1. Introduction
In the long history of human beings exploiting natural resources for their basic living needs, natural plants have always been the utmost valued species used in human activities such as religious events and disease curing. Traditional Chinese medicine is an example of this kind of tries, and have accumulated a huge amount of experience in using natural plants for medicinal purposes, which could be a key to future therapies by finding the essential active natural products in these natural plants through modern chemistry and biochemistry methods. Nowadays in China, there are 11,000 species of natural plants used in traditional Chinese medicine, with only 30% among them studied by natural pharmacologists in terms of chemical composition and biological activity, providing a rich and reliable resource for natural product research related to traditional Chinese medicine.
Terpenes and terpenoids (terpenes with hetero atoms), a large family of natural products derived from isoprene units, are a promising source of new therapeutics due to their broad spectrum of biological activities, which are always the key active compounds of the herbs used in traditional Chinese medicine[1, 2]. Phainanoids, a recently isolated class of triterpenoid (built with six isoprene subunits) from Paranephelium hainanensis Merr., one of the natural plants used in traditional Chinese medicine for medical treatment of diabetes, infections and hepatitis B, found only on the Hainan Island of China, have shown their cytotoxicity against various cancer cells and strong potency as Immunosuppressant, arousing the broad interest of pharmacologists and synthetic chemists. [3] (Figure 1)[4]
With environmental problems and changes in human genes, strengthening the immune system is necessary to fight common infections and diseases. Immuno-antibody drugs can suppress or reduce the strength of the body’s immune system. The World Health Organization currently has only two immunosuppressive drugs on its list of essential medicines: cyclosporine and azathioprine, of which cyclosporine is the only natural product. After 1972 it was successively discovered that cyclosporine can be used for immunosuppression in kidney transplantation and liver transplantation. However, in some cases, it can reach other body systems and may lead to various complications of crisis illnesses, such as rheumatoid arthritis, multiple lymphoma, lupus, Crohn’s disease, and traumatic cholangitis. In 2014 a paper from Jianmin Yue et al. reported the isolation of Phainanoids for the first time[3]. In this paper six new compounds called Phainanoids A-F were introduced with their possible immunosuppressive activity. A continuing study on Phainanoids is published in 2017 by Jianmin Yue et al. shows another four newly established compounds belong to Phainanoids, including Phainanolide A and Phainanoids G-I[5]. Compounds belong to these new class of triterpene natural products are found to be cytotoxic to certain tumor material lines (Phaninanoid G-I) and exhibit immunosuppressive activity similar to that of the developed drug cyclosporin A2, with the most potent one Phainanoid F showing 7 and 221 times more active than cyclosporin[3].
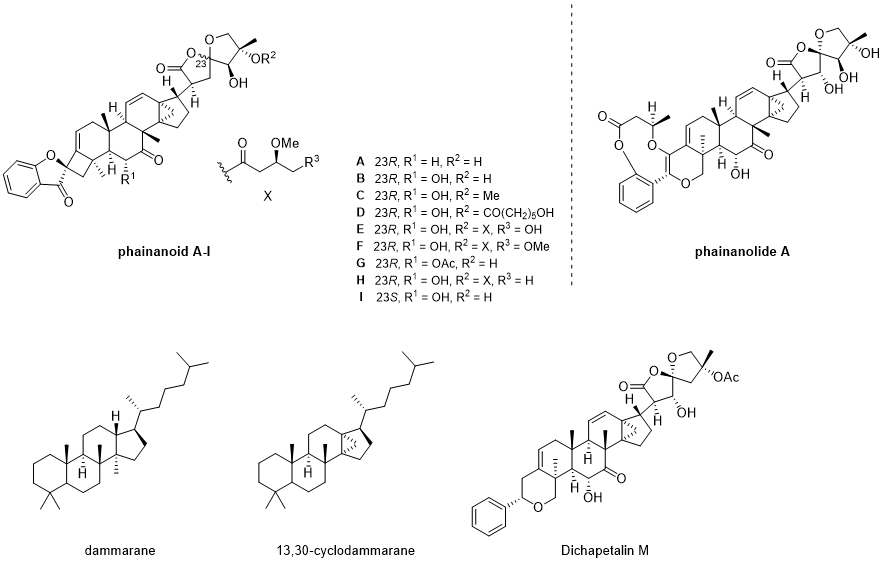
Figure 1. Phainanoid/dichapetalin-type 13,30-cyclodammarane triterpenoids.
