1. Introduction
Attention deficit hyperactivity disorder (ADHD) is one of the most frequent neuropsychiatric disorders that is characterized mainly by inattention, hyperactivity, and impulsivity. ADHD has become one of the most common disorders in school-aged children [1], with ADHD symptoms appearing at the peak in childhood. The prevalence of ADHD in childhood and adolescence is about 7.1% [2]. A minority of patients with ADHD have their symptoms persist into adulthood (even sometimes muted), with a prevalence of 2.8% in adults [3,4].
ADHD can co-occur with a variety of disorders, such as bipolar disorders, substance use disorders (SUDs), obsessive–compulsive disorder (OCD), and depression [5]. Children who have ADHD are at higher risk for a range of unfavorable outcomes [6,7], including the outgrowth of substance use disorders [8,9]. In general, the risk of developing a substance abuse disorder increases during adolescence and maximizes in early adulthood. And people with ADHD develop SUDs faster compared to the general population [10,11]. Discovered predictors of substance abuse in ADHD patients include constant ADHD [12] and ADHD diagnosed in late adolescence or adulthood [13].
Substance use and substance use disorders are prevalent throughout the world [14]. An important hallmark of ADHD is impulsivity [15], which refers broadly to deficiencies in inhibition control that result in behavioral problems that may have long-term negative repercussions in daily living, including various public health conditions [16]. Impulsivity also plays a major role in other psychiatric disorders including SUDs [17].
Thus, ADHD has a high relevance with SUDs, such as ADHD-smoking comorbidity [18] and alcohol use disorders (AUDs) [19]. In these studies, they proposed respectively that ADHD-smoking comorbidity or AUDs involve dysregulation of dopaminergic circuits. For instance, ADHD symptoms are associated with cognitive control and attentional biases that may lead to greater sensitivity to the rewarding effects of alcohol and/or other drugs [20,21]. In previous research, many researchers have reported that the dopamine reward system is associated with addiction and dependence, including alcohol dependence [22]. They also found some evidence that alcohol may increase striatal dopamine release [23,24], which provided support for the dopamine theory of addiction.
As previously mentioned, ADHD could combine with co-occurring disorders (depression, anxiety, etc.), and it would raise the risk of addiction [25,26]. It is universally acknowledged that Alcohol is one of the most popular used and abused substances, leading to many undesirable outcomes, so there are many issues that need to be addressed. A study of college students with ADHD reported higher depression symptoms and frequent alcohol use than non-ADHD students as well as increased substance use, and college students with ADHD who have most substance use (e.g., alcohol abuse) showed a lower GPA than non-substance-used students [27]. ADHD students have more risk for developing substance abuse problems and emotional difficulties, and they have more pressure in their lives. This issue should be brought to the attention of the general public, as much worse situations might occur for ADHD students.
People often have an expectation that they will like the things they want [28]. A report suggested that expectations may be multiply determined by both biological (genotypic) and behavioral factors, such as the correlation between the 7-repeat (7-R) allele of the DRD4 (dopamine D4 receptor) gene, ADHD, and expectations about the effects of alcohol on behavior and mood [29]. Other researchers also suggested that DRD4 genes predicted expectations of being ‘wild and crazy’ after alcohol consumption [30]. In the previous study, DRD4 genes have been found those related to substance abuse [31]. Meanwhile, DRD4 genes are also related to the onset of ADHD [32].
It is interesting that some treatments for ADHD and SUDs are gene-linked. For example, Methylphenidate (MPH) primarily acts as a norepinephrine–dopamine reuptake inhibitor (NDRI), which is an effective drug for most ADHD patients. It has also been found to treat SUDs and it is dependent on individual’s genotypes, such as DAT1 (dopamine transporter) and DRD4 genes [33].
Furthermore, dopaminergic and glutamatergic neurotransmission are also relative, and some areas of the brain where glutamate receptors (GluRs) are located are also associated with ADHD, specifically impulsivity and hyperactivity [34-36]. Some studies have also reported some information on the genetic linkage of glutamate systems, such as C-kinase-1(PICK1) [37] and N-methyl D-aspartate (NMDA) receptor subtype 2B (GRIN2B) genes [38]. They also have individual diversities. Therefore, ADHD and SUDs are highly comorbid and may share a genetic vulnerability. It is easy to deduce that ADHD and SUDs are associated with a wider range of genotypes.
As we see that genotype may be a new target for the treatment of ADHD, SUDs and related disorders, we wondered if there were more physiological systems like the dopamine and glutamate systems that were related to genotype. In this study, we first searched a series of papers to make references and then conducted a meta-analysis of ADHD and SUDs genotype data, but the results of the analysis deviated significantly from our expectations and references. We identified several potential gene loci through meta-analysis on databases that may have significance worth exploring deeply, and we hope to inspire further research in this area.
2. Methods and Materials
2.1. Data Acquisition
The primary source of our data was from the NHGRI-EBI GWAS Catalog. The GWAS Catalog is an open-access database that aggregates and annotates results from published genome-wide association studies from across the globe [39]. This continually increasing resource provides a robust repository for researchers, giving us a comprehensive understanding of gene-disease associations. In addition to the GWAS catalog, we also integrated additional GWAS data from the iPSYCH/Psychiatric Genomics Consortium (PGC) [40]. Recognized for its rich repository of psychiatric genomic data, the PGC offered insights into Alcohol Dependence in 2019 [41] and Cannabis Use Disorder (CUD) [42] to enrich our collection of SUDs data.
By examining the GWAS Catalog, we were able to pinpoint genes that showed overlaps between ADHD and SUDs. This examination yielded a preliminary list of over 40 studies that explored these intersecting genes. To ensure the integrity and reproducibility of our findings, all data sourced were verified against original publications where possible. Each of these studies was subjected to a detailed review to determine its relevance and reliability. Criteria for evaluation included sample size, methods of gene identification, validation of findings, and the subject of study.
We have subsequently narrowed down our selection to 5 key GWAS datasets from our initial list (table 1). These datasets spanned a variety of conditions: 3 datasets were dedicated solely to ADHD, while 2 others focused explicitly on risky substance use disorders.
We have also extracted 2 datasets from the PGC, in which contains GWAS data relevant to Cannabis use disorder and Alcohol use disorder. All the data were processed and analyzed using the GRCh37 (hg19) assembly.
Table 1. Data was sourced from GWAS catalog and PGC.
Study Accession | Source | Notes | Sample Size |
GCST012597 | GWAS Catalog | Attention deficit hyperactivity disorder | 4,945 European ancestry female cases, 16,246 European ancestry female controls |
GCST005362 | GWAS Catalog | Attention deficit hyperactivity disorder | 14,154 European ancestry male cases, 17,948 European ancestry male controls |
GCST007543 | GWAS Catalog | Attention deficit hyperactivity disorder | 19,099 European ancestry cases, 72 non-European ancestry cases, 1,012 Han Chinese ancestry cases, 34,194 European ancestry controls, 72 non-European ancestry controls, 925 Han Chinese ancestry controls |
GCST008414 (PGC | PMID: 33096046) | PGC | Cannabis use disorder | 5,501 Icelandic ancestry cases, 301,041 Icelandic ancestry controls |
GCST90027271 | GWAS Catalog
| Psychiatric symptomatology and risky substance use | 604 Mexican ancestry cases, 1,487 Mexican ancestry controls |
GCST90027269 | GWAS Catalog | Risky substance use | 1,165 Mexican ancestry cases, 1,487 Mexican ancestry controls |
PGC | PMID 30482948 | PGC | Alcohol dependence 2020 | 14,904 individuals with AD and 37,944 controls from 28 case-control and family-based studies |
2.2. Statistical method
We applied meta-analysis on the 7 selected GWAS datasets using PLINK 1.9 (https://www.cog-genomics.org/plink/). PLINK 1.9 is an open-source whole-genome association analysis toolset designed for large-scale analyses. The tool offers improved features and speed improvements compared to its predecessor, making it well-suited for managing and analyzing extensive datasets [43]. Data management, basic statistical evaluations, and association meta-analyses were conducted using PLINK’s functionalities. Since the analysis includes multiple studies, we adapt a Random-effect Model in the analysis to account for the heterogeneity to account for variations across studies.
