

Volume 116
Published on June 2025Volume title: Proceedings of the 3rd International Conference on Modern Medicine and Global Health
Motor and non-motor symptoms are hallmarks of Parkinson's disease (PD), a chronic, progressive neurodegenerative illness caused by the decline of dopamine-producing neurones in the substantia nigra. Although the exact cause is still unknown, environmental variables like pesticide exposure and genetic alterations (such as SNCA, LRRK2, and PARK7) are involved in its development. Tremors, bradykinesia, stiffness, and postural instability are the hallmark motor symptoms that are caused by a dopamine deficit in the basal ganglia. Quality of life is impacted by non-motor symptoms such as mood problems, sleep disruptions, and cognitive deterioration. By restoring dopamine levels in the brain, Levodopa efficiently treats motor symptoms and is still the gold standard for treating Parkinson's disease. When combined with carbidopa to increase delivery and reduce side effects, Levodopa dramatically enhances motor function, particularly in the early and middle stages of the disease. However, problems, including dyskinesia and motor irregularities, are linked to prolonged use. Another treatment option is provided by dopamine agonists, which directly activate dopamine receptors, especially in younger or early-stage PD patients. They reduce the risk of early dyskinesia by delaying Levodopa and providing long-lasting symptom alleviation, although less effective than Levodopa. They do, however, have an increased risk of behavioural and cognitive adverse effects, including impulse control issues and hallucinations. Combination therapy maximises symptom management by utilising the advantages of both levodopa and dopamine agonists, but it necessitates close observation. Treatment plans must change as PD worsens to strike a balance between effectiveness and adverse effects. Future studies on sophisticated medication regimens and neuroprotective therapies have the potential to improve long-term results in the treatment of Parkinson's disease.

 View pdf
View pdf


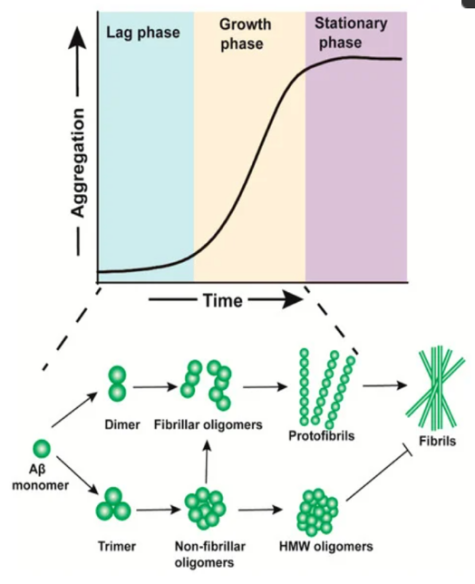
So far, the primary culprits in the onset of Alzheimer's disease are the deposits of amyloid-β (Aβ) proteins and the formation of tau protein knots. These markers are crucial for a conclusive pathological diagnosis. Despite the significance of both amyloid-β deposits and tau protein knots, the nature of their interaction remains largely unexplained, and the scientific inquiries into this relationship yield a patchy and inconclusive body of research. In this paper, we find out Aβ plaques accelerate neurotic plaque tau aggregation and propagation and apply fluorescent biotechnique to detect and measure Aβ oligomers in normal and tau seed condition. The findings indicate that upon introducing varied concentrations of Aβ aggregates into culture mediums devoid of lipofectamine, both Aβ filaments and newly synthesized Aβ enhance tau nucleation in correlation with their concentration. Moreover, Aβ oligomers exhibit a more pronounced stimulatory effect, strongly suggesting an interaction between Aβ and tau.

 View pdf
View pdf


From the work of Moreno-Jimenez et al., it is known that the generation of new neurons dramatically declines in patients with Alzheimer’s disease compared with mentally healthy individuals, marked by DCX+ cells (immature neurons) failing to produce other structures characteristic of mature neurons. The same researchers also observed that such a phenomenon worsened as Alzheimer’s disease progressed but did not offer any explanation. This research proposal introduces an experiment that could reveal the mechanism behind the decrease of adult hippocampal neurogenesis in patients with Alzheimer’s disease. Focusing on Aβ1-42, a hallmark of Alzheimer’s disease, this paper examines its role in reducing the number of new neurons produced. Techniques such as immunofluorescence, chromatin immunoprecipitation sequencing, and RNA sequencing are employed.