Phainanoids can be structurally devided into 3 parts including two unique motifs of 3H-spiro[benzofuran-2,1′-cyclobutan]-3-one and 1,6-dioxaspiro[4.4]nonan-2-one, with the latter resembling existing anti-tumor dichapetalin-type triterpenoids and the former not being found previously in natural product, definitely playing an important role in the structure-activity relation. as 13,30-cyclodammarane-type triterpenoids share the common [4.3.1] propellane architecture embedded in the core. For Phainanolide it has an unprecedented 6/9/6 heterotricyclic system which differs from its Phainanoids analogues. These unique structure features make its synthesis a challenge, and Phainanoids have only 1 total synthesis reported up to now when the synthesis of their two special motifs have been studied by several groups and some achievements have been made.
This article will begin with an overall introduction of terpenes and terpenoids. Then, the structure of Phainanoids will be talked about, followed with the effort synthesists taken for chemical syntheses of Phainanoids coping with the readily clarified structure. Its full syntheses will be taken full insight, considering its abnormal skeleton and functional groups. The strategies taken to achieve the challenging novel fragments will also be mentioned. At the end of this article, the structure-activity relationship will be concluded and its medicinal use will be talked with the comparison with the existing drugs to show its potency.
2. Terpenes and Terpenoids
Terpenes, or Terpenoids , the oxidized ones, are a diverse group of compounds found abundantly in nature, typically as terpenes and their oxygenated forms. They are most varied in plants, serving as the richest category of secondary metabolites with diverse molecular structures.
Terpenoids are built from isoprene units and are classified into hemiterpenes (C5), monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), triterpenes (C30), and tetraterpenes (C40) based on isoprene unit count. They can further be categorized as acyclic, monocyclic, bicyclic, or tricyclic based on their carbon ring structures.
Monoterpenes are a subclass of terpenoids characterized by a ten-carbon framework, consisting of two isoprene units. They are commonly found in secretory tissues of plants, such as glandular trichomes and oil glands. Monoterpenes exhibit structural diversity, including acyclic monoterpenes, monocyclic monoterpenes, bicyclic monoterpenes, and monocyclic ether monoterpenes.Acyclic monoterpenes are composed of two isoprene units in a linear arrangement. Representative compounds in this category include geraniol and citronellol.Monocyclic monoterpenes derive from linear monoterpenes, and their structural variation arises from differences in cyclization pathways. Among these, menthol is a prominent and representative compound.Bicyclic monoterpenes are a subset of bicyclic terpenoid compounds, primarily formed from two isoprene units. The p-menthane and camphane-type structures are the most stable among them, with camphor being a well-known representative compound.Monocyclic ether monoterpenes constitute a distinct subgroup of monoterpenes, often existing in the form of glycosides. Notable representatives in this category include gardenoside, loganin, and gentiopicroside.
Sesquiterpenes are a natural class of terpenoids composed of 15 carbon atoms, formed from three isoprene units. They are predominantly found in plant volatile oils, often coexisting with monoterpenes.From a total of 11 species spanning six genera, including Chloranthus, Sarcandra, and Hedyosmum, 134 sesquiterpene monomers, dimers, or polymers have been isolated. This collection encompasses 49 novel compounds, 10 new skeletal structures, and 50 bioactive compounds [20,72,84-95]. Notably, some sesquiterpene dimers exhibit remarkable anti-malarial activity, surpassing the efficacy of artemisinin by up to 1000-fold. This discovery holds significant promise for the development of novel anti-malarial drugs, presenting valuable candidates in this endeavor.