3. Results
The PLINK meta-analysis generated 1.6GB of analysis results. Includes 11429210 unique SNPs, which 8708996 were overlapped in two or more studies. The program had rejected 249451 SNPs. We used R [44] to process our data. With the aid of pre-available packages such as dplyr [45] and qqman [46], we plot the Q-Q plot (Figure 1) to show the distribution of the P-value and Manhattan plot (Figure 2) to highlight high relevance SNPs.
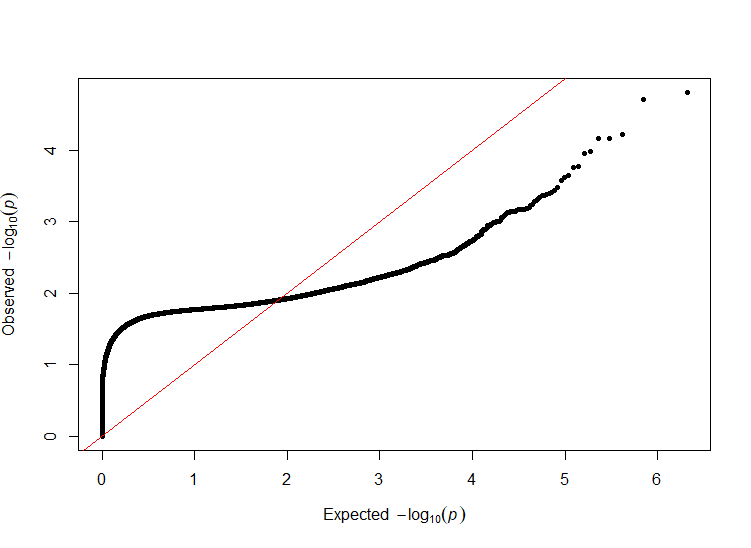
Figure 1. Q-Q plot on the random-effect p-value of our meta-analysis.
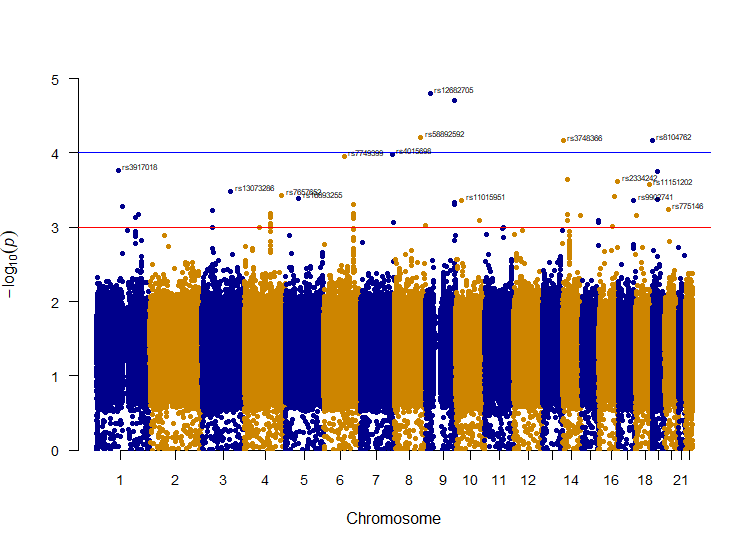
Figure 2. Manhattan plot of the SNPs. The y-axis is transformed to the –log10 of random-effect p-value. The red line indicates a primary threshold for SNPs with P < 1e-3; the blue line shows a more restrictive cut-off at P < 1e-4.
In our analysis, we have identified a variety of Single Nucleotide Polymorphisms (SNPs) that hold significance. Our emphasis was placed on SNPs that showed a recurrent presence, making an appearance in more than four GWAS studies (N > 4). This threshold was set so that at least one SUDs case was taken into account for the studies.
To provide a clear and efficient analysis, also considering the distribution of the p-value, we set a primary p-value cut-off at 1e-3 (conventionally 1e-5) to highlight SNPs of relevance (Figure 2). Further refining our search, we implemented a stricter suggestive threshold set at 1e-4 (conventionally 5e-8). These thresholds were especially useful in isolating SNPs with remarkably strong p-values, and hence showing a “skyscraper” pattern on the Manhattan plot. Of particular note, SNPs that demonstrated p-values less than 8e-4 (P < 8e-4) were regarded with moderate significance and were then isolated for a more detailed examination in the subsequent stages of our research.
Delving deeper into our findings, we have found 45 SNPs that are highly relevant to the disease. The SNPs were then searched in the SNP database (dbSNP) [47] to extract information about the gene. Among those SNPs, 12 yielded no results from the dbSNP. While the gene name and other data were located for the rest of 33 SNPs. Due to some repetitive hits, we were eventually able to identify 21 unique genes that had relevance with ADHD and SUDs.
These genes, listed in order of their located chromosomes, include VCAM1, HSD3B1, CACNA1E, CRB1, ZNF589, BFSP2, EXOSC9, CCNA2, BBS7, LINC02268, CWC27, ARFGEF3, ACTR3C, MLLT3, VAV2, LOC107987137, MKX, ZFHX3, GGA3, DLGAP1, and MISP. The recurrent appearance of these genes across multiple GWAS studies and their p-values accentuates their likely importance in the genomic landscape surrounding ADHD and Substance Use Disorder.
Among the genes identified, ARFGEF4 and BBS7 stand out due to their consistent hits within a small region. This is visually represented by a distinct “skyscraper” pattern on the Manhattan plot (Figure 2) for chromosomes 4 and 6, respectively. Within the peak for ARFGEF4 on chromosome 4, the gene CCNA2 was also detected, forming part of the “skyscraper” formation. Such patterns could suggest robust associations with the diseases under investigation. Beyond these prominent peaks, another noticeable elevation was observed on chromosome 14. Although it did not surpass our established threshold, this elevation directs attention to SNPs associated with the LOC105370504 gene.
4. Discussion
The aim of the present study was to show the relationship between several genes and ADHD, SUDs. Several investigations found that genes that we found do have some relationship with ADHD and SUDs. GWAS revealed genetic variants that affect both ADHD and SUDs. We have found several SUDs-associated genes that contribute to ADHD. There has always been a close association between ADHD and SUDs, but their genetic associations have not been well documented. GWAS is a powerful tool for identifying genetic variants for complex traits. It reveals significant variants that contribute to highly comorbid disease highly effective.
It provides novel genomic findings that prove well-established genetic loci for ADHD have relationship with SUDs. Several investigations found that individuals with ADHD have a greater probability to get SUDs, and Adult ADHD may also contribute to the development and maintenance of substance use disorders [48]. Functional variants, rs16946051 and rs2049161 on DLGAP1 have been proved that are related with executive function in ADHD children [49]. Recently, A research about alcohol use disorder reported that DLGAP1 might be a blood-based biomarker of alcohol use disorder [50]. We discovered that DLGAP1 might make difference in abusing behavior for individuals with attention deficit hyperactivity disorder.
