1. Introduction
Triple-negative breast cancer (TNBC) is a special subtype of breast cancer that originates from malignant tumors of the ductal epithelium of the breast. To be more specific, TNBC refers to breast cancer subtypes with ER and PR expression not reaching to 1% in immunohistochemical staining, and no HER-2 overexpression or gene amplification, and accounts for about 15-20% of all types of breast cancer (BC) [1]. Moreover, TNBC is the most problematic subtype to treat, with significant proliferative activity and growth rate, highly invasive, poor prognosis and a high rate of recurrence. According to the latest literature, the median survival period after recurrence is only 12 months. For the moment, the patient’s response to chemotherapy is better, but the effect of adjuvant radiotherapy after surgery is poor. Residual lesions led to tumor recurrence, with a recurrence rate of 80%. Therefore, chemotherapy is still the most important treatment at present [2]. However, long-time process of chemotherapy will absolutely lead to drug resistance, which reduces the efficacy of the treatment and is prone to tumor recurrence and metastasis to distant places. Rapid drug resistance occurs through a variety of mechanisms [3]. Due to its significant heterogeneity, the clinical treatment and prognosis of different patients vary greatly, and there is an urgent need to find new therapeutic targets and explore new therapeutic methods. In recent years, more research is based on the targeted therapy of cancer pathogenesis and targeted agents improve the prognosis of early and metastatic TNBC.
Recently, the heterogeneity of ion death in TNBC has revealed a potential immunotherapy combination therapy [4]. GPX4 inhibitors in combination with other effective drugs provide an innovative treatment method for tumors with biologically similar characteristics to LAR but still need to be validated in clinical practice [4].
To master this part, the molecular and transcriptomic characterization of TNBC may be beneficial to the research [4]. With the proliferation development of histological analysis, potential therapeutic targets have emerged in large numbers, and many of them have strong clinical practical value [5]. This review presents clinically important pathogenesis of this tumor. In addition, related therapeutic targets are further discussed. Overall, this article intends to give a comprehensive understanding of the overall progress of TNBC immunotherapy, providing new insights for its clinical diagnosis and latest advances.
2. Pathogenesis
TNBC exhibits a limited number of highly relapsing mutated genes, and among known drivers of BC, TP53 has a mutation rate of up to 90% in specific subtypes [6]. Moreover, the incidence of TNBC has already been identified to be related to genetic factors, and there is a family aggregation tendency. In addition, changes in hormone levels in the body caused by long-term hormone replacement therapy, lifestyle habits and environmental factors may cause such diseases as well. In a follow-up study, Pro. Shao’s research team from the Affiliated Cancer Hospital of Fudan University published an article on Cancer Cell in 2019, which analyzed the collected abundant clinical samples through multi-omics technologies such as whole exome sequencing and RNA sequencing, and finally divided them into four tumor-specific subtypes, namely a luminal androgen receptor (LAR) subtype (23%); an immunomodulatory (IM) subtype (24%); a basal-like and immune-suppressed (BLIS) subtype (39%) and a mesenchymal-like (MES) subtype (15%) [7]. The prognosis and molecular characteristics of the four types have certain differences, as shown in the table below. Among all the subtypes, the prognosis was poor except for IM. In addition, all of them were characterized by high chromosome instability and high TP53 mutation frequency. Although the survival rate of TNBC patients has improved after the precision treatment, the prognosis of some patients with subtypes, especially those with LAR and BLIS, has not reached the expectation. Based on the analysis above, it is urgent to find new therapeutic targets.
The title is set 17 point Times Bold, flush left, unjustified. The first letter of the title should be capitalized with the rest in lower case. It should not be indented. Leave 28 mm of space above the title and 10 mm after the title.
3. Potential targeted therapy
3.1. AR Signaling Pathway Upregulation in the LAR Subtype
LAR subtypes’ AR expression levels are linked to TNBC patients’ PFS (progression-free survival) and OS (overall survival). Although the researchers have found many AR inhibitors that are effective in this subtype of patients through data analysis of clinical trials, the mechanism for these findings still needs to be explored. Currently, studies that combine AR inhibitors with PARP inhibitors show great significant curative effect in BC cells by regulating the DNA damage process [8]. Additionally, studies have shown that simultaneous pharmacological blocking of PI3K and AR signaling pathways has synergistic activity in AR+ TNBC subtypes [9].