 View pdf
View pdf


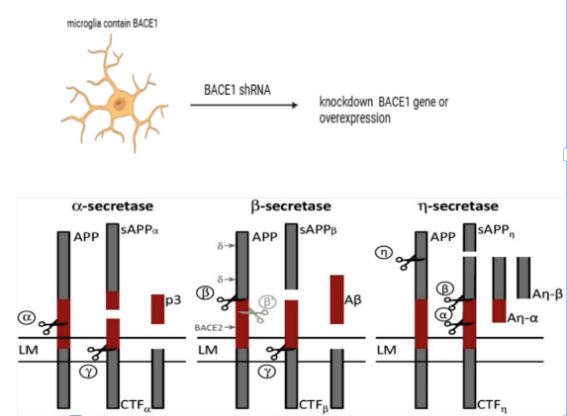
The present study tests the proposition that enhancement of BDNF by polarizing microglial cells from M1 to M2 contribute to the phenomenon of hippocampal neurogenesis and synaptic plasticity, thus reducing depressive-like behaviors. The experimental design covers both in vitro and in vivo, with BACE1-modified microglial culture, co-culture with neural progenitors, and a mouse model of depressed state. Thus, the present study intends to understand exactly how microglial polarization is connected to the alteration in BDNF and adult hippocampal neurogenesis in depression. It is possible that the results obtained will shed light on the new therapeutic approaches to influencing microglial polarization and BDNF receptor expression in depressive disorders and improving the efficacy of the therapies. The present study adds to the current knowledge about the neuroinflammation process in psychiatric diseases, as well as possibly offering hope for better biology-driven treatment options for depression in the future.

 View pdf
View pdf


The popularity of fast food has led to a rapid global increase in high-fat diets (HFD) recently. The prevalence of HFD has raised public concerns about metabolic health. Animal studies and clinical trials have implied the alternations of gut microbiota components when HFD, thereby influencing their metabolites abundances, specifically short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate, which play important roles in host physiological activities. Alternations in intestinal flora abundance and components may also exacerbate gut permeability, potentially initiating inflammation which is a start of various chronic diseases. This review primarily explores mechanisms by which HFD induces obesity-related diseases, including metabolic dysfunction-associated steatotic liver disease, atherosclerosis, and type 2 diabetes mellitus. Additionally, this review demonstrates the role and effectiveness of intestinal flora, especially probiotics, and their derived SCFAs in preventing disease progression and promoting tissue regeneration in HFD-induced disorders. An intensive study on the significance of intestinal flora and their derived SCFAs to disease progression and therapeutic targets can help supplement the loop between microbial and host homeostasis.

 View pdf
View pdf


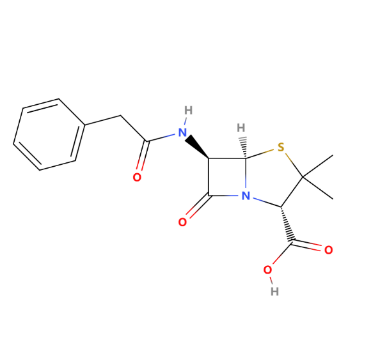
β-Lactam antibiotics, discovered in the 1920s with the development of penicillin by Alexander Fleming, revolutionized the treatment of bacterial infections. These antibiotics are characterized by their β-lactam ring as their typical structure, leading to the development of various derivatives such as cephalosporins and carbapenems. To date, they remain one of the most widely used and effective antibiotics. β-Lactam antibiotics exert their antibacterial action by inhibiting the synthesis of the bacterial cell wall, specifically by targeting and binding to penicillin-binding proteins. Furthermore, this binding disrupts the cross-linking of peptidoglycan chains, leading to cell wall weakening and ultimately causing bacterial cell lysis and death. The development of resistance to β-lactam antibiotics primarily occurs through the production of β-lactamases, which hydrolyze the β-lactam ring. Obviously, resistance to β-lactam antibiotics can also develop through other mechanisms, but this is the most significant and most urgent to address. In the wide history of β-Lactam antibiotics, researchers paid much effort to overcome the resistance and the side effects. Researchers have also increased the stability of β-lactam rings through chemical modifications, such as adding side chains or introducing enzyme-resistant groups, to withstand the hydrolytic action of β-lactamases produced by bacteria. By modifying the structure of the R group, medicinal chemists can design more effective and targeted antibiotics to address various bacterial infections. This review systematically reviews the development of β-lactam antibiotics and the influence of different side chains on the functionality of β-lactam antibiotics, such as side effects, clinical applications, drug stability, etc. Finally, we propose the future development of β-lactam antibiotics.

