1. Introduction
Diabetes mellitus (DM) is a chronic condition. It affects how the body processes blood sugar. The pathogenesis of diabetes is genetics and environmental factors that predispose individuals to Type 1 and Type 2 diabetes. Specifically, insulin resistance produces Type 2 diabetes, and the autoimmune process also causes Type 1 diabetes. The disease known as heart failure occurs when the heart is unable to adequately pump blood to fulfill the demands of the body. The pathogenesis of the heart failure is molecular mechanisms and genetics. In clinical diagnosis, diabetes and heart failure often coexist. Understanding how diabetes causes the development and progress of heart failure is the focus of recent research. The findings indicate that those who are obese, have insulin resistance, and have hyperinsulinemia are more likely to develop diabetic cardiomyopathy and heart failure [1]. Helena C. Kenny et al. investigate the impact of glucose-lowering agents in Type 2 diabetes to cause heart failure in 2020. Their study concluded that different anti-hyperglycemic agents may have different effects on heart failure, with some of the agents potentially reducing the risk of heart failure [2]. But few studies have summarized all the major mechanisms and treatment options. So this paper is going to investigate the pathogenesis and treatment of the diabetes patients with heart failure with the literature research method. Through this study, more people can understand the relationship between these two diseases, and it can also provide more prevention and treatment recommendations.
2. Epidemiological status of diabetic heart failure
DM is mainly divided into Type 1 diabetes mellitus (T1DM) and Type 2 diabetes mellitus(T2DM). T1DM is related to genetic, environmental, autoimmune and other factors, leading to the destruction of pancreatic beta-cells and functional failure. T2DM is caused by insulin deficiency and insulin resistance due to genetic, environmental, and defective function of islet betacells. From these two types of diabetes, different mechanisms affect the role of betacells to lead to diabetes and then to the clinical manifestation of hyperglycemia. When hyperglycemia occurs, all the forms of diabetes are at risk of developing the same complications such as microvascular and macrovascular complications [3].
A chronic medical illness known as heart failure occurs when the heart cannot pump blood effectively enough to meet the body's needs. This can result from the heart being too weak or too stiff to fill and pump blood effectively. Heart failure patients might have mid-range ejection fraction (HFmrEF), preserved ejection fraction (HFpEF), or low or reduced ejection fraction (HFrEF). In HFrEF, the heart muscle is weak and cannot pump blood out to the body as effectively, a condition commonly referred to as systolic heart failure. While in HFpEF, the heart muscle is stiff and cannot relax properly, leading to inadequate filling of the heart; this condition is often termed diastolic heart failure. Clinically, HFpEF patients are frequently characterized by advanced age, female gender, obesity, and a history of hypertension and/or atrial fibrillation [4].
The global prevalence of diabetes in adults reached 537 million (10.5%) in 2021, and the International Diabetes Federation (IDF) indicated an estimated increase to 783 million by 2045. Research indicates that patients with diabetes face more than double the risk of developing heart failure compared to those without diabetes [5]. Additionally, recent estimates suggest that over 64 million individuals worldwide are living with heart failure, with approximately 30% to 40% of these patients also having comorbid diabetes.
3. The effects of diabetes on the heart
Whether diabetes is Type 1 or Type 2, the most typical sign is elevated blood sugar. Elevated blood glucose levels have the potential to harm blood vessels, resulting in the accumulation of plaque that may lead to atherosclerosis. Heart attacks are more likely as a result of the arteries becoming narrower and the heart receiving less blood flow. Additionally, diabetes-related cardiomyopathy can be caused by elevated blood sugar levels directly harming heart muscle cells. Heart failure may ensue from this disorder, which impairs the heart's capacity to pump blood efficiently [6]. Peripheral arterial disease (PAD), a condition in which the arteries in the legs and other body parts constrict or obstruct, is another complication of diabetes. Because of the decreased blood flow, this raises the risk of heart attacks and strokes.
Diabetes is linked to oxidative stress and persistent low-grade inflammation, both of which promote the onset and advancement of heart disease. Clinically speaking, diabetes and high blood pressure frequently coexist, adding to the stress on the heart and hastening the onset of heart disease. Diabetes can lead to autonomic neuropathy, a disorder of the nervous system characterized by damage to the neurons that regulate the heart and blood vessels. The risk of cardiovascular events may increase as a result of improper blood pressure and heart rate regulation.