Another study has demonstrated that the LAR TNBC cell line is more dependent on the CDK (cyclin-dependent kinases) 4/6 axis for cell cycle progression and maybe more sensitive to CDK4/6 inhibition than other TNBC subtypes [10]. Therefore, it is worth conducting more clinical trials to find whether AR inhibitors combined with other targets will bring amazing healing effects and explore internal mechanisms as well.
3.2. PI3K/AKT/mTOR Signaling Pathway greatly linked to proliferation and apoptosis in cancer cells
The PI3K/AKT/mTOR signaling pathway is one of the classical signaling pathways of intracellular signaling, which is associated with many human diseases. Overactivation of PI3K results in proliferation of cancer cells. A study has shown that the mutation rate of PIK3CA in TNBC is about 25%, and was linked to increased resistance to chemotherapy, especially in LAR and MES subtypes [5]. AKT1, AKT2 and AKT3, which are closely related to PI3K, are the downstream effector proteins of PI3K.
Alpelisib is the first approved PI3K inhibitor for breast cancer indication [11]. In addition to several PI3K inhibitors, several AKT inhibitors are either on the market or in the research stage as well [5]. For example, AKT inhibitors Ipatasertib (GDC-0068) and Capivasertib (AZD-5363) are both in clinical studies. For patients with PIK3CA mutation after CDK4/6 inhibitor resistance, Alpelisib combined with endocrine therapy can be preferred [11].
3.3. Immune checkpoint inhibitors for neoadjuvant chemotherapy
TNBC has greater immunological invasiveness and higher PD-L1 expression levels as compared to other BC subtypes. Combining the immunological checkpoint receptor PD-1 produced by tumor cells with the immune checkpoint receptor PD-1 on the surface of activated T cells allows for immune evasion by sending a negative regulatory signal to T cells. Research on PD1/PD-L1 inhibitors has demonstrated its ability to effectively inhibit a variety of tumor types. The creation of these inhibitors may offer a fresh approach to the management of BC.
Combining albumin-paclitaxel with atezolizumab is another adjuvant strategy. Phase III clinical research on this treatment is presently being conducted globally. With its positive outcomes in stage Ib, this therapy is anticipated to be a viable option for treating BC [12].
3.4. Overexpression of EGFR in the BL2 Subtype
Epidermal growth factor receptor (EGFR) is a member of the transmembrane glycoprotein, tyrosine protein kinase ERBB receptor family, which also includes HER-2/NEU, HER-3, and HER-4. EGFR overexpression plays an important role in the evolution of malignant tumors, which are found in kidney cancer, lung cancer, BC et al. Clinical trials have shown that tyrosine kinase inhibitors targeting EGFR mutations are far more effective than conventional chemotherapy for tumors that contain classical EGFR-activating mutations [5]. Moreover, the EGFR signaling pathway regulates the interaction between tumor cells and the tumor microenvironment (TME) as well.
EGFR-targeting drugs have been approved for clinical use, including gefitinib and monoclonal antibodies [5]. At present, more and more inhibitors targeting triple EGFR mutations have been reported, including TQB3804, BPI-361175, HS-10375, DAJH-1050766, and URP1444, which have been approved for clinical trials.
However, current clinical trials of EGFR-targeted therapy in BC patients have not yet shown significant curative effects. For example, Panitumumab is an anti-EGFR monoclonal antibody that inactivates the EGFR signaling pathway by binding to EGFR and blocking EGF ligand binding.
It’s worth noting that TME is a driving factor of the clinical phenotype and invasiveness. Therefore, future studies will continue to understand the correlation and potential regulatory mechanisms between targeted signaling pathways such as EGFR and TME, thereby improving the clinical prognosis of patients.
3.5. Overactivation of FGFR interacted with hormone receptor signaling
Approximately 15% of TNBC patients have fibroblast growth factor receptor (FGFR) gene copy amplification, mutation, and fusion, which means that overactivation of the FGFR signaling pathway in the tumor leads to tumor growth and is directly associated with tumor metastasis and reduced survival. Therefore, FGFR is considered as a potential therapeutic target for BC. Studies have shown that FGFR-targeted therapy has significant efficacy in the treatment of BC, especially when FGFR is amplified [5]. However, drug resistance to FGFR inhibitors in BC patients is currently the biggest obstacle to clinical approval. Therefore, systematic research on the resistance mechanism of FGFR inhibitors and design of more accurate and effective combination therapies will undoubtedly bring more hope for patients with tumor-carrying FGFR gene abnormalities.