Diterpenes are a subclass of terpenoids characterized by 20 carbon atoms, consisting of four isoprene units. They are primarily found in nature in the forms of resins, lactones, or glycosides. Their structural diversity categorizes them into acyclic diterpenes, monocyclic diterpenes, bicyclic diterpenes, tricyclic diterpenes, and more.Acyclic diterpenes are relatively rare in nature and are commonly found in plants containing chlorophyll. Monocyclic diterpenes, such as labdane-type diterpenes, can be further subdivided into various subclasses, including halimane, clerodane, cembrane, serrulatane, and viscidane-type diterpenes. Among these, halimane-type diterpenes feature a decalin nucleus with a variable structural configuration, characterized by a trans-fused A/B ring system, and a methyl group at the C-10 position [5].Clerodane-type diterpenes share the basic decalin skeleton with halimane-type diterpenes and are considered a structural rearrangement of the latter.
Triterpenes are a class of terpenoids characterized by a fundamental nucleus composed of 30 carbon atoms, derived from six isoprene units. They are typically found in plants in the form of glycosides or esters. Among triterpenes, tetracyclic triterpenes are widely distributed in traditional Chinese medicine, exhibiting a diverse range of compounds, with representative examples including ginsenosides. Pentacyclic triterpenes are prevalent in the plant kingdom and often possess medicinal properties. This category includes compounds such as ursolic acid and glycyrrhizic acid. Ursolic acid, in particular, is a naturally occurring substance known for its medicinal value and biological effects.
Another widely discussed subclass of triterpenes are Dammaranes. Biosynthetically, like other terpenes and terpenoids, Dammaranes ultimately is the product of iterative reductive couplings of dimethylallyl pyrophosphate (DMAPP) and isopentenyl pyrophosphate (IPP), both active basic C5 units coming from isoprene. Under the catalysis of prenyltransferase, two IPPs and one DMAPP construct fanesyl diphosphate (FPP), which 2 FPPS afterwards form squalene with the existence of squanlene synthase. Subsequently, squalene undergoes enzymatic oxidation, with the help of squalene epoxidase, to give 2,3-oxidosqualene, the precursor of most triterpenes. Dammaranes are then derived from the cyclization of 2,3-oxidosqualene catalyzed by dammaranediol-II synthase, one of the oxidosqualene cyclases, with the basic skeleton determined and the following chemical modification such as oxidation, glycosylation and acylation on dammaranediol-II obtained directly after cyclization.
3. Structural overview of Phainanoids
Apparently Phainanoids fall into the category of Dammaranes. To be more specific, this kind of triterpenoids can be defined as 13,30-cyclodammarane, which emphasized the distinct cyclopropane embedded into the four rings system shared by Dammaranes, forming a unique [4.3.1] propellane.[4] Structurally, Phainanoids can be divided into three part: the 5,5-oxaspirolatone motif from the northeastern branch, the 4,5-spirocycle motif at southwest, and the remained central ring system containing the [4.3.1] propellane structure feature. Comparing with Dichapetalin M, one of the dichapetalin triterpenoids known for their anticancer activity, the similar 5,5-oxaspirolatone and [4.3.1] propellane structure can be easily identified, but unlike the dichapetalins phainanoids 1-9 shares an unprecedented 4,5-spirocyclic motif, showing the phainanoids are more highly oxidized. Apparently, these novel structures and highly strained rings, the stereoselectivity of these complicated natural products and the heavily oxygenated 5,5-oxaspirolactones with the sensitive function groups make the total synthesis of Phainanoids a challenge.
Currently, efforts have been made by synthesists on the synthesis of Phainanoids and some certain achievements have been achieved. The asymmetric total synthesis of (+)-phainanoid A has been reported in 2023 by Guangbin Dong et al.[4] based on the previous research on the construction of the essential fragments and the connection of these fragments, and further structure-activity relationship has been explored coupling with the unnatural active analogues synthesized by their research group. Besides the significant works from Dong’s group, there are several meaningful studies focusing on the synthesis and modification of the representative motifs, with model compounds and model reactions established. For example, in 2017, Nan et al.[6] have successfully synthesized the 5,5-oxaspirolactone motif of phainanoid F through furan oxidative spirocyclization. Also in 2023, Liu et al.[7] reported a stereo-divergent preparation of 5,5-oxaspirolactones from phainanoids directed by the different substitution on cyclopropanol precursors.