In addition, ZFHX3 is required for the differentiation of late born D1-type medium spiny neurons [51]. That reveals that ZFHX3 play an important role in the growth of dopamine system. Inhibition of the protein that is coded by VCAM1 dopamine neurons protects the dopamine neurons from acute inflammation [52]. GGA3 interacts with ADRA2B which is a G protein-coupled receptor that plays a part in the adrenergic system [53]. VAV2 regulates the reward system mesolimbic pathway which shows there is some associations between VAV2 and substance abuse [54]. In this point of view, the finding linked development of catecholamine system to ADHD and SUDs. This might help finding potential biologically meaningful targets for ADHD and SUDs treatment method.
Interestingly, we found peaks in BBS7, CCNA2, ARFGEF4 and LOC105370504 in our analysis. ARFGEF4 and BBS7 as “skyscrapers” may show significant impacts on the connection of ADHD and SUDs. LOC105370504 is a non-coding RNA, which is still controversial for whether it’s functional or not [55]. The finding reveals that there might be some functions of non-coding transcripts that are still not discovered. BBS7 is a gene that are associated with the Bardet–Biedl syndrome [56]. There’s no research that explains the association between BBS7, ADHD and SUDs. Thus, it could be a potential target to be researched in the future. CCNA2 encodes Cyclin A2 which is a protein associated with cell cycle progression [57]. It means that there might be some relationship between cell cycle progression and neurological disorders like ADHD and SUDs to be discovered in the future. Lack of ARFGEF4 enhances spatial and object recognition memory and plays multiple functional roles in neurons [58]. Therefore, it may show some links between cognitive ability, ADHD and SUDs.
There are some limitations in our research. GWAS cannot identify all genetic determinants of complex traits and it might not pinpoint all causal variants and genes. And GWAS couldn’t reveal some mutations which are too rare to be identified. But GWAS do play an important role in the development of genomics today.
In conclusion, we found several genetic loci that are associated with both ADHD and SUDs. The research revealed some biological targets for further study that may be potential targets for ADHD and SUDs therapies. Meanwhile, we still have many unresolved questions because of time and resource constraints. There remains much future work to understand the reasons for the linkages between ADHD and SUDs.
5. Conclusion
In an effort to explore the genetic interplay between Attention-Deficit/Hyperactivity Disorder (ADHD) and Substance Use Disorders (SUDs), we examined various genes. While past research has alluded to a potential link between ADHD and SUDs, the specific genetic foundations had not been fully delineated. By employing Genome-Wide Association Studies (GWAS), we identified several genetic variants that seemingly influence both disorders. Notably, the DLGAP1 gene, previously linked with executive function in ADHD-affected children, appears to also have implications in substance abuse. Similarly, the ZFHX3 gene, connected with dopamine system development, emerges as a significant player, considering the known associations of the dopamine system with both ADHD and SUDs. Additionally, the genes VCAM1 and GGA3, connected to the dopamine and adrenergic systems respectively, also hint at roles in substance use. Our observations suggest that the evolution of the catecholamine system might be central in understanding the nuances of ADHD and SUDs. An unexpected finding of our study was the potential association of genes like BBS7, CCNA2, ARFGEF4, and LOC105370504, which had not been previously tied to these disorders. While GWAS has been an invaluable tool in this endeavor, it’s imperative to recognize its limitations, such as not capturing all genetic determinants. In sum, our work sheds light on numerous genetic intersections between ADHD and SUDs. Still, the intricate nexus between ADHD and SUDs warrants further exploration.
Authors’ contribution
Y.G. G.S. and Y.X. formulated the research question. Y.G. C.S. and Y.X. collected and selected the related paper. Y.G. G.S. and Y.X. co-drafted the manuscript. G.S. designed the approach, coded the program of analysis, collected and analyzed data. Y.G. and C.S. revised and finalized the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Yaqing Gao, Chen Sang, Guotai Shen and Yuan Xu contributed equally to this work and should be considered co-first authors.
References
[1]. Maxwell, A. (2011). Are some individuals diagnosed with ADHD prone to alcohol abuse? Journal of Attention Disorders, 17(2), 98–101. https://doi.org/10.1177/1087054711427400
[2]. Thomas, R., Sanders, S., Doust, J., Beller, E., & Glasziou, P. (2015). Prevalence of attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Pediatrics, 135(4). https://doi.org/10.1542/peds.2014-3482
[3]. Biederman, Joseph. (2005). Attention-deficit/hyperactivity disorder: A selective overview. Biological Psychiatry, 57(11), 1215–1220. https://doi.org/10.1016/j.biopsych.2004.10.020
[4]. Fayyad, J., Sampson, N. A., Hwang, I., Adamowski, T., Aguilar-Gaxiola, S., Al-Hamzawi, A., Andrade, L. H., Borges, G., de Girolamo, G., Florescu, S., Gureje, O., Haro, J. M., Hu, C., Karam, E. G., Lee, S., Navarro-Mateu, F., O’Neill, S., Pennell, B.-E., Piazza, M., … Kessler, R. C. (2016). The descriptive epidemiology of DSM-IV ADULT ADHD in the World Health Organization World Mental Health Surveys. ADHD Attention Deficit and Hyperactivity Disorders, 9(1), 47–65. https://doi.org/10.1007/s12402-016-0208-3
[5]. Hao, W., & Lu, L. (2018). In Jing Shen Bing Xue = psychiatry (9th ed., pp. 125–149). essay, Ren min wei sheng chu ban she.
[6]. Dalsgaard, S., Mortensen, P. B., Frydenberg, M., & Thomsen, P. H. (2013). Long-term criminal outcome of children with attention deficit hyperactivity disorder. Criminal Behaviour and Mental Health, 23(2), 86–98. https://doi.org/10.1002/cbm.1860
[7]. Dalsgaard, S., Østergaard, S. D., Leckman, J. F., Mortensen, P. B., & Pedersen, M. G. (2015). Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: A nationwide cohort study. The Lancet, 385(9983), 2190–2196. https://doi.org/10.1016/s0140-6736(14)61684-6
[8]. Wilens, T. E. (2006). Attention deficit hyperactivity disorder and substance use disorders. American Journal of Psychiatry, 163(12), 2059–2063. https://doi.org/10.1176/ajp.2006.163.12.2059
[9]. Dalsgaard, S., Mortensen, P. B., Frydenberg, M., & Thomsen, P. H. (2014). ADHD, stimulant treatment in childhood and subsequent substance abuse in adulthood — a naturalistic long-term follow-up study. Addictive Behaviors, 39(1), 325–328. https://doi.org/10.1016/j.addbeh.2013.09.002
[10]. Molina, B. S. G., Howard, A. L., Swanson, J. M., Stehli, A., Mitchell, J. T., Kennedy, T. M., Epstein, J. N., Arnold, L. E., Hechtman, L., Vitiello, B., & Hoza, B. (2018). Substance use through adolescence into early adulthood after childhood-diagnosed ADHD: Findings from the MTA Longitudinal Study. Journal of Child Psychology and Psychiatry, 59(6), 692–702. https://doi.org/10.1111/jcpp.12855
[11]. Wimberley, T., Agerbo, E., Horsdal, H. T., Ottosen, C., Brikell, I., Als, T. D., Demontis, D., Børglum, A. D., Nordentoft, M., Mors, O., Werge, T., Hougaard, D., Bybjerg‐Grauholm, J., Hansen, M. B., Mortensen, P. B., Thapar, A., Riglin, L., Langley, K., & Dalsgaard, S. (2020). Genetic liability to ADHD and substance use disorders in individuals with ADHD. Addiction, 115(7), 1368–1377. https://doi.org/10.1111/add.14910
[12]. Huntley, Z., & Young, S. (2012). Alcohol and substance use history among ADHD adults. Journal of Attention Disorders, 18(1), 82–90. https://doi.org/10.1177/1087054712446171
[13]. Faraone, S. V., Wilens, T. E., Petty, C., Antshel, K., Spencer, T., & Biederman, J. (2007). Substance use among ADHD adults: Implications of late onset and subthreshold diagnoses. American Journal on Addictions, 16(s1), 24–34. https://doi.org/10.1080/10550490601082767
[14]. Griswold, M. G., Fullman, N., Hawley, C., Arian, N., Zimsen, S. R., Tymeson, H. D., Venkateswaran, V., Tapp, A. D., Forouzanfar, M. H., Salama, J. S., Abate, K. H., Abate, D., Abay, S. M., Abbafati, C., Abdulkader, R. S., Abebe, Z., Aboyans, V., Abrar, M. M., Acharya, P., … Gakidou, E. (2018). Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the global burden of disease study 2016. The Lancet, 392(10152), 1015–1035. https://doi.org/10.1016/s0140-6736(18)31310-2
[15]. American Psychiatric Association. (2022). Diagnostic and statistical manual of mental disorders: DSM-5-TR. American Psychiatric Association Publishing.
