1. Introduction
Cancer has been with us throughout human history. However, until the advent of modern cancer chemotherapy in the 1940s [1], there was little that humans could do about cancer pharmaceutically. Fortunately, over the past 80 years, the chemotherapy regimens for different cancers have been continuously improved and refined, so that the treatment of small molecule chemotherapy drugs to kill cancer has successfully saved the lives of a large number of cancer patients. However, the lack of selectivity of small molecule drugs used in traditional chemotherapy leads to the inevitable damage to the rest of the body’s healthy tissues.
Since the development of hybridoma technology in the 1970s, the medical community has begun to try to use monoclonal antibodies to treat tumors in order to improve the defects of chemotherapy that cannot recognize tumor tissue [2-4]. In recent decades, an increasing number of approved monoclonal antibodies have been actively used in the treatment of solid tumors and hematological tumors. However, the use of monoclonal antibodies alone for tumor treatment is not enough: one of the main reasons is that the killing effect of single antibodies on cancer cells is very low [5]. Therefore, the concept of antibody-drug conjugate has been proposed by the pharmaceutical community, which provides a possibility to combine the above two options into one [6].
Antibody-coupled drugs (ADCs) are a new class of drugs that have been on the market since the beginning of this century, mainly used for the targeted therapy of cancer and some other diseases [7]. The concept of ADC drugs appears very early: in 1900, Nobel Prize winner Paul Ehrlich put forward the idea of “magic bullet” drugs, and pointed out that this will be a specific tumor drug that only targets tumor cells without affecting normal cells [8]. As is shown in Figure 1, ADC drugs are different from the traditional biosynthetic or chemical synthetic drugs derived from a single source, but generally composed of cytotoxic small molecule drugs and macromolecular monoclonal antibodies, and they are connected together through special connectors to form complex drug molecules [9]. The drug enters the cancer cells by inducing endocytosis of the recipient cancer cells, and kills the cancer cells by using the intracellular cytotoxic drugs [10]. This structure is designed to exert the targeting ability of monoclonal antibodies and the ability of cytotoxic drugs to kill cancer cells at the same time, achieving good anti- cancer effect while minimizing the negative impact on normal tissues.
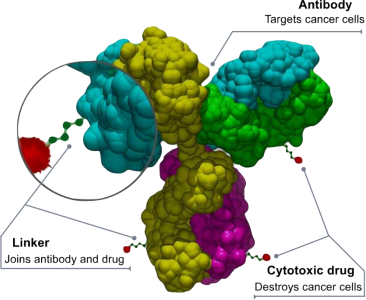
Figure 1. Schematic representation of the basic structure of an ADC drug
In 2000, gemtuzumab ozomicin became the first ADC drug approved by the FDA [3]; As of today gemtuzumab (2023), 14 ADC drugs have been approved by the FDA worldwide. At the same time, more than 100 ADC drug candidates from different companies are currently under clinical investigation [8].
There is no doubt that ADC drugs have great prospects. But at the same time, there are some urgent problems that need to be solved. In 2010, ozomicin was withdrawn from the market due to serious side effects [12,13]. At the same time, other marketed ADC drugs also have negative effects on human health that cannot be ignored due to the limitations of their own small molecule drugs or connectors. Therefore, ADC drugs marketed and developed in recent years have made many improvements in structure and function compared with the original ADC drugs, resulting in the generational division of ADC drugs.
2. Crucial considerations addressed in the development of ADC drugs
A classic ADC drug molecule consists of three parts: a monoclonal antibody, a cytotoxic payload, and a chemical linker. Consider solid tumors: in the most ideal case, an ADC drug should remain stable in the blood circulation, accurately reach the therapeutic target with body fluid circulation, and eventually release a cytotoxic payload near or inside cancer cells [14]. In practice, however, the situation is not always perfect. Each factor will influence the ultimate efficacy and safety of an ADC, and in general, the development of an ADC needs to consider all of these critical factors, including the target antigen, antibody, cytotoxic payload, linker, as well as the choice of conjugation method [15].