Currently, clinical data have shown that in patients with trastuzumab-resistant HER2-positive BC, the combination of FGFR inhibitors and anti-HER2 therapy can overcome drug resistance [12]. Therefore, it is urgent to explore the practicability of FGFR inhibitors and other treatments for TNBC patients. In addition, the prospect of combining FGFR inhibitors with CDK4/6 inhibitors is also worth exploring [12]. No clinical data is available now.
3.6. High correlation in Epigenetic Regulation to Carcinogenesis in BC
In a study, researchers have revealed that epigenetic regulation is the main mechanism of tumor inhibition. The research team studied 215 recurrent breast cancer genes by establishing somatic CRISPR/Cas9 mutation screens [13]. In vivo CRISPR screens out tumor suppressors that function on epigenetic regulation. Ultimately, it was found that the loss of epigenetic regulatory factors accelerated the frequency of tumor development, and revealed that abnormal gene expression was a potential signal of tumor development [13].
Interestingly, this work found that epigenetic regulation in conjunction with proto-oncogenes is involved in a novel mechanism of breast cancer development. This process, which the researchers call “alveogenic mimicry,” refers to the fact that the massive proliferation of acines during pregnancy/lactation is precisely coordinated, and tumorigenesis “hijack” this process for rapid cell proliferation compared to physiological conditions [13].
Therefore, more attention should be paid to the relationship between abnormal epigenetic regulation and changes in tumor heterogeneity/cell lineage characteristics during tumorigenesis.
3.7. Constitutively activated of STAT3 in TNBC cells
There is a family of cytoplasmic transcription factors that can bind to DNA and are named signal transducers and activators of transcription (STATs). After being activated, STATs can move from the cytoplasm to the nucleus and promote transcription after binding to specific sites. STAT3 in this family has been detected to be overexpressed in many tumors, confirming its strong correlation with tumor formation [7].
In BC-related research, it was found that STAT3 promotes the proliferation of BC cells by combining with the promoter of surviving. This gene is also involved in the metastasis of TNBC, thereby leading to the poor prognosis of TNBC.
In recent years, a large number of STAT3 inhibitors have entered clinical trials, but only some have been approved by the FDA, which may be related to crosstalk between pathways and resistance to STAT3 [14]. Therefore, STAT3 remains a potential target for the prevention and treatment for TNBC.
3.8. An innovative immunotherapy combination strategy correlated to Ferroptosis heterogeneity in TNBC
According to a new study, by integrating and analyzing multi-omics data from a large TNBC cohort (n = 465), researchers found that TNBC is characterized by heterogeneity in metabolites and metabolic pathways associated with iron death [15]. They confirmed that the LAR subtype of TNBC is particularly sensitive to GPX4 inhibitors [15]. Moreover, the effect of GPX4 inhibitor combined with anti-PD1 is better than that of monotherapy [15].
However, there are some limitations. First, more clinical trials are needed to test the efficacy of the combination of GPX4 inhibitors with immunotherapy strategy [15]. In addition, results from mouse cell line models treated with GPX4 inhibitor monotherapy may not directly applicable to human LAR cell lines [15].
Most importantly, GPX4 inhibitors in combination with immune checkpoint blockade provides an new targeted therapy for LAR subtypes in TNBC [15].
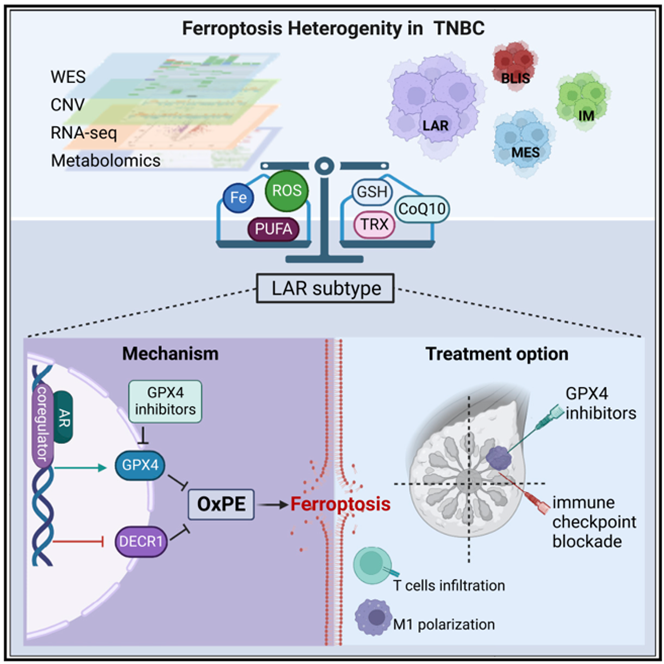
Figure 1. Ferroptosis Heterogeneity in TNBC [15]
4. Conclusion
TNBC is a highly drug-resistant form of breast cancer, making its treatment challenging. Chemotherapy is currently the most effective conventional treatment. However, it has unavoidable adverse effects on the human body, which makes target development especially crucial. Although several targets have been investigated, the most often used and researched targets are ER, HER2, and PR.