[16]. Ivanov, I., Newcorn, J., Morton, K., & Tricamo, M. (2011). Inhibitory control deficits in childhood: Definition, measurement, and clinical risk for Substance Use Disorders. Inhibitory Control and Drug Abuse Prevention, 125–144. https://doi.org/10.1007/978-1-4419-1268-8_7
[17]. Moeller, F. G., Barratt, E. S., Dougherty, D. M., Schmitz, J. M., & Swann, A. C. (2001). Psychiatric aspects of impulsivity. American Journal of Psychiatry, 158(11), 1783–1793. https://doi.org/10.1176/appi.ajp.158.11.1783
[18]. McClernon, F. J., & Kollins, S. H. (2008). ADHD and smoking. Annals of the New York Academy of Sciences, 1141(1), 131–147. https://doi.org/10.1196/annals.1441.016
[19]. Shirley, M. C., & Sirocco, K. Y. (2014). Introduction to special section: ADHD, impulsivity, and alcohol abuse. Experimental and Clinical Psychopharmacology, 22(2), 97–99. https://doi.org/10.1037/a0036124
[20]. Elkins, I. J., McGue, M., & Iacono, W. G. (2007). Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Archives of General Psychiatry, 64(10), 1145. https://doi.org/10.1001/archpsyc.64.10.1145
[21]. Lee, S. S., Humphreys, K. L., Flory, K., Liu, R., & Glass, K. (2011). Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: A meta-analytic review. Clinical Psychology Review, 31(3), 328–341. https://doi.org/10.1016/j.cpr.2011.01.006
[22]. Nutt, D. J., Lingford-Hughes, A., Erritzoe, D., & Stokes, P. R. (2015). The dopamine theory of addiction: 40 years of highs and lows. Nature Reviews Neuroscience, 16(5), 305–312. https://doi.org/10.1038/nrn3939
[23]. Boileau, I., Assaad, J.-M., Pihl, R. O., Benkelfat, C., Leyton, M., Diksic, M., Tremblay, R. E., & Dagher, A. (2003). Alcohol promotes dopamine release in the human nucleus accumbens. Synapse, 49(4), 226–231. https://doi.org/10.1002/syn.10226
[24]. Urban, N. B. L., Kegeles, L. S., Slifstein, M., Xu, X., Martinez, D., Sakr, E., Castillo, F., Moadel, T., O’Malley, S. S., Krystal, J. H., & Abi-Dargham, A. (2010). Sex differences in striatal dopamine release in young adults after oral alcohol challenge: A Positron emission tomography imaging study with [11C]Raclopride. Biological Psychiatry, 68(8), 689–696. https://doi.org/10.1016/j.biopsych.2010.06.005
[25]. Biederman, J., Faraone, S. V. i, Spencer, T. J., Milberger, S., Mick, E., & Wilens, T. (1995). Psychoactive substance use disorders in adults with attention deficit hyperactivity disorder (ADHD): Effects of ADHD and psychiatric comorbidity. American Journal of Psychiatry, 152(11), 1652–1658. https://doi.org/10.1176/ajp.152.11.1652
[26]. Disney, E. R., Elkins, I. J., McGue, M., & Iacono, W. G. (1999). Effects of ADHD, conduct disorder, and gender on substance use and abuse in adolescence. American Journal of Psychiatry, 156(10), 1515–1521. https://doi.org/10.1176/ajp.156.10.1515
[27]. Mochrie, K. D., Whited, M. C., Cellucci, T., Freeman, T., & Corson, A. T. (2018). ADHD, depression, and substance abuse risk among beginning college students. Journal of American College Health, 68(1), 6–10. https://doi.org/10.1080/07448481.2018.1515754
[28]. Berridge, K. C., & Robinson, T. E. (2003). Parsing reward. Trends in Neurosciences, 26(9), 507–513. https://doi.org/10.1016/s0166-2236(03)00233-9
[29]. Lee, S. S., & Humphreys, K. L. (2014). Interactive association of dopamine receptor (DRD4) genotype and ADHD on alcohol expectancies in children. Experimental and Clinical Psychopharmacology, 22(2), 100–109. https://doi.org/10.1037/a0035338
[30]. Clark, D. B., de Wit, H., & Iacono, W. G. (2014). ADHD, impulsivity and alcohol abuse: Methods, results, and implications. Experimental and Clinical Psychopharmacology, 22(2), 141–143. https://doi.org/10.1037/a0036140
[31]. Comings, D. E., Gonzalez, N., Wu, S., Gade, R., Muhleman, D., Saucier, G., Johnson, P., Verde, R., Rosenthal, R. J., Lesieur, H. R., Rugle, L. J., Miller, W. B., & MacMurray, J. P. (1999). Studies of the 48 BP repeat polymorphism of the DRD4 gene in impulsive, compulsive, addictive behaviors: Tourette syndrome, ADHD, pathological gambling, and substance abuse. American Journal of Medical Genetics, 88(4), 358–368. https://doi.org/10.1002/(sici)1096-8628(19990820)88:4<358::aid-ajmg13>3.0.co;2-g
[32]. Grady, D. L., Chi, H.-C., Ding, Y.-C., Smith, M., Wang, E., Schuck, S., Flodman, P., Spence, M. A., Swanson, J. M., & Moyzis, R. K. (2003). High prevalence of rare dopamine receptor D4 alleles in children diagnosed with attention-deficit hyperactivity disorder. Molecular Psychiatry, 8(5), 536–545. https://doi.org/10.1038/sj.mp.4001350