The target antigens recognized by monoclonal antibodies are usually specific proteins that are overexpressed on the surface of cancer cells. At present, most of the monoclonal antibodies used for ADC drugs are immunoglobulin G (IgG) antibodies, including four subtypes IgG1, IgG2, IgG3 and IgG4. IgG1 is a commonly used subtype of ADC drugs because IgG1 is the most abundant in serum [16]. Through its high binding affinity to Fc receptors, strong effector functions such as antibody- dependent cell-mediated cytotoxicity (ADCC), antibody-dependent phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC) are induced. In the selection and optimization of target proteins and monoclonal antibodies, the affinity between antibodies and target proteins needs to be paid the most attention, and Figure 2 briefly shows the presentation of different specific antigens on the surface of cancer cells and their recognition by ADC drugs. Too low affinity will reduce the targeting ability of ADC drugs and the rate of internalization of drug molecules by cancer cells, and may cause severe blood toxicity [17]. At the sametime,too high affinity may also lead to the inability of ADC drug molecules to penetrate into solid tumors, resulting in low therapeutic efficiency [18]. In fact, this balance is difficult to maintain in clinical treatment, so early ADC drugs are almost all targeted at hematological tumors.
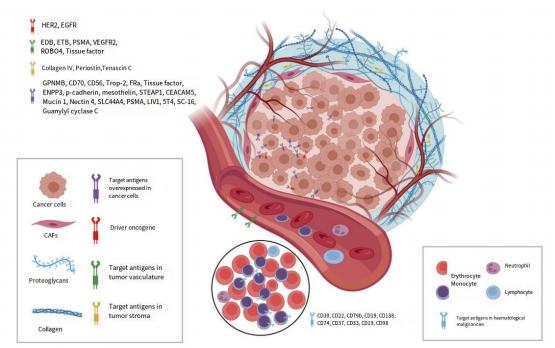
Figure 2. Schematic representation of the main target proteins recognized by ADC drugs [8]
The linker is responsible for connecting monoclonal antibodies with small molecule drugs, and can be subdivided into chemical cleavage connectors and enzymatic cleavage connectors [19]. The former mainly uses hydrazone and disulfide bonds [20], and the latter mainly uses glucuronic acid bonds with specific peptide bonds [21]. Although there are ADC drugs that do not actively separate monoclonal antibodies and cytotoxic small molecules, in order to achieve the best therapeutic effect, both generally require precise cleavage of the linker in the tumor microenvironment for release purposes [20]. These connectors are envisioned to be influenced by the concentration of ions, pH, or proteases in the microenvironment and to release small-molecule drugs at the appropriate site, typically inside the tumor cell. However, this is not an easy task: in the complex environment of the human body, it is difficult for the connectors to completely avoid false cleavage. For example, hydrazone based connectors that are not completely metabolized in the body have the probability of releasing a large number of cytotoxic small molecular substances in the acidic microenvironment in the renal tubules or digestive tract, causing kidney failure or digestive tract damage in patients. Excessive early cleavage will greatly increase the off-target toxicity of the drug, resulting in the destruction of normal tissues, and then cause adverse reactions [22].
Compared with monoclonal antibodies and connectors, the choice of small molecule cytotoxic drugs for ADC drugs is relatively conservative [23]. Most of the “bullets” of ADC drugs that have been marketed and entered clinical trials are still derived from small molecule chemotherapy drugs. At present, the small molecule cytotoxic drugs in ADC drugs can be divided into three categories according to their principles of action [24]. Among them, DNA damage agents (such as caricamycin [25]) and tubulin inhibitors (such as microtubule-lysin) are mature options for ADC drugs. Dna-damaging agents are more effective than tubulin inhibitors but may cause more off-target toxicity. It is noteworthy that some new small-molecule immunomodulators, such as toll-like receptor agonists [26], have emerged in ADC drug development in recent years, and some of them have begun to enter clinical trials. This new type of small molecule drug is expected to reduce the cytotoxicity of ADC drugs while maintaining the therapeutic efficacy, and obtain better survival expectancy.