 View pdf
View pdf


This study investigates the relationship between 40 Hz gamma frequency entrainment and microglial activation in Alzheimer's disease, focusing on how brain wave stimulation affects amyloid-beta clearance. It analyzes the correlation between gamma entrainment and microglial phenotype transitions, shedding light on potential therapeutic approaches in a changing medical landscape. Additionally, the research examines whether gamma entrainment can facilitate enhanced amyloid-beta clearance through microglial activation. The study's insights inform medical practitioners, researchers, and healthcare providers on enhancing treatment efficacy, reducing neurodegeneration, and maintaining cognitive function in the context of increasing Alzheimer's disease prevalence. It contributes to the discourse on neurodegenerative disease treatment amid technological advancement.

 View pdf
View pdf


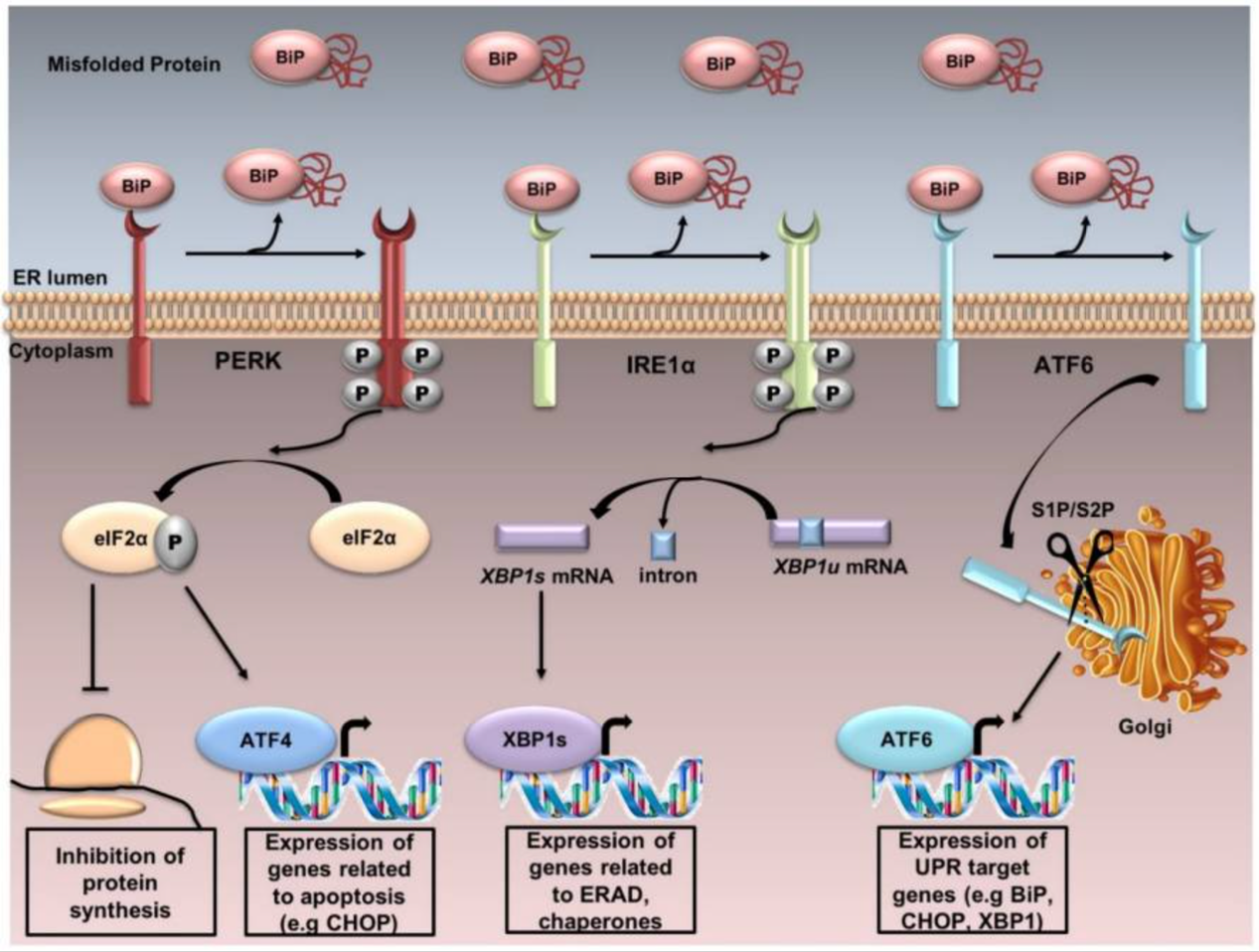
This research proposal aims to study the relationship between CFTR gene and ER stress and its potential involvement in the aetiology of AD. Using CRISPR Cas9 technology, we intend to induce the F508del mutation in the CFTR gene of a mouse model in a way that mimics the pathophysiological state of Cystic Fibrosis. It describes a study design, including the assessment of spatial learning capability in the Morris Water Maze experiment, and the analysis of Aβ protein aggregation in the hippocampus. The results may give clues as to the molecular mechanisms of Alzheimer's disease and help identify new targets for therapeutic intervention.