4. The Asymmetric Total Synthesis of (+)-Phainanoid A
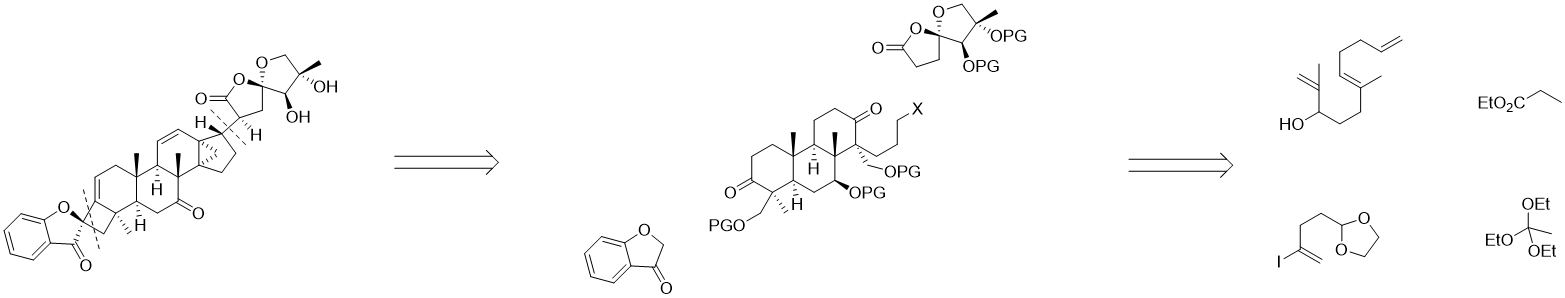
Figure 2. Retrosynthetic analysis from Dong’s synthesis of Phainanoid A.
To achieve this total synthesis, the Dong’s group[4] takes an in-depth analysis on the structure and disconnects phainanoid A at the 4,5-spirocycle and [4.3.1] propellane as they are highly strained, easy to be broken which makes it better to reach them at the very late stage of synthesis. Thus, phainanoid A has been divided into three simpler fragments. The tricyclic core can be easily approached by polyene cyclization of linear precursor, and 3-coumaranone is commercially available. The construction of 5,5-oxaspirolactone moieties, the connection of these three separated parts and the formation of the 4,5-spirocycle and [4.3.1] propellane are still challenging. Model compounds are designed to study connection strategies, instructing the synthases of key fragments.
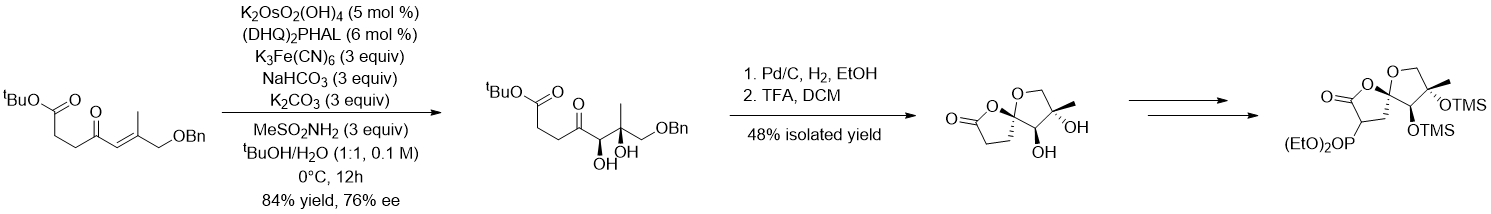
Figure 3. Synthesis of the 5,5-Oxaspirolactone Fragment in Dong’s synthesis of Phainanoid A.