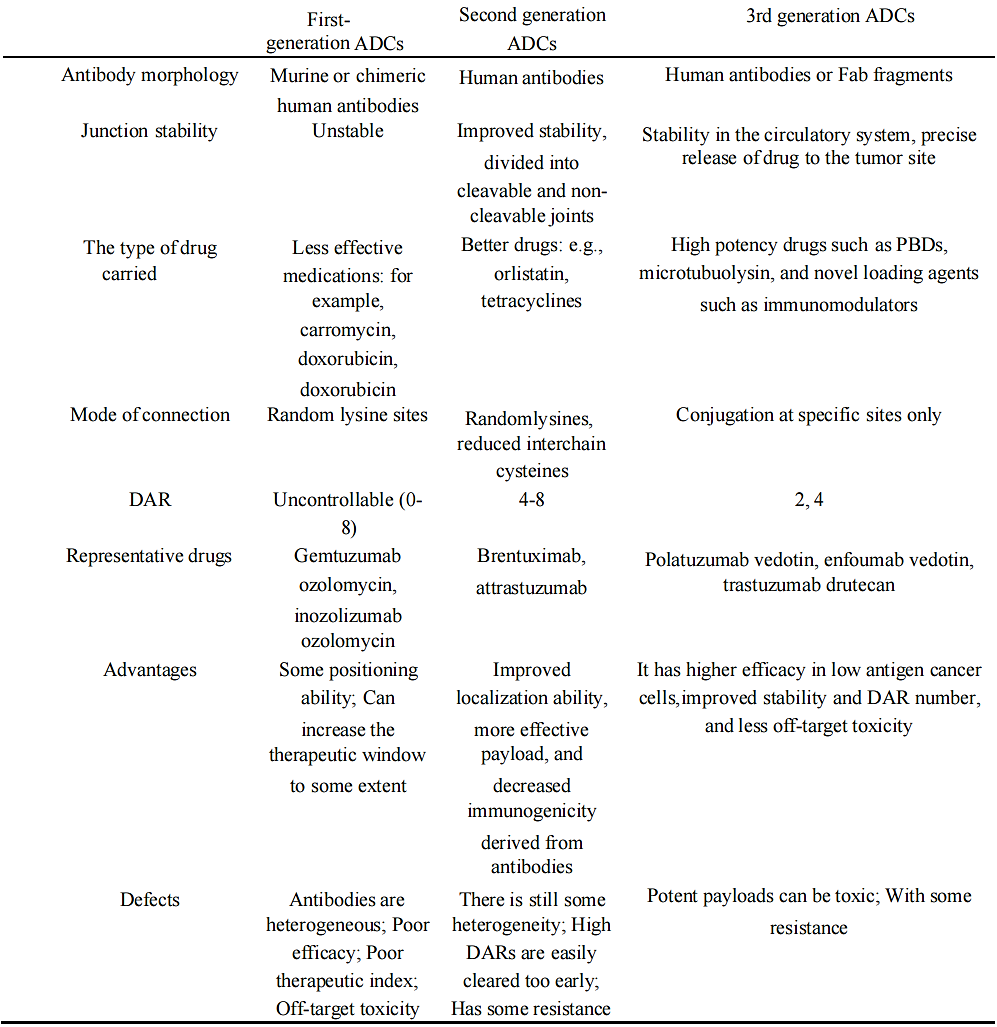
Based on the above factors and the actual clinical application of ADC drugs, the ADC drugs in use and development can be divided into three generations. The brief information of these three generations of ADCs is shown in Table 1.
In general, the development of ADC drugs is in line with the trend of gradually decreasing molecular weight and rejection of antibodies, gradually increasing drug efficacy, and gradually decreasing targeted toxicity.
Table 1. The mainstream generational division of ADC drugs [8]
3. Statistics and brief analysis of FDA pending ADC drugs
At present, the development and experiment of ADC drugs have received a lot of attention and investment from relevant industries. According to the Clinic Trial data, by the end of August 2023, there were 533 ADC drug research projects in the direction of oncology, of which 51 projects had entered clinical Ⅲ/Ⅳ. Figure 3 shows the distribution of tumor types targeted by ADCs that have entered Phase III/IV in the current Clinic Trial.