 View pdf
View pdf


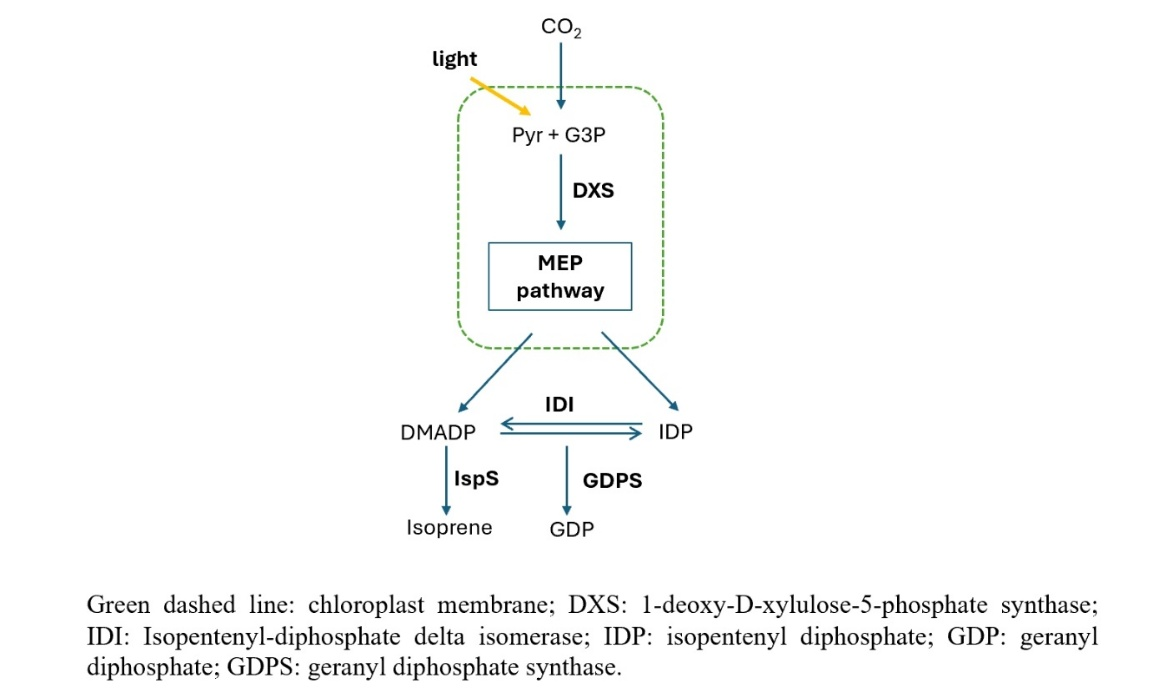
World energy is facing multiple threats. On the one hand, non-renewable energy sources such as fossil energy, which account for a large proportion, are gradually depleting, but the demand is still high. On the other hand, most of the energy currently used, such as oil and coal, is directly related to climate change and air pollution. Renewable biosynthetic pathways currently account for a very small proportion of energy consumption and therefore have great potential for development. Isoprene has a wide range of uses and can be used as a target product for biosynthesis. The MEP pathway of Chlamydomonas reinhardtii UPN22 can be used to synthesize this terpene compound, and there is the possibility of further improving the synthesis efficiency through the combined effects of genetic engineering and the external environment. At this stage, the characteristics of the UPN22 strain may affect the collection of isoprene products. In addition, there is a lack of real data on the entire MEP pathway, and it is impossible to confirm how these strategies will affect the synthesis of isoprene.