Following this idea of retrosynthetic analysis, the five-membered ring in the [4.3.1] propellane can be constructed by the reductive Heck reaction, followed by a biosynthesis inspired one-step β-elimination cyclization through cyclopropylcarbinyl cation intermediate to obtain the cyclopropane and C11-12 olefinic structures; the northeastern fragment of the 5,5-oxaspirolactone can be introduced through Horner-Wadsworth-Emmons (HWE) olefination while the 5,5-oxaspirolactone is constructed by acid-catalyzed spiroketonization. The 4,5-spirocycle on the southwestern part of the molecule was constructed thanks to the authors’ earlier published methodology of palladium-catalyzed intramolecular ketone α-alkenylation[8], where the 3-benzofuranone structure could be introduced by a formal aldol condensation through the addition of the powerful organolithium nucleophilic prepared from 3-benzofuranone to the aldehyde. With the tricyclic core generated via radical-based polyene cyclization and semipinacol rearrangement, all the syntheses of fragments and strategies for connections are soundly established, leading to the fulfillment of the total synthesis. In the construction of the tricyclic core, it is worth mentioning that the introduction of the carbonyl group at the C7 position is also a difficult issue that should not be overlooked, which is cleverly introduced by using the cyclopentanone carbonyl-directed selective C-H oxidation reaction developed by Prof. Schönecker.[9] To reach the asymmetric total synthesis, Corey-Bakshi-Shibata reduction-mediated kinetic resolution strategy[10] is used to enantioselectively construct the tricyclic core, while the other parts are readily formed with high stereoselectivity.
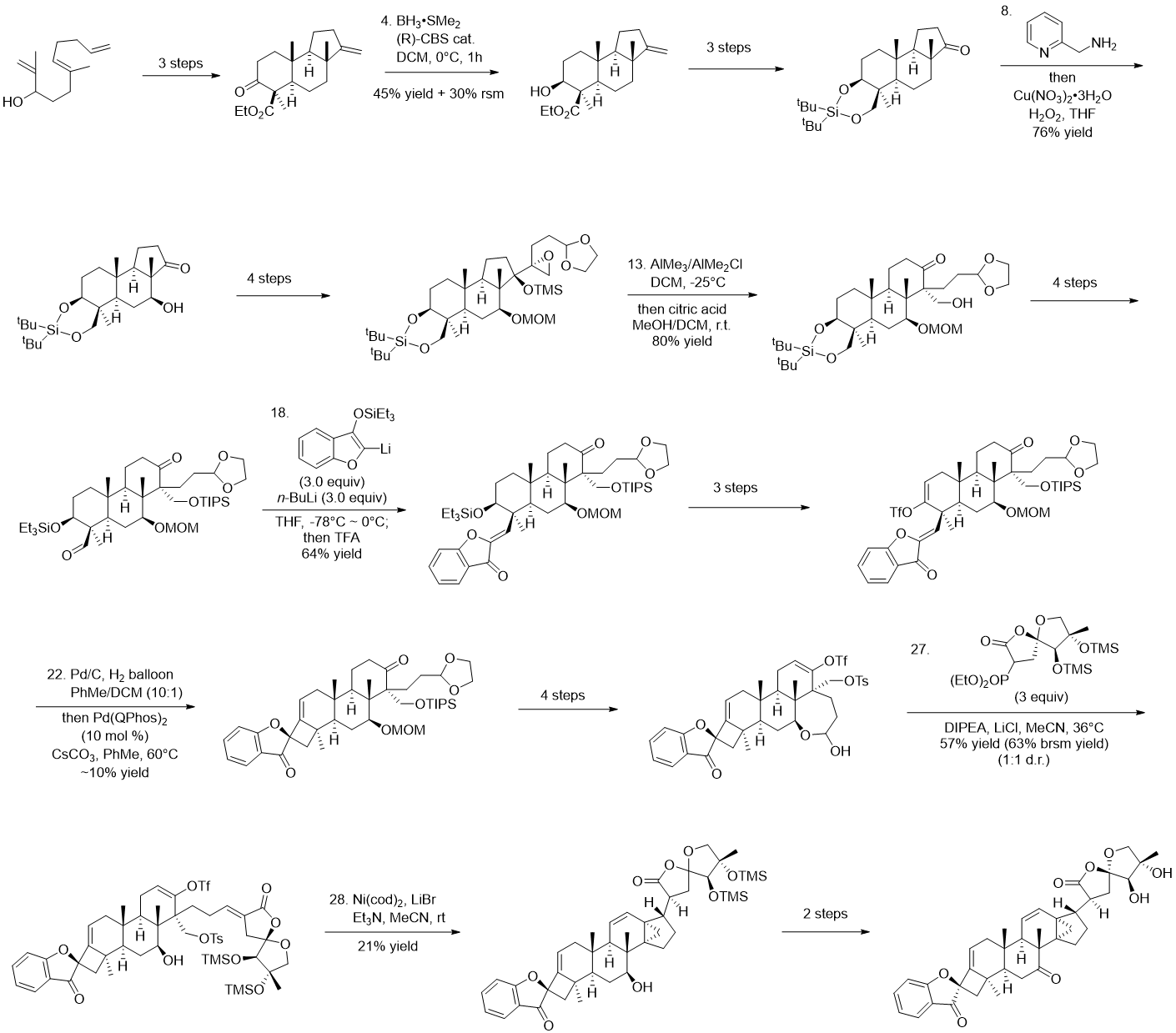
Figure 4. Key steps in Dong’s synthesis of Phainanoid A.