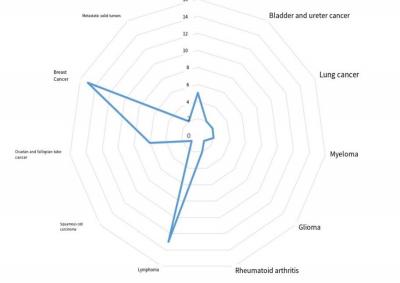
Figure 3. Distribution of research directions of ADC drug-related phase III/IV clinical projects
Among the projects entered into Ⅲ/Ⅳ, 14 projects have produced results, and the remaining 37 projects have not yet had any results. It should be noted that not all of the programs are independent testing of novel ADC drugs: these programs cover the testing of many novel drugs as well as the replication of existing marketed drugs. This article will focus on the results of more mature phase III/IV trials, and provide a brief analysis of the drugs that are awaiting marketing.
The same drug maybe in several trials at the same time. Excluding duplicate drug clinical trials, a total of 22 drugs have entered phase III/IV testing, of which 14 are new drugs that have not yet completed clinical trials for marketing, or are difficult to achieve marketing conditions in the near future. The 14 drugs are summarized in the Table 2 below:
Table 2. Phase III/IV drugs not yet marketed in Clinic trials
Drug names | Clinic Trial No | Cancer category | Specific cancer types |
ARX788 | NCT05426486 | Breast cancer | Her2-positive breast cancer |
Daratumumab | NCT04246047 | Myeloma | Relapsed/refractory multiple myeloma |
Depatuxizumab mafodotin | NCT02573324 | Glioma | Glioblastoma |
Deruxtecan | NCT03734029 | Breast cancer | Her2-low breast cancer |
Durvalumab | NCT05629585 | Lung cancer | Non-small cell lung cancer (NSCLC) |
FS- 1502 | NCT05755048 | Breast cancer | Unresectable, locally advanced or metastatic breast cancer |
GM-CSF | NCT00089115 | Lymphoma | Non-hodgkin’s lymphoma |
MRG002 | NCT04924699 | Breast cancer | Her2-positive unresectable locally advanced or metastatic breast cancer |
MRG003 | NCT05751512 | Squamous cell carcinoma | Recurrent/metastatic Head and neck squamous cell carcinoma (RM-SCCHN) |
Pembrolizumab | NCT05609968; NCT05711628 | Lung cancer; lymphoma | Non-small cell lung cancer (NSCLC); Relapsed or refractory Hodgkin lymphoma |
RC48-ADC | NCT04400695 | Breast cancer | Her2-low breast cancer |
SYD985 | NCT03262935 | Breast cancer | Her2-positive, locally advanced or metastatic breast cancer |
Vadastuximab Talirine | NCT02785900 | Leukemia | Acute myeloid leukemia (CASCADE) |
XMT- 1536 | NCT05329545 | Ovarian and Fallopian tube cancer | Platinum-sensitive recurrent ovarian cancer |
style='position:absolute;left:0;text-align:left;margin-left:169.75pt;margin-top:-85.75pt;width:136.8pt;height:23.4pt;z-index:251658240;mso-position-horizontal-relative:text;mso-position-vertical-relative:text' />It is important to note that the current clinical programs of phase III and IV drugs target an uneven variety of cancers. Most ADC drug development focuses on breast cancer and hematological tumors, which are generally highly affected by genetic factors and have relatively stable expression logic and surface target proteins. By contrast, few ADC drugs are being tested in gastrointestinal cancers, which are highly influenced by acquired environment.
Since most of the experimental projects in progress have not disclosed the effective results, it is difficult to make a precise analysis and proportion statistics of the drugs in development. Instead, we choose representative examples to describe.