 View pdf
View pdf


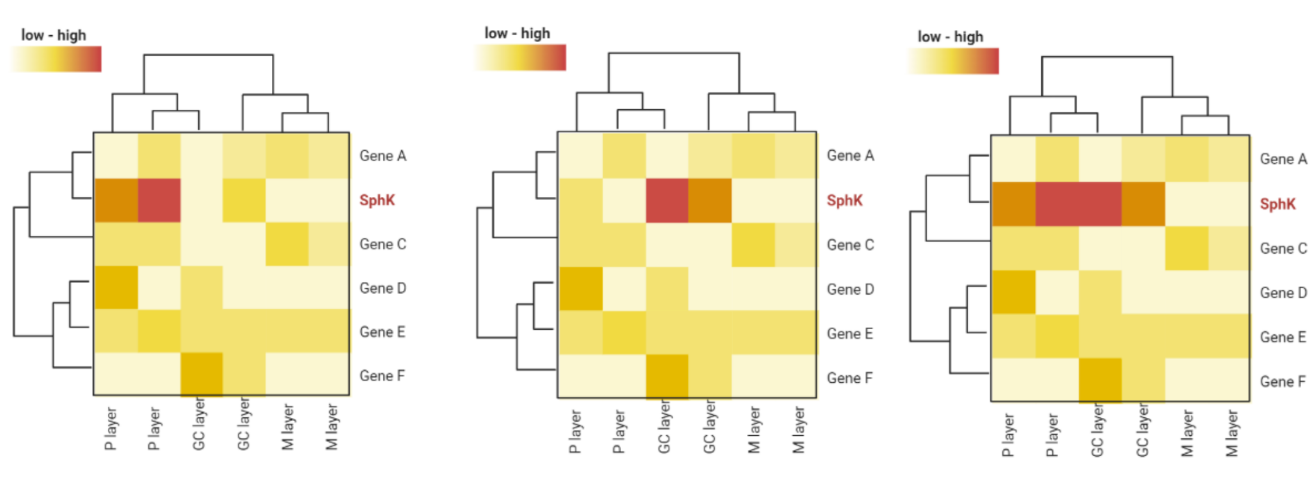
This work studies the functions of sphingosine-1-phosphate (S1P) and Reelin in the horizontal-to-radial transitioning of freshly formed dentate granule cells (DGCs) in the hippocampus. We determined the location of S1P synthesis in the dentate gyrus, investigated the cooperative character of S1P and Reelin signaling, and looked at the regulatory link between the two. According to our research, the polymorphic layer or granule cell of the dentate gyrus is where S1P is formed. Moreover, we show that the horizontal-to-radial transitioning of DGCs is facilitated by the joint contribution of S1P and Reelin signaling. Crucially, our findings imply that Reelin participates in this developmental process upstream of S1P. These discoveries deepen our knowledge of hippocampus neurogenesis and could have consequences for neurological conditions linked to abnormal.

 View pdf
View pdf