5. Synthesis of Phainanoid F
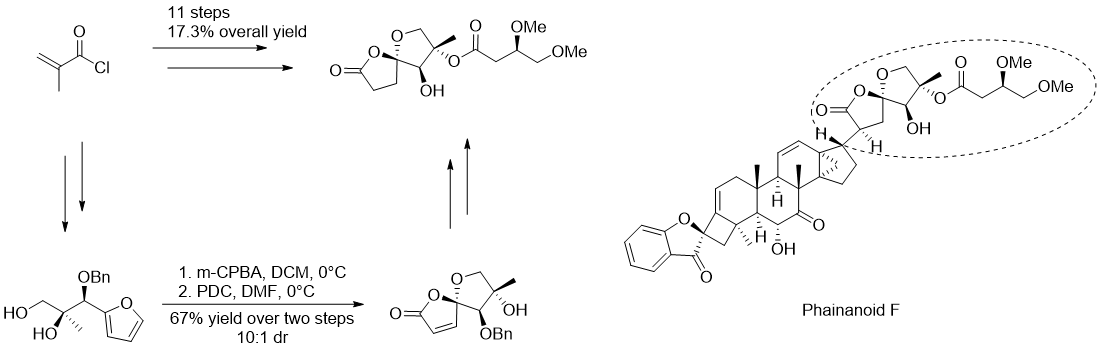
Figure 5. Key step in Nan’s synthesis of the 5,5-oxaspirolactone of Phainanoid F
Fa-Jun Nan successfully developed an efficient synthesis of the 1,6-dioxaspiro[4.4]nonan-2-one motif of Phainanoid F by employing a furanoxidative spirocyclization as the pivotal step. This synthetic pathway encompasses eleven sequential steps and attains an impressive overall yield of 17.3%. Nevertheless, there exists ample room for enhancement in terms of stereoselectivity during reduction.[6]
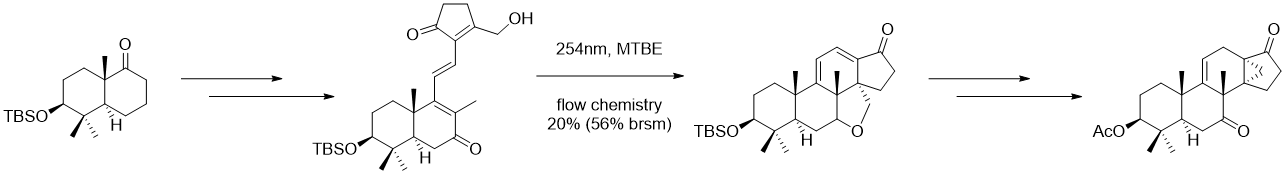
Figure 6. Key cyclization forming the 13,30-cyclodammarane skeleton of Phainanoid F in Li’s work
Yuan-He Li presents an efficient strategy for synthesizing the central ring system of phainanoid F containing the [4.3.1] propellane structure feature, showcasing a remarkable feat in the construction of the 13,30-cyclodammarane skeleton through a photoinduced 6π-electrocyclization and homoallylic elimination. Notably, this represents a rare instance where electrocyclization reactions are employed to simultaneously forge two adjacent quaternary carbons in total synthesis. The elucidated approach serves as the cornerstone for our comprehensive synthesis of Phainanoid F while also offering a versatile methodology applicable to other natural products featuring analogous 13,30-cyclodammarane frameworks.[11]
6. Physiological Activity of Phainanoid
Presently, certain researchers have embarked on investigations into the potential therapeutic applications of Phainanoids and conducted preliminary animal experiments.[3, 6] These experiments evince that Phainanoids can effectively impede tumor growth with minimal impact on normal tissues. Among them, Phainanoid F stands out for its exceptional potency in inhibiting the proliferation of T and B lymphocytes, with activity levels reaching single-digit nanomolar concentrations. Therefore, Phainanoids have the potential to emerge as a groundbreaking class of anti-tumor medication. Studies have unequivocally demonstrated that Phainanoids possess remarkable anti-tumor activity by effectively impeding the growth and proliferation of malignant cells while inducing apoptosis.