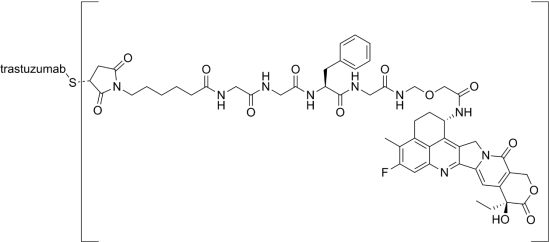
Figure 4. Schematic representation of the small-molecule drug moiety of Deruxtecan [27]
Deruxtecan (NCT03734029) is an ADC drug targeting trop2. Trop2 is a transmembrane glycoprotein that signals to cells for self-renewal, proliferation, invasion and survival, and has stem-like properties. Trop2 is highly expressed in many (but not all) cancers and differentially expressed in some normal tissues. Monoclonal antibodies to Deruxtecan inhibit cancer cell proliferation in part by recognizing Trop2, which is highly expressed on the surface of cancer cells, and then releasing topoisomerase I inhibitor (DXd, already shown in Figure 4, average DAR = 4) into the cancer cells. The latest results show that patients treated with DS1062 (6 mg/kg) have good safety and efficacy. Among 125 patients whose response could be evaluated, the probability of sustained response at 6 months was more than 80%, and the disease control rate (CR + PR + SD) was 79%, a fairly good outcome in the ongoing phase III/IV clinical trial [8].
4. Further development of ADC drugs in the future
As mentioned above, the development and clinical trials of many ADC drugs are not going well. ADC drugs that have been marketed are also facing many doubts about their poor efficacy and side effects.
Gemtuzumab is the first drug approved for the treatment of ADC in the world. It consists of an engineered human monoclonal IgG4 antibody targeting CD33 and cytotoxic N-acetyl-γ-calicheamicin linked by a cleavable hydrazone linker. In principle, endocytosis of the drug would release the cytotoxic small molecule calicheamicin.
However, the hydrazone based linker in gemtuzumab is not completely stable, resulting in frequent premature release of the cytotoxin in the blood, increasing off-target toxicity. The results of the SOG- S0106A study showed that a high rate of severe fatal toxicity was observed in patients receiving combination therapy, but there was no significant clinical benefit response [13].
For the foreseeable future, any ADC drug will include the “triplex” described above: a monoclonal antibody, a connector, and a small-molecule cytotoxic agent. This also means that all the design and optimization of ADC drugs should be carried out from these three aspects.
Based on this information, the current ideas for improvement can be classified and discussed:
One of themisto improve the small molecule cytotoxic drugs carried by ADC drugs. As mentioned above, most of the small molecule drugs used in ADC drugs are classical chemotherapy drugs, which can still inevitably cause serious toxicity to normal tissues in the human body. At present, some relevant institutions are trying to use signaling molecules that promote the apoptosis of cancer cells or disrupt their metabolism. At present, it is still in the early clinical stage.
The other is to improve the protein structure of the monoclonal antibody fraction of ADC drugs. Like most IgG antibodies, ADC drugs accumulate in the human body and are difficult to penetrate tumor tissue. The current idea is to tailor the monoclonal antibody by molecular biological means to improve the penetration and endocytosis efficiency of ADC without affecting the therapeutic effect.
5. Conclusion
For over two decades, substantial resources from both the academic and business sectors have been dedicated to the development and experimentation of ADC drugs. These efforts have yielded significant successes, leading to the preservation of numerous cancer patients’lives while mitigating their suffering. Nevertheless, certain issues persist within current ADC drugs, necessitating further attention through subsequent research and clinical trials. In the long term, the trajectory of ADC drug development appears to be trending towards a “short and powerful” direction. This involves reducing molecular weight and simplifying their structures to enhance penetration efficiency. Concurrently, improvements in linkers and small molecule drugs aim to augment their targeting capabilities and cytotoxic effectiveness. ADC drugs, indeed, stand as promising future contenders in the realm of cancer therapy. As we look ahead, ADC drugs are poised to assume a more significant role not only in oncology but potentially extending beyond the scope of cancer treatment.
References
[1]. Christakis P. (2011). The birth of chemotherapy at Yale. Bicentennial lecture series: Surgery Grand Round. The Yale journal of biology and medicine, 84(2), 169-172.
[2]. Köhler, G., & Milstein, C. (1975). Continuous cultures of fused cells secreting antibody of predefined specificity. Nature, 256(5517), 495-497.
[3]. Ferrara, N., Hillan, K. J., & Novotny, W. (2005). Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochemical and biophysical research communications, 333(2), 328-335.