[33]. Szobot, C. M., Roman, T., Hutz, M. H., Genro, J. P., Shih, M. C., Hoexter, M. Q., Júnior, N., Pechansky, F., Bressan, R. A., & Rohde, L. A. P. (2010). Molecular imaging genetics of methylphenidate response in ADHD and substance use comorbidity. Synapse, 65(2), 154–159. https://doi.org/10.1002/syn.20829
[34]. Bauer, J., Werner, A., Kohl, W., Kugel, H., Shushakova, A., Pedersen, A., & Ohrmann, P. (2016). Hyperactivity and impulsivity in adult attention-deficit/hyperactivity disorder is related to glutamatergic dysfunction in the anterior cingulate cortex. The World Journal of Biological Psychiatry, 19(7), 538–546. https://doi.org/10.1080/15622975.2016.1262060
[35]. Huang, X., Wang, M., Zhang, Q., Chen, X., & Wu, J. (2019). The role of glutamate receptors in attention‐deficit/hyperactivity disorder: From physiology to disease. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 180(4), 272–286. https://doi.org/10.1002/ajmg.b.32726
[36]. Luderer, M., Ramos Quiroga, J. A., Faraone, S. V., Zhang-James, Y., & Reif, A. (2021). Alcohol use disorders and ADHD. Neuroscience & Biobehavioral Reviews, 128, 648–660. https://doi.org/10.1016/j.neubiorev.2021.07.010
[37]. Tajbakhsh, A., Alimardani, M., Asghari, M., Abedini, S., Saghafi Khadem, S., Nesaei Bajestani, A., Alipoor, F., Alidoust, M., Savardashtaki, A., Hashemian, P., & Pasdar, A. (2021). Association of PICK1 and BDNF variations with increased risk of methamphetamine dependence among Iranian population: A case–control study. BMC Medical Genomics, 14(1). https://doi.org/10.1186/s12920-021-00873-7
[38]. Kim, J. I., Kim, J.-W., Park, J.-E., Park, S., Hong, S.-B., Han, D. H., Cheong, J. H., Choi, J.-W., Lee, S., & Kim, B.-N. (2016). Association of the grin2b RS2284411 polymorphism with methylphenidate response in attention-deficit/hyperactivity disorder. Journal of Psychopharmacology, 31(8), 1070–1077. https://doi.org/10.1177/0269881116667707
[39]. Sollis E, Mosaku A, Abid A, Buniello A, Cerezo M, Gil L, Groza T, Güneş O, Hall P, Hayhurst J, Ibrahim A, Ji Y, John S, Lewis E, MacArthur JAL, McMahon A, Osumi-Sutherland D, Panoutsopoulou K, Pendlington Z, Ramachandran S, Stefancsik R, Stewart J, Whetzel P, Wilson R, Hindorff L, Cunningham F, Lambert SA, Inouye M, Parkinson H, Harris LW. The NHGRI-EBI GWAS Catalog: knowledgebase and deposition resource. Nucleic Acids Res. 2022 Nov 9:gkac1010. doi: 10.1093/nar/gkac1010. Epub ahead of print. PMID: 36350656.
[40]. Sullivan, P. F., Agrawal, A., Bulik, C. M., Andreassen, O. A., Børglum, A. D., Breen, G., Cichon, S., Edenberg, H. J., Faraone, S. V., Gelernter, J., Mathews, C. A., Nievergelt, C. M., Smoller, J. W., O’Donovan, M. C., & Psychiatric Genomics Consortium (2018). Psychiatric Genomics: An Update and an Agenda. The American journal of psychiatry, 175(1), 15–27. https://doi.org/10.1176/appi.ajp.2017.17030283
[41]. Sanchez-Roige, S., Palmer, A. A., Fontanillas, P., Elson, S. L., 23andMe Research Team, the Substance Use Disorder Working Group of the Psychiatric Genomics Consortium, Adams, M. J., Howard, D. M., Edenberg, H. J., Davies, G., Crist, R. C., Deary, I. J., McIntosh, A. M., & Clarke, T. K. (2019). Genome-Wide Association Study Meta-Analysis of the Alcohol Use Disorders Identification Test (AUDIT) in Two Population-Based Cohorts. The American journal of psychiatry, 176(2), 107–118. https://doi.org/10.1176/appi.ajp.2018.18040369
[42]. Johnson, E. C., Demontis, D., Thorgeirsson, T. E., Walters, R. K., Polimanti, R., Hatoum, A. S., Sanchez-Roige, S., Paul, S. E., Wendt, F. R., Clarke, T. K., Lai, D., Reginsson, G. W., Zhou, H., He, J., Baranger, D. A. A., Gudbjartsson, D. F., Wedow, R., Adkins, D. E., Adkins, A. E., Alexander, J., … Agrawal, A. (2020). A large-scale genome-wide association study meta-analysis of cannabis use disorder. The lancet. Psychiatry, 7(12), 1032–1045. https://doi.org/10.1016/S2215-0366(20)30339-4
[43]. Purcell, S., Neale, B., Todd-Brown, K., Thomas, L., Ferreira, M. A., Bender, D., Maller, J., Sklar, P., de Bakker, P. I., Daly, M. J., & Sham, P. C. (2007). PLINK: a tool set for whole-genome association and population-based linkage analyses. American journal of human genetics, 81(3), 559–575. https://doi.org/10.1086/519795
[44]. R Core Team (2023). _R: A Language and Environment for Statistical Computing_. R Foundation for Statistical Computing, Vienna, Austria. <https://www.R-project.org/>.
[45]. Wickham H, François R, Henry L, Müller K, Vaughan D (2023). _dplyr: A Grammar of Data Manipulation_. R package version 1.1.2, <https://CRAN.R-project.org/package=dplyr>.