The pathology of TNBC involves several signaling pathways. Based on pertinent signaling pathways, the creation of molecular markers can aid in the delivery of more targeted treatment for TNBC.
“Fudan typing” plays a vital role in collating TNBC multi-omics data, classifying it and studying the corresponding subtypes, which can definitely improve the accuracy of the genome, which will also improve the targeted therapy for TNBC.
Monoclonal antibody is an effective treatment strategy for BC, but it has disadvantages such as cost, duration frequency of treatment, drug resistance and tolerance. In addition, once the tumor has genetic mutation, it’s more likely to transfer and develop drug resistance thus affecting the patient’s prognosis.
Tumor vaccine therapy can use the specific antigens of cancer cells to activate the immune system for a long time, especially because the body has a long immune memory, which can reduce the chance of cancer recurrence. On the other hand, vaccines don’t need to be given frequently, and historically, vaccines have been relatively safer than chemotherapy.
Despite the current slow progress and challenges in clinical translation, the development of clinical BC vaccines has great implications for both prevention and treatment. Therefore, TNBC targeted therapies still have a long way to go.
References
[1]. Zhu Y Zhu X Tang C et al 2021 Progress and challenges of immunotherapy in triple-negative breast cancer Biochim Biophys Acta Rev Cancer Dec 1876(2) 188593
[2]. MacDonald I Nixon NA Khan OF 2022 Triple-Negative Breast Cancer: A Review of Current Curative Intent Therapies. Curr Oncol Jul 7 29(7) 4768-4778
[3]. Li Y Zhang H Merkher Y et al Recent advances in therapeutic strategies for triple-negative breast cancer J Hematol Oncol 2022 Aug 29 15(1) 121
[4]. Yang F Xiao Y Ding JH et al 2023 Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy Cell Metab Jan 3 35(1) 84-100e8
[5]. Li Y, Zhang H, Merkher Y et al 2022 Recent advances in therapeutic strategies for triple-negative breast cancer J Hematol Oncol Aug 29 15(1) 121
[6]. Derakhshan F, Reis-Filho JS 2022 Pathogenesis of Triple-Negative Breast Cancer Annu Rev Pathol Jan 24 17 181-204
[7]. Jiang YZ, Ma D et al 2019 Genomic and Transcriptomic Landscape of Triple-Negative Breast Cancers Subtypes and Treatment Strategies Cancer Cell Mar 18 35(3) 428-440e5
[8]. Min A, Jang H, Kim S et al 2018 Androgen Receptor Inhibitor Enhances the Antitumor Effect of PARP Inhibitor in Breast Cancer Cells by Modulating DNA Damage Response Mol Cancer Ther Dec 17(12) 2507-2518
[9]. Lehmann BD, Abramson VG, Sanders ME, et al 2020 Translational Breast Cancer Research Consortium TBCRC 032 IB/II Multicenter Study Molecular Insights to AR Antagonist and PI3K Inhibitor Efficacy in Patients with AR+ Metastatic Triple-Negative Breast Cancer Clin Cancer Res May 1 26(9) 2111-2123
[10]. Choupani E Madjd Z Saraygord-Afshari N et al 2022 Combination of androgen receptor inhibitor enzalutamide with the CDK4/6 inhibitor ribociclib in triple negative breast cancer cells PLoS One Dec 22 17(12) e0279522
[11]. Chinese Anti-Cancer Association Cancer Drug Clinical Research Professional Committee, National Cancer Quality Control Center Breast Cancer Expert Committee, Chinese Anti-Cancer Association Cancer Pathology Professional Committee, etc. 2022 Experts on the clinical application of PI3K/AKT/mTOR signaling pathway inhibitors in the treatment of breast cancer Consensus Chinese J Oncol 44(7) 673-692
[12]. Qiusheng G Wenming C Xiaojia W 2022 Research progress of fibroblast growth factor receptor signaling pathway in breast cancer J Chinese Academy of Medical Sci 44(01) 136-141
