungi, presenting a promising future in antifungal drug development.
3.4. Antiviral peptides
Viruses has always been a major threat to human health. Currently vaccination is the main treatment against viruses, but it still has multiple disadvantages: vaccines can’t be produced until a big enough sample of the virus has been collected and studied, vaccines specifically designed for one virus may be ineffective against its mutants, and vaccines still has lots of possible side-effects that can sometimes be serious. Therefore, a more generic antiviral treatment is needed, and a few scorpion peptides happen to be potential antiviral peptides (AVPs).
Hp1090, found in the venom of Hetermetrus petersii, shows a 50% in vitro inhibition of hepatitis C virus (HCV) infection at 5mM. It works by directly interacting and damaging the phospholipid membranes of the virus [13]. This makes it a promising candidate for a new treatment for HCV. Other peptides have also shown inhibiting effects against viruses such as the SARS coronavirus, Influenza A virus H5N1 [4]. These studies indicate that a generic antiviral treatment may be developed using scorpion venom-derived peptides and their derivatives, and brings hope to the development of new treatments against unsolved diseases such as AIDS.
3.5. Antiparasitic peptides
Currently parasites are threatening the health of billions of people worldwide, leading to millions of morbidities and mortalities. However, current therapeutics for parasites still have high risk for toxic side effects, which means there is still an urgent need for novel therapeutics that is safer and more effective. Up to now multiple scorpion venom derivatives has been found to have antiparasitic properties, which brings huge potential to the development of novel treatments against parasties.
The first peptide with antiparasitic properties to be separated from scorpion venom is scorpine, first discovered in the venom of Pandinus imperator, which is a disulfide-bridged peptide with 75 amino acids and 3 disulfide bridges. Scorpine presented a 98% mortality rate against Plasmodium berghei, a common malaria parasite, in its sexual stages at only 15 μM, and a 100% effectiveness against Plasmodium falciparum parasitemia at 5 μM, both without harming mammalian cells [14].
Other scorpion venom-derived peptides also showed a therapeutic effect on different parasitic infections. Hge36, a peptide isolated from the venom of Hoffmannihadrurus gertschi, inhibits the growth of Taenia Crassiceps (a common type of pork tapeworm) at a submicromolar dose, with little to no damage to human tissue. Stigmurin showed an active effect against the trypomastigote forms of Trypanosoma Cruzi, which is the parasite responsible for Chagas disease) [15]. These antiparasitic peptides showed a promising future in the development of novel antiparasitic treatments, for its high specialized effect on the parasite and little side effects on human body.
4. Conclusion
Hundreds of million years of existence has given scorpions unique and complex venoms to defend themselves and attack others. Despite its toxicity, as a complex cocktail of bioactive components, scorpion venoms are a valuable source for potential drug components. Scorpion venoms hold a huge diversity of bioactive peptides, many of which have therapeutic properties and the potential as a cure for various medical problems including cancer, bacterial infection, viral infection, fungal infection, parasitic infection, among others. In vivo and in vitro experiments have already proved the therapeutic properties of some of these peptides, and some have already been put to test as a novel treatment. Development on the medical uses of scorpion venom-derived peptides should be focused on in the near future, and more therapeutic properties of these peptides may also be discovered, leaving us with infinite potentials to turn scorpion venoms from deadly threats to valuable medicines.
References
[1]. Simard MJ, Watt DD. Venoms and toxins, Stanford: Stanford University Press, 1990
[2]. Possani L D, Becerril B, Delepierre M, et al. 1999 European journal of biochemistry 264(2) 287-300
[3]. Cheng D, MD Scorpion Envenomation. Emergency Medicine, 2021
[4]. Ortiz E, Gurrola G B, Schwartz E F, et al. 2015 Toxicon 93 125-135
[5]. Zeng X C, Luo F, Li W X. 2006 Peptides 27(7) 1745-1754
[6]. Zeng X C, Corzo G, Hahin R. 2005 IUBMB life 57(1) 13-21
[7]. Lui V C H, Lung S S S, Pu J K S, et al. 2010 Anticancer Research 30(11) 4515-4524
[8]. Xiang Y, Liang L, Wang X, et al. 2011 Journal of controlled release 152(3) 402-410
[9]. Bloch M, Ousingsawat J, Simon R, et al. 2007 Oncogene 26(17) 2525-2534
[10]. Guo X, Ma C, Du Q, et al. 2013 Biochimie 95(9) 1784-1794
[11]. Moerman L, Bosteels S, Noppe W, et al. 2002 European journal of biochemistry 269(19) 4799-4810
[12]. Garcia F, Villegas E, Espino-Solis G P, et al. 2013 The Journal of antibiotics 66(1) 3-10
[13]. Yan R, Zhao Z, He Y, et al. 2011 Peptides 32(1) 11-19
[14]. Carballar-Lejarazú R, Rodriguez M H, de la Cruz Hernández-Hernández F, et al. 2008 Cellular and Molecular Life Sciences 65 3081-3092
[15]. Amorim-Carmo B, Daniele-Silva A, Parente A, et al. 2019 International journal of molecular sciences 20(3) 623
Cite this article
Du,Y. (2024). Therapeutic effect and potential application of scorpion venom peptide. Theoretical and Natural Science,29,178-183.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Simard MJ, Watt DD. Venoms and toxins, Stanford: Stanford University Press, 1990
[2]. Possani L D, Becerril B, Delepierre M, et al. 1999 European journal of biochemistry 264(2) 287-300
[3]. Cheng D, MD Scorpion Envenomation. Emergency Medicine, 2021
[4]. Ortiz E, Gurrola G B, Schwartz E F, et al. 2015 Toxicon 93 125-135
[5]. Zeng X C, Luo F, Li W X. 2006 Peptides 27(7) 1745-1754
[6]. Zeng X C, Corzo G, Hahin R. 2005 IUBMB life 57(1) 13-21
[7]. Lui V C H, Lung S S S, Pu J K S, et al. 2010 Anticancer Research 30(11) 4515-4524
[8]. Xiang Y, Liang L, Wang X, et al. 2011 Journal of controlled release 152(3) 402-410
[9]. Bloch M, Ousingsawat J, Simon R, et al. 2007 Oncogene 26(17) 2525-2534
[10]. Guo X, Ma C, Du Q, et al. 2013 Biochimie 95(9) 1784-1794
[11]. Moerman L, Bosteels S, Noppe W, et al. 2002 European journal of biochemistry 269(19) 4799-4810
[12]. Garcia F, Villegas E, Espino-Solis G P, et al. 2013 The Journal of antibiotics 66(1) 3-10
[13]. Yan R, Zhao Z, He Y, et al. 2011 Peptides 32(1) 11-19
[14]. Carballar-Lejarazú R, Rodriguez M H, de la Cruz Hernández-Hernández F, et al. 2008 Cellular and Molecular Life Sciences 65 3081-3092
[15]. Amorim-Carmo B, Daniele-Silva A, Parente A, et al. 2019 International journal of molecular sciences 20(3) 623