[46]. Stephen D. Turner (2018). qqman: an R package for visualizing GWAS results using Q-Q and manhattan plots. Journal of Open Source Software, 3(25), 731 doi:10.21105/joss.00731
[47]. Sherry, S. T., Ward, M. H., Kholodov, M., Baker, J., Phan, L., Smigielski, E. M., & Sirotkin, K. (2001). dbSNP: the NCBI database of genetic variation. Nucleic acids research, 29(1), 308–311. https://doi.org/10.1093/nar/29.1.308
[48]. Wilson, J. J., & Levin, F. R. (2001). Attention deficit hyperactivity disorder (ADHD) and Substance Use Disorders. Current Psychiatry Reports, 3(6), 497–506. https://doi.org/10.1007/s11920-001-0044-8
[49]. Fan, Z., Qian, Y., Lu, Q., Wang, Y., Chang, S., & Yang, L. (2018). DLGAP1 and NMDA receptor-associated postsynaptic density protein genes influence executive function in attention deficit hyperactivity disorder. Brain and Behavior, 8(2). https://doi.org/10.1002/brb3.914
[50]. Clark, S. L., Chan, R. F., Zhao, M., Xie, L. Y., Copeland, W. E., Penninx, B. W., Aberg, K. A., & van den Oord, E. J. (2021). Dual methylation and hydroxymethylation study of alcohol use disorder. Addiction Biology, 27(2). https://doi.org/10.1111/adb.13114
[51]. Zhang, Z., Wei, S., Du, H., Su, Z., Wen, Y., Shang, Z., Song, X., Xu, Z., You, Y., & Yang, Z. (2019). ZFHX3 is required for the differentiation of late born D1-type medium spiny neurons. Experimental Neurology, 322, 113055. https://doi.org/10.1016/j.expneurol.2019.113055
[52]. Becchi, S., Buson, A., & Balleine, B. W. (2021). Inhibition of vascular adhesion protein 1 protects dopamine neurons from the effects of acute inflammation and restores habit learning in the striatum. Journal of Neuroinflammation, 18(1). https://doi.org/10.1186/s12974-021-02288-8
[53]. Zhang, M., Davis, J. E., Li, C., Gao, J., Huang, W., Lambert, N. A., Terry, A. V., & Wu, G. (2016). GGA3 interacts with a G protein-coupled receptor and modulates its cell surface export. Molecular and Cellular Biology, 36(7), 1152–1163. https://doi.org/10.1128/mcb.00009-16
[54]. Zhu, S., Zhao, C., Wu, Y., Yang, Q., Shao, A., Wang, T., Wu, J., Yin, Y., Li, Y., Hou, J., Zhang, X., Zhou, G., Gu, X., Wang, X., Bustelo, X. R., & Zhou, J. (2015). Identification of a VAV2-dependent mechanism for GDNF/ret control of Mesolimbic Dat Trafficking. Nature Neuroscience, 18(8), 1084–1093. https://doi.org/10.1038/nn.4060
[55]. Brosius, J. (2005). Waste not, want not – transcript excess in multicellular eukaryotes. Trends in Genetics, 21(5), 287–288. https://doi.org/10.1016/j.tig.2005.02.014
[56]. Badano, J. L., Ansley, S. J., Leitch, C. C., Lewis, R. A., Lupski, J. R., & Katsanis, N. (2003). Identification of a novel Bardet-Biedl syndrome protein, BBS7, that shares structural features with BBS1 and BBS2. The American Journal of Human Genetics, 72(3), 650–658. https://doi.org/10.1086/368204
[57]. Pagano, M., Pepperkok, R., Verde, F., Ansorge, W., & Draetta, G. (1992). Cyclin A is required at two points in the human cell cycle. The EMBO Journal, 11(3), 961–971. https://doi.org/10.1002/j.1460-2075.1992.tb05135.x
[58]. Yoo, K.-S., Lee, K., Lee, Y.-S., Oh, W.-J., & Kim, H. K. (2020). Rho guanine nucleotide exchange factor 4 (ARHGEF4) deficiency enhances spatial and object recognition memory. Experimental Neurobiology, 29(5), 334–343. https://doi.org/10.5607/en20049
Cite this article
Gao,Y.;Sang,C.;Shen,G.;Xu,Y. (2024). Discovery of genes prominent in ADHD and SUDs: A meta-analysis and review. Theoretical and Natural Science,45,102-112.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Maxwell, A. (2011). Are some individuals diagnosed with ADHD prone to alcohol abuse? Journal of Attention Disorders, 17(2), 98–101. https://doi.org/10.1177/1087054711427400
[2]. Thomas, R., Sanders, S., Doust, J., Beller, E., & Glasziou, P. (2015). Prevalence of attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Pediatrics, 135(4). https://doi.org/10.1542/peds.2014-3482
[3]. Biederman, Joseph. (2005). Attention-deficit/hyperactivity disorder: A selective overview. Biological Psychiatry, 57(11), 1215–1220. https://doi.org/10.1016/j.biopsych.2004.10.020
[4]. Fayyad, J., Sampson, N. A., Hwang, I., Adamowski, T., Aguilar-Gaxiola, S., Al-Hamzawi, A., Andrade, L. H., Borges, G., de Girolamo, G., Florescu, S., Gureje, O., Haro, J. M., Hu, C., Karam, E. G., Lee, S., Navarro-Mateu, F., O’Neill, S., Pennell, B.-E., Piazza, M., … Kessler, R. C. (2016). The descriptive epidemiology of DSM-IV ADULT ADHD in the World Health Organization World Mental Health Surveys. ADHD Attention Deficit and Hyperactivity Disorders, 9(1), 47–65. https://doi.org/10.1007/s12402-016-0208-3
[5]. Hao, W., & Lu, L. (2018). In Jing Shen Bing Xue = psychiatry (9th ed., pp. 125–149). essay, Ren min wei sheng chu ban she.
[6]. Dalsgaard, S., Mortensen, P. B., Frydenberg, M., & Thomsen, P. H. (2013). Long-term criminal outcome of children with attention deficit hyperactivity disorder. Criminal Behaviour and Mental Health, 23(2), 86–98. https://doi.org/10.1002/cbm.1860
[7]. Dalsgaard, S., Østergaard, S. D., Leckman, J. F., Mortensen, P. B., & Pedersen, M. G. (2015). Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: A nationwide cohort study. The Lancet, 385(9983), 2190–2196. https://doi.org/10.1016/s0140-6736(14)61684-6
[8]. Wilens, T. E. (2006). Attention deficit hyperactivity disorder and substance use disorders. American Journal of Psychiatry, 163(12), 2059–2063. https://doi.org/10.1176/ajp.2006.163.12.2059
[9]. Dalsgaard, S., Mortensen, P. B., Frydenberg, M., & Thomsen, P. H. (2014). ADHD, stimulant treatment in childhood and subsequent substance abuse in adulthood — a naturalistic long-term follow-up study. Addictive Behaviors, 39(1), 325–328. https://doi.org/10.1016/j.addbeh.2013.09.002
[10]. Molina, B. S. G., Howard, A. L., Swanson, J. M., Stehli, A., Mitchell, J. T., Kennedy, T. M., Epstein, J. N., Arnold, L. E., Hechtman, L., Vitiello, B., & Hoza, B. (2018). Substance use through adolescence into early adulthood after childhood-diagnosed ADHD: Findings from the MTA Longitudinal Study. Journal of Child Psychology and Psychiatry, 59(6), 692–702. https://doi.org/10.1111/jcpp.12855
[11]. Wimberley, T., Agerbo, E., Horsdal, H. T., Ottosen, C., Brikell, I., Als, T. D., Demontis, D., Børglum, A. D., Nordentoft, M., Mors, O., Werge, T., Hougaard, D., Bybjerg‐Grauholm, J., Hansen, M. B., Mortensen, P. B., Thapar, A., Riglin, L., Langley, K., & Dalsgaard, S. (2020). Genetic liability to ADHD and substance use disorders in individuals with ADHD. Addiction, 115(7), 1368–1377. https://doi.org/10.1111/add.14910
[12]. Huntley, Z., & Young, S. (2012). Alcohol and substance use history among ADHD adults. Journal of Attention Disorders, 18(1), 82–90. https://doi.org/10.1177/1087054712446171
[13]. Faraone, S. V., Wilens, T. E., Petty, C., Antshel, K., Spencer, T., & Biederman, J. (2007). Substance use among ADHD adults: Implications of late onset and subthreshold diagnoses. American Journal on Addictions, 16(s1), 24–34. https://doi.org/10.1080/10550490601082767
[14]. Griswold, M. G., Fullman, N., Hawley, C., Arian, N., Zimsen, S. R., Tymeson, H. D., Venkateswaran, V., Tapp, A. D., Forouzanfar, M. H., Salama, J. S., Abate, K. H., Abate, D., Abay, S. M., Abbafati, C., Abdulkader, R. S., Abebe, Z., Aboyans, V., Abrar, M. M., Acharya, P., … Gakidou, E. (2018). Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the global burden of disease study 2016. The Lancet, 392(10152), 1015–1035. https://doi.org/10.1016/s0140-6736(18)31310-2
[15]. American Psychiatric Association. (2022). Diagnostic and statistical manual of mental disorders: DSM-5-TR. American Psychiatric Association Publishing.