[4]. Plosker, G. L., & Figgitt, D. P. (2003). Rituximab: a review of its use in non-Hodgkin’s lymphoma and chronic lymphocytic leukaemia. Drugs, 63(8), 803-843.
[5]. Shefet-Carasso, L., & Benhar, I. (2015). Antibody-targeted drugs and drug resistance--challenges and solutions. Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy, 18, 36-46.
[6]. Sievers, E. L., & Senter, P. D. (2013). Antibody-drug conjugates in cancer therapy. Annual review of medicine, 64, 15-29.
[7]. Norsworthy, K. J., Ko, C. W., Lee, J. E., Liu,J., John, C. S., Przepiorka, D., Farrell, A. T., & Pazdur, R. (2018). FDA Approval Summary: Mylotarg for Treatment of Patients with Relapsed or Refractory CD33-Positive Acute Myeloid Leukemia. The oncologist, 23(9), 1103- 1108.
[8]. Fu,Z., Li, S., Han, S., Shi,C., & Zhang,Y. (2022). Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal transduction and targeted therapy, 7(1), 93.
[9]. De Cecco, M., Galbraith, D. N., & McDermott, L. L. (2021). What makes a good antibody-drug conjugate? Expert opinion on biological therapy, 21(7), 841-847.
[10]. Xu S. (2015). Internalization, Trafficking, Intracellular Processing and Actions of Antibody-Drug Conjugates. Pharmaceutical research, 32(11), 3577-3583.
[11]. https://en.wikipedia.org/wiki/Antibody-drug_conjugate#cite_note-:0-2
[12]. Petersdorf, S. H., Kopecky, K. J., Slovak, M., Willman, C., Nevill, T., Brandwein, J., Larson, R. A., Erba, H. P., Stiff, P. J., Stuart, R. K., Walter, R. B., Tallman, M. S., Stenke, L., & Appelbaum, F. R. (2013). A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood, 121(24), 4854-4860.
[13]. Wadleigh, M., Richardson, P. G., Zahrieh, D., Lee, S. J., Cutler, C., Ho, V., Alyea, E. P., Antin, J.H., Stone, R. M., Soiffer, R. J., & DeAngelo, D. J. (2003). Prior gemtuzumab ozogamicin exposure significantly increases the risk of veno-occlusive disease in patients who undergo myeloablative allogeneic stem cell transplantation. Blood, 102(5), 1578- 1582.
[14]. Xiao, Y., & Yu, D. (2021). Tumor microenvironment as a therapeutic target in cancer. Pharmacology & therapeutics, 221, 107753.
[15]. Kaytor, M. D., Wilkinson, K. D., & Warren, S. T. (2004). Modulating huntingtin half-life alters polyglutamine-dependent aggregate formation and cell toxicity. Journal of neurochemistry, 89(4), 962- 973.
[16]. Natsume, A., Niwa, R., & Satoh, M. (2009). Improving effector functions of antibodies for cancer treatment: Enhancing ADCC and CDC. Drug design, development and therapy, 3, 7- 16.
[17]. Tsumura, R., Manabe, S., Takashima, H., Koga, Y., Yasunaga, M., & Matsumura, Y. (2018). Influence of the dissociation rate constant on the intra-tumor distribution of antibody-drug conjugate against tissue factor. Journal of controlled release : official journal of the Controlled Release Society, 284, 49-56.
[18]. Saunders K. O. (2019). Conceptual Approaches to Modulating Antibody Effector Functions and Circulation Half-Life. Frontiers in immunology, 10, 1296.
[19]. Bargh, J. D., , Isidro-Llobet, A., , Parker, J. S., , & Spring, D. R., (2019). Cleavable linkers in antibody-drug conjugates. Chemical Society reviews, 48(16), 4361-4374.
[20]. NoltingB. (2013). Linker technologies for antibody-drug conjugates. Methods in molecular biology (Clifton, N.J.), 1045, 71- 100.
[21]. Jeffrey, S. C., Nguyen, M. T., Moser, R. F., Meyer, D. L., Miyamoto, J. B., & Senter, P. D. (2007). Minor groove binder antibody conjugates employing a water soluble beta-glucuronide linker. Bioorganic & medicinal chemistry letters, 17(8), 2278-2280.