[13]. Langille E, Al-Zahrani KN, Ma Z, et al 2022 Loss of Epigenetic Regulation Disrupts Lineage Integrity, Induces Aberrant Alveogenesis, and Promotes Breast Cancer Cancer Discov Dec 2 12(12) 2930-2953
[14]. Shuo G Jinhua W 2020 Research progress of STAT3 signaling pathway in triple-negative breast cancer Chinese J Cell Biol 42(12): 2266-2273
[15]. Yang F, Xiao Y, Ding JH et al 2022 Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy Cell Metab 2023 Jan 3 35(1) 84-100e8 doi 101016/jcmet202209021 Epub Oct 17
Cite this article
Tao,S. (2024). Current therapeutic targets and promising strategies for triple-negative breast cancer . Theoretical and Natural Science,32,210-215.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Zhu Y Zhu X Tang C et al 2021 Progress and challenges of immunotherapy in triple-negative breast cancer Biochim Biophys Acta Rev Cancer Dec 1876(2) 188593
[2]. MacDonald I Nixon NA Khan OF 2022 Triple-Negative Breast Cancer: A Review of Current Curative Intent Therapies. Curr Oncol Jul 7 29(7) 4768-4778
[3]. Li Y Zhang H Merkher Y et al Recent advances in therapeutic strategies for triple-negative breast cancer J Hematol Oncol 2022 Aug 29 15(1) 121
[4]. Yang F Xiao Y Ding JH et al 2023 Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy Cell Metab Jan 3 35(1) 84-100e8
[5]. Li Y, Zhang H, Merkher Y et al 2022 Recent advances in therapeutic strategies for triple-negative breast cancer J Hematol Oncol Aug 29 15(1) 121
[6]. Derakhshan F, Reis-Filho JS 2022 Pathogenesis of Triple-Negative Breast Cancer Annu Rev Pathol Jan 24 17 181-204
[7]. Jiang YZ, Ma D et al 2019 Genomic and Transcriptomic Landscape of Triple-Negative Breast Cancers Subtypes and Treatment Strategies Cancer Cell Mar 18 35(3) 428-440e5
[8]. Min A, Jang H, Kim S et al 2018 Androgen Receptor Inhibitor Enhances the Antitumor Effect of PARP Inhibitor in Breast Cancer Cells by Modulating DNA Damage Response Mol Cancer Ther Dec 17(12) 2507-2518
[9]. Lehmann BD, Abramson VG, Sanders ME, et al 2020 Translational Breast Cancer Research Consortium TBCRC 032 IB/II Multicenter Study Molecular Insights to AR Antagonist and PI3K Inhibitor Efficacy in Patients with AR+ Metastatic Triple-Negative Breast Cancer Clin Cancer Res May 1 26(9) 2111-2123
[10]. Choupani E Madjd Z Saraygord-Afshari N et al 2022 Combination of androgen receptor inhibitor enzalutamide with the CDK4/6 inhibitor ribociclib in triple negative breast cancer cells PLoS One Dec 22 17(12) e0279522
[11]. Chinese Anti-Cancer Association Cancer Drug Clinical Research Professional Committee, National Cancer Quality Control Center Breast Cancer Expert Committee, Chinese Anti-Cancer Association Cancer Pathology Professional Committee, etc. 2022 Experts on the clinical application of PI3K/AKT/mTOR signaling pathway inhibitors in the treatment of breast cancer Consensus Chinese J Oncol 44(7) 673-692
[12]. Qiusheng G Wenming C Xiaojia W 2022 Research progress of fibroblast growth factor receptor signaling pathway in breast cancer J Chinese Academy of Medical Sci 44(01) 136-141
[13]. Langille E, Al-Zahrani KN, Ma Z, et al 2022 Loss of Epigenetic Regulation Disrupts Lineage Integrity, Induces Aberrant Alveogenesis, and Promotes Breast Cancer Cancer Discov Dec 2 12(12) 2930-2953
[14]. Shuo G Jinhua W 2020 Research progress of STAT3 signaling pathway in triple-negative breast cancer Chinese J Cell Biol 42(12): 2266-2273
[15]. Yang F, Xiao Y, Ding JH et al 2022 Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy Cell Metab 2023 Jan 3 35(1) 84-100e8 doi 101016/jcmet202209021 Epub Oct 17