[16]. Ivanov, I., Newcorn, J., Morton, K., & Tricamo, M. (2011). Inhibitory control deficits in childhood: Definition, measurement, and clinical risk for Substance Use Disorders. Inhibitory Control and Drug Abuse Prevention, 125–144. https://doi.org/10.1007/978-1-4419-1268-8_7
[17]. Moeller, F. G., Barratt, E. S., Dougherty, D. M., Schmitz, J. M., & Swann, A. C. (2001). Psychiatric aspects of impulsivity. American Journal of Psychiatry, 158(11), 1783–1793. https://doi.org/10.1176/appi.ajp.158.11.1783
[18]. McClernon, F. J., & Kollins, S. H. (2008). ADHD and smoking. Annals of the New York Academy of Sciences, 1141(1), 131–147. https://doi.org/10.1196/annals.1441.016
[19]. Shirley, M. C., & Sirocco, K. Y. (2014). Introduction to special section: ADHD, impulsivity, and alcohol abuse. Experimental and Clinical Psychopharmacology, 22(2), 97–99. https://doi.org/10.1037/a0036124
[20]. Elkins, I. J., McGue, M., & Iacono, W. G. (2007). Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Archives of General Psychiatry, 64(10), 1145. https://doi.org/10.1001/archpsyc.64.10.1145
[21]. Lee, S. S., Humphreys, K. L., Flory, K., Liu, R., & Glass, K. (2011). Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: A meta-analytic review. Clinical Psychology Review, 31(3), 328–341. https://doi.org/10.1016/j.cpr.2011.01.006
[22]. Nutt, D. J., Lingford-Hughes, A., Erritzoe, D., & Stokes, P. R. (2015). The dopamine theory of addiction: 40 years of highs and lows. Nature Reviews Neuroscience, 16(5), 305–312. https://doi.org/10.1038/nrn3939
[23]. Boileau, I., Assaad, J.-M., Pihl, R. O., Benkelfat, C., Leyton, M., Diksic, M., Tremblay, R. E., & Dagher, A. (2003). Alcohol promotes dopamine release in the human nucleus accumbens. Synapse, 49(4), 226–231. https://doi.org/10.1002/syn.10226
[24]. Urban, N. B. L., Kegeles, L. S., Slifstein, M., Xu, X., Martinez, D., Sakr, E., Castillo, F., Moadel, T., O’Malley, S. S., Krystal, J. H., & Abi-Dargham, A. (2010). Sex differences in striatal dopamine release in young adults after oral alcohol challenge: A Positron emission tomography imaging study with [11C]Raclopride. Biological Psychiatry, 68(8), 689–696. https://doi.org/10.1016/j.biopsych.2010.06.005
[25]. Biederman, J., Faraone, S. V. i, Spencer, T. J., Milberger, S., Mick, E., & Wilens, T. (1995). Psychoactive substance use disorders in adults with attention deficit hyperactivity disorder (ADHD): Effects of ADHD and psychiatric comorbidity. American Journal of Psychiatry, 152(11), 1652–1658. https://doi.org/10.1176/ajp.152.11.1652
[26]. Disney, E. R., Elkins, I. J., McGue, M., & Iacono, W. G. (1999). Effects of ADHD, conduct disorder, and gender on substance use and abuse in adolescence. American Journal of Psychiatry, 156(10), 1515–1521. https://doi.org/10.1176/ajp.156.10.1515
[27]. Mochrie, K. D., Whited, M. C., Cellucci, T., Freeman, T., & Corson, A. T. (2018). ADHD, depression, and substance abuse risk among beginning college students. Journal of American College Health, 68(1), 6–10. https://doi.org/10.1080/07448481.2018.1515754
[28]. Berridge, K. C., & Robinson, T. E. (2003). Parsing reward. Trends in Neurosciences, 26(9), 507–513. https://doi.org/10.1016/s0166-2236(03)00233-9
[29]. Lee, S. S., & Humphreys, K. L. (2014). Interactive association of dopamine receptor (DRD4) genotype and ADHD on alcohol expectancies in children. Experimental and Clinical Psychopharmacology, 22(2), 100–109. https://doi.org/10.1037/a0035338
[30]. Clark, D. B., de Wit, H., & Iacono, W. G. (2014). ADHD, impulsivity and alcohol abuse: Methods, results, and implications. Experimental and Clinical Psychopharmacology, 22(2), 141–143. https://doi.org/10.1037/a0036140
[31]. Comings, D. E., Gonzalez, N., Wu, S., Gade, R., Muhleman, D., Saucier, G., Johnson, P., Verde, R., Rosenthal, R. J., Lesieur, H. R., Rugle, L. J., Miller, W. B., & MacMurray, J. P. (1999). Studies of the 48 BP repeat polymorphism of the DRD4 gene in impulsive, compulsive, addictive behaviors: Tourette syndrome, ADHD, pathological gambling, and substance abuse. American Journal of Medical Genetics, 88(4), 358–368. https://doi.org/10.1002/(sici)1096-8628(19990820)88:4<358::aid-ajmg13>3.0.co;2-g
[32]. Grady, D. L., Chi, H.-C., Ding, Y.-C., Smith, M., Wang, E., Schuck, S., Flodman, P., Spence, M. A., Swanson, J. M., & Moyzis, R. K. (2003). High prevalence of rare dopamine receptor D4 alleles in children diagnosed with attention-deficit hyperactivity disorder. Molecular Psychiatry, 8(5), 536–545. https://doi.org/10.1038/sj.mp.4001350
[33]. Szobot, C. M., Roman, T., Hutz, M. H., Genro, J. P., Shih, M. C., Hoexter, M. Q., Júnior, N., Pechansky, F., Bressan, R. A., & Rohde, L. A. P. (2010). Molecular imaging genetics of methylphenidate response in ADHD and substance use comorbidity. Synapse, 65(2), 154–159. https://doi.org/10.1002/syn.20829
[34]. Bauer, J., Werner, A., Kohl, W., Kugel, H., Shushakova, A., Pedersen, A., & Ohrmann, P. (2016). Hyperactivity and impulsivity in adult attention-deficit/hyperactivity disorder is related to glutamatergic dysfunction in the anterior cingulate cortex. The World Journal of Biological Psychiatry, 19(7), 538–546. https://doi.org/10.1080/15622975.2016.1262060
[35]. Huang, X., Wang, M., Zhang, Q., Chen, X., & Wu, J. (2019). The role of glutamate receptors in attention‐deficit/hyperactivity disorder: From physiology to disease. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 180(4), 272–286. https://doi.org/10.1002/ajmg.b.32726
[36]. Luderer, M., Ramos Quiroga, J. A., Faraone, S. V., Zhang-James, Y., & Reif, A. (2021). Alcohol use disorders and ADHD. Neuroscience & Biobehavioral Reviews, 128, 648–660. https://doi.org/10.1016/j.neubiorev.2021.07.010
[37]. Tajbakhsh, A., Alimardani, M., Asghari, M., Abedini, S., Saghafi Khadem, S., Nesaei Bajestani, A., Alipoor, F., Alidoust, M., Savardashtaki, A., Hashemian, P., & Pasdar, A. (2021). Association of PICK1 and BDNF variations with increased risk of methamphetamine dependence among Iranian population: A case–control study. BMC Medical Genomics, 14(1). https://doi.org/10.1186/s12920-021-00873-7
[38]. Kim, J. I., Kim, J.-W., Park, J.-E., Park, S., Hong, S.-B., Han, D. H., Cheong, J. H., Choi, J.-W., Lee, S., & Kim, B.-N. (2016). Association of the grin2b RS2284411 polymorphism with methylphenidate response in attention-deficit/hyperactivity disorder. Journal of Psychopharmacology, 31(8), 1070–1077. https://doi.org/10.1177/0269881116667707
[39]. Sollis E, Mosaku A, Abid A, Buniello A, Cerezo M, Gil L, Groza T, Güneş O, Hall P, Hayhurst J, Ibrahim A, Ji Y, John S, Lewis E, MacArthur JAL, McMahon A, Osumi-Sutherland D, Panoutsopoulou K, Pendlington Z, Ramachandran S, Stefancsik R, Stewart J, Whetzel P, Wilson R, Hindorff L, Cunningham F, Lambert SA, Inouye M, Parkinson H, Harris LW. The NHGRI-EBI GWAS Catalog: knowledgebase and deposition resource. Nucleic Acids Res. 2022 Nov 9:gkac1010. doi: 10.1093/nar/gkac1010. Epub ahead of print. PMID: 36350656.