[22]. Flygare, J. A., Pillow, T. H., & Aristoff, P. (2013). Antibody-drug conjugates for the treatment of cancer. Chemical biology & drug design, 81(1), 113-121.
[23]. Diamantis, N., & Banerji, U. (2016). Antibody-drug conjugates--an emerging class of cancer treatment. British journal of cancer, 114(4), 362-367.
[24]. Yang, H., Ganguly,A., & Cabral, F. (2010). Inhibition of cell migration and cell division correlates with distinct effects of microtubule inhibiting drugs. The Journal of biological chemistry, 285(42), 32242-32250.
[25]. Zein, N., Sinha, A. M., McGahren, W. J., & Ellestad, G. A. (1988). Calicheamicin gamma 1I: an antitumor antibiotic that cleaves double-stranded DNA site specifically. Science (New York, N.Y.), 240(4856), 1198- 1201.
[26]. Gregson, S. J., Masterson, L. A., Wei, B., Pillow, T. H., Spencer, S. D., Kang, G. D., Yu, S. F., Raab, H., Lau, J., Li, G., Lewis Phillips, G. D., Gunzner-Toste, J., Safina, B. S., Ohri, R., Darwish, M., Kozak, K. R., Dela Cruz-Chuh, J., Polson, A., Flygare, J. A., & Howard, P. W. (2017). Pyrrolobenzodiazepine Dimer Antibody-Drug Conjugates: Synthesis and Evaluation of Noncleavable Drug-Linkers. Journal of medicinal chemistry, 60(23), 9490-9507.
[27]. https://en.wikipedia.org/wiki/Gemtuzumab_ozogamicin
Cite this article
Li,A. (2024). An overview of the principles and prospects of ADC drugs. Theoretical and Natural Science,35,5-13.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Christakis P. (2011). The birth of chemotherapy at Yale. Bicentennial lecture series: Surgery Grand Round. The Yale journal of biology and medicine, 84(2), 169-172.
[2]. Köhler, G., & Milstein, C. (1975). Continuous cultures of fused cells secreting antibody of predefined specificity. Nature, 256(5517), 495-497.
[3]. Ferrara, N., Hillan, K. J., & Novotny, W. (2005). Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochemical and biophysical research communications, 333(2), 328-335.
[4]. Plosker, G. L., & Figgitt, D. P. (2003). Rituximab: a review of its use in non-Hodgkin’s lymphoma and chronic lymphocytic leukaemia. Drugs, 63(8), 803-843.
[5]. Shefet-Carasso, L., & Benhar, I. (2015). Antibody-targeted drugs and drug resistance--challenges and solutions. Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy, 18, 36-46.
[6]. Sievers, E. L., & Senter, P. D. (2013). Antibody-drug conjugates in cancer therapy. Annual review of medicine, 64, 15-29.
[7]. Norsworthy, K. J., Ko, C. W., Lee, J. E., Liu,J., John, C. S., Przepiorka, D., Farrell, A. T., & Pazdur, R. (2018). FDA Approval Summary: Mylotarg for Treatment of Patients with Relapsed or Refractory CD33-Positive Acute Myeloid Leukemia. The oncologist, 23(9), 1103- 1108.
[8]. Fu,Z., Li, S., Han, S., Shi,C., & Zhang,Y. (2022). Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal transduction and targeted therapy, 7(1), 93.
[9]. De Cecco, M., Galbraith, D. N., & McDermott, L. L. (2021). What makes a good antibody-drug conjugate? Expert opinion on biological therapy, 21(7), 841-847.
[10]. Xu S. (2015). Internalization, Trafficking, Intracellular Processing and Actions of Antibody-Drug Conjugates. Pharmaceutical research, 32(11), 3577-3583.
[11]. https://en.wikipedia.org/wiki/Antibody-drug_conjugate#cite_note-:0-2
[12]. Petersdorf, S. H., Kopecky, K. J., Slovak, M., Willman, C., Nevill, T., Brandwein, J., Larson, R. A., Erba, H. P., Stiff, P. J., Stuart, R. K., Walter, R. B., Tallman, M. S., Stenke, L., & Appelbaum, F. R. (2013). A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood, 121(24), 4854-4860.