Hints of structure-activity relationship is revealed through the biological evaluation on synthesized (+)-phainanoid A and its analogues by Dong et al. in 2023. The significance of 4,5-spirocycle for the immunosuppressive activities may be overestimated, because the simple 4,5-spirocycles possess no such activities, while some analogues without 4,5-spirocycles show moderate reactivity. Besides, the polar alcohol-type moieties are proved important to the activities, but the 5,5-oxaspirolactone itself is inactive. Analogues having [4.3.1] propellane feature with the existence of northeastern polar moieties and southwestern aromatic rings also show moderate activities.[4]
7. Conclusion
Although significant progress has been achieved in the research of Phainanoids, there still remain numerous unresolved questions that necessitate attention. In the forthcoming future, further investigations are imperative to acquire a more profound comprehension of the mechanisms and in vivo activities exhibited by Phainanoids. Moreover, to fully unlock the potential of Phainanoids, additional preclinical studies encompassing their pharmacokinetics, safety profile, and synergistic effects with other pharmaceuticals are indispensable. Furthermore, refinement and optimization of both extraction and synthesis methods for Phainanoids are warranted to enhance their yield and purity levels. Ultimately, it is plausible to attain large-scale production through continuous improvement and optimization of synthetic pathways via methodologies such as synthetic biology.
Acknowledgement
Chengmin Xie and Jie Luo contributed equally to this work and should be considered co-first authors.
References
[1]. NEWMAN D J, CRAGG G M. Natural Products as Sources of New Drugs from 1981 to 2014 [J]. Journal of Natural Products, 2016, 79(3): 629-61.
[2]. HARVEY A L, EDRADA-EBEL R, QUINN R J. The re-emergence of natural products for drug discovery in the genomics era [J]. Nature Reviews Drug Discovery, 2015, 14(2): 111-29.
[3]. FAN Y-Y, ZHANG H, ZHOU Y, et al. Phainanoids A–F, A New Class of Potent Immunosuppressive Triterpenoids with an Unprecedented Carbon Skeleton from Phyllanthus hainanensis [J]. Journal of the American Chemical Society, 2015, 137(1): 138-41.
[4]. XIE J, ZHENG Z, LIU X, et al. Asymmetric Total Synthesis of (+)-Phainanoid A and Biological Evaluation of the Natural Product and Its Synthetic Analogues [J]. Journal of the American Chemical Society, 2023, 145(8): 4828-52.
[5]. FAN Y-Y, GAN L-S, LIU H-C, et al. Phainanolide A, Highly Modified and Oxygenated Triterpenoid from Phyllanthus hainanensis [J]. Organic Letters, 2017, 19(17): 4580-3.
[6]. ZHANG C-L, NAN F-J. An efficient synthesis of the 1,6-dioxaspiro[4.4]nonan-2-one motif of the immunosuppressive triterpenoid Phainanoid F [J]. Tetrahedron Letters, 2017, 58(46): 4357-9.