[40]. Sullivan, P. F., Agrawal, A., Bulik, C. M., Andreassen, O. A., Børglum, A. D., Breen, G., Cichon, S., Edenberg, H. J., Faraone, S. V., Gelernter, J., Mathews, C. A., Nievergelt, C. M., Smoller, J. W., O’Donovan, M. C., & Psychiatric Genomics Consortium (2018). Psychiatric Genomics: An Update and an Agenda. The American journal of psychiatry, 175(1), 15–27. https://doi.org/10.1176/appi.ajp.2017.17030283
[41]. Sanchez-Roige, S., Palmer, A. A., Fontanillas, P., Elson, S. L., 23andMe Research Team, the Substance Use Disorder Working Group of the Psychiatric Genomics Consortium, Adams, M. J., Howard, D. M., Edenberg, H. J., Davies, G., Crist, R. C., Deary, I. J., McIntosh, A. M., & Clarke, T. K. (2019). Genome-Wide Association Study Meta-Analysis of the Alcohol Use Disorders Identification Test (AUDIT) in Two Population-Based Cohorts. The American journal of psychiatry, 176(2), 107–118. https://doi.org/10.1176/appi.ajp.2018.18040369
[42]. Johnson, E. C., Demontis, D., Thorgeirsson, T. E., Walters, R. K., Polimanti, R., Hatoum, A. S., Sanchez-Roige, S., Paul, S. E., Wendt, F. R., Clarke, T. K., Lai, D., Reginsson, G. W., Zhou, H., He, J., Baranger, D. A. A., Gudbjartsson, D. F., Wedow, R., Adkins, D. E., Adkins, A. E., Alexander, J., … Agrawal, A. (2020). A large-scale genome-wide association study meta-analysis of cannabis use disorder. The lancet. Psychiatry, 7(12), 1032–1045. https://doi.org/10.1016/S2215-0366(20)30339-4
[43]. Purcell, S., Neale, B., Todd-Brown, K., Thomas, L., Ferreira, M. A., Bender, D., Maller, J., Sklar, P., de Bakker, P. I., Daly, M. J., & Sham, P. C. (2007). PLINK: a tool set for whole-genome association and population-based linkage analyses. American journal of human genetics, 81(3), 559–575. https://doi.org/10.1086/519795
[44]. R Core Team (2023). _R: A Language and Environment for Statistical Computing_. R Foundation for Statistical Computing, Vienna, Austria. <https://www.R-project.org/>.
[45]. Wickham H, François R, Henry L, Müller K, Vaughan D (2023). _dplyr: A Grammar of Data Manipulation_. R package version 1.1.2, <https://CRAN.R-project.org/package=dplyr>.
[46]. Stephen D. Turner (2018). qqman: an R package for visualizing GWAS results using Q-Q and manhattan plots. Journal of Open Source Software, 3(25), 731 doi:10.21105/joss.00731
[47]. Sherry, S. T., Ward, M. H., Kholodov, M., Baker, J., Phan, L., Smigielski, E. M., & Sirotkin, K. (2001). dbSNP: the NCBI database of genetic variation. Nucleic acids research, 29(1), 308–311. https://doi.org/10.1093/nar/29.1.308
[48]. Wilson, J. J., & Levin, F. R. (2001). Attention deficit hyperactivity disorder (ADHD) and Substance Use Disorders. Current Psychiatry Reports, 3(6), 497–506. https://doi.org/10.1007/s11920-001-0044-8
[49]. Fan, Z., Qian, Y., Lu, Q., Wang, Y., Chang, S., & Yang, L. (2018). DLGAP1 and NMDA receptor-associated postsynaptic density protein genes influence executive function in attention deficit hyperactivity disorder. Brain and Behavior, 8(2). https://doi.org/10.1002/brb3.914
[50]. Clark, S. L., Chan, R. F., Zhao, M., Xie, L. Y., Copeland, W. E., Penninx, B. W., Aberg, K. A., & van den Oord, E. J. (2021). Dual methylation and hydroxymethylation study of alcohol use disorder. Addiction Biology, 27(2). https://doi.org/10.1111/adb.13114
[51]. Zhang, Z., Wei, S., Du, H., Su, Z., Wen, Y., Shang, Z., Song, X., Xu, Z., You, Y., & Yang, Z. (2019). ZFHX3 is required for the differentiation of late born D1-type medium spiny neurons. Experimental Neurology, 322, 113055. https://doi.org/10.1016/j.expneurol.2019.113055
[52]. Becchi, S., Buson, A., & Balleine, B. W. (2021). Inhibition of vascular adhesion protein 1 protects dopamine neurons from the effects of acute inflammation and restores habit learning in the striatum. Journal of Neuroinflammation, 18(1). https://doi.org/10.1186/s12974-021-02288-8
[53]. Zhang, M., Davis, J. E., Li, C., Gao, J., Huang, W., Lambert, N. A., Terry, A. V., & Wu, G. (2016). GGA3 interacts with a G protein-coupled receptor and modulates its cell surface export. Molecular and Cellular Biology, 36(7), 1152–1163. https://doi.org/10.1128/mcb.00009-16
[54]. Zhu, S., Zhao, C., Wu, Y., Yang, Q., Shao, A., Wang, T., Wu, J., Yin, Y., Li, Y., Hou, J., Zhang, X., Zhou, G., Gu, X., Wang, X., Bustelo, X. R., & Zhou, J. (2015). Identification of a VAV2-dependent mechanism for GDNF/ret control of Mesolimbic Dat Trafficking. Nature Neuroscience, 18(8), 1084–1093. https://doi.org/10.1038/nn.4060
[55]. Brosius, J. (2005). Waste not, want not – transcript excess in multicellular eukaryotes. Trends in Genetics, 21(5), 287–288. https://doi.org/10.1016/j.tig.2005.02.014
[56]. Badano, J. L., Ansley, S. J., Leitch, C. C., Lewis, R. A., Lupski, J. R., & Katsanis, N. (2003). Identification of a novel Bardet-Biedl syndrome protein, BBS7, that shares structural features with BBS1 and BBS2. The American Journal of Human Genetics, 72(3), 650–658. https://doi.org/10.1086/368204
[57]. Pagano, M., Pepperkok, R., Verde, F., Ansorge, W., & Draetta, G. (1992). Cyclin A is required at two points in the human cell cycle. The EMBO Journal, 11(3), 961–971. https://doi.org/10.1002/j.1460-2075.1992.tb05135.x
[58]. Yoo, K.-S., Lee, K., Lee, Y.-S., Oh, W.-J., & Kim, H. K. (2020). Rho guanine nucleotide exchange factor 4 (ARHGEF4) deficiency enhances spatial and object recognition memory. Experimental Neurobiology, 29(5), 334–343. https://doi.org/10.5607/en20049