[13]. Wadleigh, M., Richardson, P. G., Zahrieh, D., Lee, S. J., Cutler, C., Ho, V., Alyea, E. P., Antin, J.H., Stone, R. M., Soiffer, R. J., & DeAngelo, D. J. (2003). Prior gemtuzumab ozogamicin exposure significantly increases the risk of veno-occlusive disease in patients who undergo myeloablative allogeneic stem cell transplantation. Blood, 102(5), 1578- 1582.
[14]. Xiao, Y., & Yu, D. (2021). Tumor microenvironment as a therapeutic target in cancer. Pharmacology & therapeutics, 221, 107753.
[15]. Kaytor, M. D., Wilkinson, K. D., & Warren, S. T. (2004). Modulating huntingtin half-life alters polyglutamine-dependent aggregate formation and cell toxicity. Journal of neurochemistry, 89(4), 962- 973.
[16]. Natsume, A., Niwa, R., & Satoh, M. (2009). Improving effector functions of antibodies for cancer treatment: Enhancing ADCC and CDC. Drug design, development and therapy, 3, 7- 16.
[17]. Tsumura, R., Manabe, S., Takashima, H., Koga, Y., Yasunaga, M., & Matsumura, Y. (2018). Influence of the dissociation rate constant on the intra-tumor distribution of antibody-drug conjugate against tissue factor. Journal of controlled release : official journal of the Controlled Release Society, 284, 49-56.
[18]. Saunders K. O. (2019). Conceptual Approaches to Modulating Antibody Effector Functions and Circulation Half-Life. Frontiers in immunology, 10, 1296.
[19]. Bargh, J. D., , Isidro-Llobet, A., , Parker, J. S., , & Spring, D. R., (2019). Cleavable linkers in antibody-drug conjugates. Chemical Society reviews, 48(16), 4361-4374.
[20]. NoltingB. (2013). Linker technologies for antibody-drug conjugates. Methods in molecular biology (Clifton, N.J.), 1045, 71- 100.
[21]. Jeffrey, S. C., Nguyen, M. T., Moser, R. F., Meyer, D. L., Miyamoto, J. B., & Senter, P. D. (2007). Minor groove binder antibody conjugates employing a water soluble beta-glucuronide linker. Bioorganic & medicinal chemistry letters, 17(8), 2278-2280.
[22]. Flygare, J. A., Pillow, T. H., & Aristoff, P. (2013). Antibody-drug conjugates for the treatment of cancer. Chemical biology & drug design, 81(1), 113-121.
[23]. Diamantis, N., & Banerji, U. (2016). Antibody-drug conjugates--an emerging class of cancer treatment. British journal of cancer, 114(4), 362-367.
[24]. Yang, H., Ganguly,A., & Cabral, F. (2010). Inhibition of cell migration and cell division correlates with distinct effects of microtubule inhibiting drugs. The Journal of biological chemistry, 285(42), 32242-32250.
[25]. Zein, N., Sinha, A. M., McGahren, W. J., & Ellestad, G. A. (1988). Calicheamicin gamma 1I: an antitumor antibiotic that cleaves double-stranded DNA site specifically. Science (New York, N.Y.), 240(4856), 1198- 1201.
[26]. Gregson, S. J., Masterson, L. A., Wei, B., Pillow, T. H., Spencer, S. D., Kang, G. D., Yu, S. F., Raab, H., Lau, J., Li, G., Lewis Phillips, G. D., Gunzner-Toste, J., Safina, B. S., Ohri, R., Darwish, M., Kozak, K. R., Dela Cruz-Chuh, J., Polson, A., Flygare, J. A., & Howard, P. W. (2017). Pyrrolobenzodiazepine Dimer Antibody-Drug Conjugates: Synthesis and Evaluation of Noncleavable Drug-Linkers. Journal of medicinal chemistry, 60(23), 9490-9507.
[27]. https://en.wikipedia.org/wiki/Gemtuzumab_ozogamicin