[7]. XUN L, ZHANG Z, ZHOU Y, et al. Stereodivergent Construction of [5,5]-Ox
[8]. XIE J, WANG J, DONG G. Synthetic Study of Phainanoids. Highly Diastereoselective Construction of the 4,5-Spirocycle via Palladium-Catalyzed Intramolecular Alkenylation [J]. Organic Letters, 2017, 19(11): 3017-20.
[9]. SCHöNECKER B, ZHELDAKOVA T, LIU Y, et al. Biomimetic Hydroxylation of Nonactivated CH2 Groups with Copper Complexes and Molecular Oxygen [J]. Angewandte Chemie International Edition, 2003, 42(28): 3240-4.
[10]. COREY E J, LINK J O. A new chiral catalyst for the enantioselective synthesis of secondary alcohols and deuterated primary alcohols by carbonyl reduction [J]. Tetrahedron Letters, 1989, 30(46): 6275-8.
[11]. LIU H-Y, ZHANG Z-Y, ZHOU Y-K, et al. Synthesis towards Phainanoid F: Photo-induced 6π-Electrocyclization for Constructing Contiguous All-Carbon Quaternary Centers [J]. Chemistry – An Asian Journal, 2023, e202300622.
Cite this article
Xie,C.;Luo,J. (2024). Phainanoids, a promising natural product from traditional Chinese medicine. Theoretical and Natural Science,44,162-169.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. NEWMAN D J, CRAGG G M. Natural Products as Sources of New Drugs from 1981 to 2014 [J]. Journal of Natural Products, 2016, 79(3): 629-61.
[2]. HARVEY A L, EDRADA-EBEL R, QUINN R J. The re-emergence of natural products for drug discovery in the genomics era [J]. Nature Reviews Drug Discovery, 2015, 14(2): 111-29.
[3]. FAN Y-Y, ZHANG H, ZHOU Y, et al. Phainanoids A–F, A New Class of Potent Immunosuppressive Triterpenoids with an Unprecedented Carbon Skeleton from Phyllanthus hainanensis [J]. Journal of the American Chemical Society, 2015, 137(1): 138-41.
[4]. XIE J, ZHENG Z, LIU X, et al. Asymmetric Total Synthesis of (+)-Phainanoid A and Biological Evaluation of the Natural Product and Its Synthetic Analogues [J]. Journal of the American Chemical Society, 2023, 145(8): 4828-52.
[5]. FAN Y-Y, GAN L-S, LIU H-C, et al. Phainanolide A, Highly Modified and Oxygenated Triterpenoid from Phyllanthus hainanensis [J]. Organic Letters, 2017, 19(17): 4580-3.
[6]. ZHANG C-L, NAN F-J. An efficient synthesis of the 1,6-dioxaspiro[4.4]nonan-2-one motif of the immunosuppressive triterpenoid Phainanoid F [J]. Tetrahedron Letters, 2017, 58(46): 4357-9.
[7]. XUN L, ZHANG Z, ZHOU Y, et al. Stereodivergent Construction of [5,5]-Ox
[8]. XIE J, WANG J, DONG G. Synthetic Study of Phainanoids. Highly Diastereoselective Construction of the 4,5-Spirocycle via Palladium-Catalyzed Intramolecular Alkenylation [J]. Organic Letters, 2017, 19(11): 3017-20.
[9]. SCHöNECKER B, ZHELDAKOVA T, LIU Y, et al. Biomimetic Hydroxylation of Nonactivated CH2 Groups with Copper Complexes and Molecular Oxygen [J]. Angewandte Chemie International Edition, 2003, 42(28): 3240-4.
[10]. COREY E J, LINK J O. A new chiral catalyst for the enantioselective synthesis of secondary alcohols and deuterated primary alcohols by carbonyl reduction [J]. Tetrahedron Letters, 1989, 30(46): 6275-8.
[11]. LIU H-Y, ZHANG Z-Y, ZHOU Y-K, et al. Synthesis towards Phainanoid F: Photo-induced 6π-Electrocyclization for Constructing Contiguous All-Carbon Quaternary Centers [J]. Chemistry – An Asian Journal, 2023, e202300622.