With the passage of time, the repertoire of potential mechanisms contributing to diabetes mellitus-induced cardiomyopathy continues to expand, as shown in Figure 1. Established mediators that have been identified for numerous years encompass nearly all the cardiac effects attributed to diabetes [7].
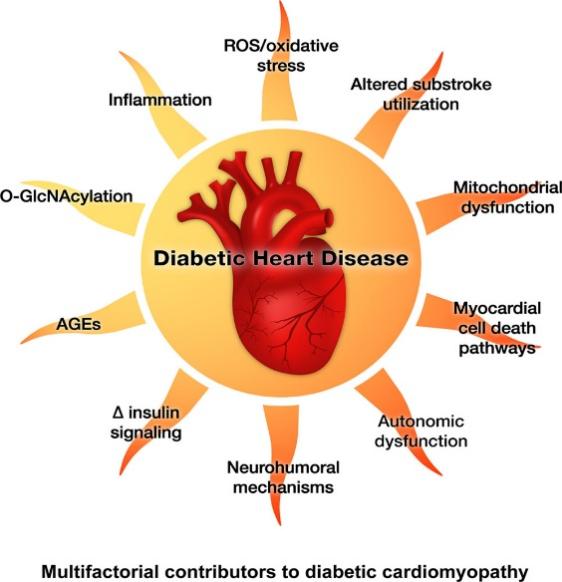
Figure 1. Mediators of myocardial remodeling and dysfunction caused by diabetes [7].
4. The mechanism of diabetic heart failure
4.1. Insulin resistance
In a normal state, the heart gets its energy from both glucose and fatty acids. Fatty acid translocase (FAT) and cluster of differentiation 36 (CD36) aid in the uptake of fatty acids, whereas glucose transporter 4 (GLUT4) mediates insulin-stimulated glucose transfer. After ingestion of nutrients, plasma insulin levels and myocardial insulin signaling increase, allowing GLUT4 and CD36 to be translocated to the muscle membrane of muscle cells to supply myocardial energy. However, in the context of insulin resistance, CD36 exhibits preferential localization to the sarcolemma, while GLUT4 undergoes internalization and returns to its intracellular location. Therefore, the impaired glucose uptake due to systemic and cardiac insulin resistance promotes a shift in substrate utilization towards enhanced free fatty acid oxidation in diabetes mellitus, leading to diminished cardiac efficiency [8]. The animal research revealed that the Zucker diabetic fatty (ZDF) rat exhibits obesity with DM and insulin resistance. Because of the insulin resistance, the ZDF rats rapidly progress to T2DM [9].
4.2. Myocardial energy metabolism abnormalities
Under physiological conditions, fatty acids are the main source of energy. It accounts for about 70% of the ATP produced by the functional heart. The remaining 30 and the remaining energy come from glucose, ketones, and branched chain amino acids (BCAAs) [10]. The heart's conversion of the chemical energy stored in fatty acids and glucose into the mechanical energy of the actin-myosin interaction in the myofibrils is the primary source of energy for the myocardium. In diabetic hearts, the utilization of glucose is restricted. To get enough energy, the uptake of the fatty acid is increased. Additionally, enhanced utilization of ketones as a source of energy is also evident in the cardiac tissue of individuals with diabetes.
4.3. Inflammatory response
First of all, the high blood sugar state of diabetic patients is conducive to the growth and reproduction of bacteria and fungi, and high blood sugar will inhibit the phagocytosis of white blood cells, thus reducing the anti-infection ability. Second, metabolic disorders in diabetic patients lead to low immune function and defective defense function. Third, diabetic patients are prone to vascular lesions, causing blood flow disorders, resulting in reduced antibody distribution, and affecting the phagocytosis function of white blood cells. Finally, diminished blood supply to the skin and mucous membranes makes diabetic patients more susceptible to ischemic lesions, providing conditions for microbial invasion. These are some of the main mechanisms by which diabetes causes inflammation. In the early stages of atherosclerosis development, monocytes adhere to the endothelial surface and subsequently migrate into the arterial wall, where they can differentiate into foam cells that accumulate around endothelial lesions. Interleukin-β (IL-β) then plays a role in modulating chemotaxis and adhesion of monocytes. Macrophages and IL-1βplay an important role in how inflammation leads to cardiovascular disease [11].
4.4. Oxidative stress
Reactive oxygen species (ROS) are created in excess by mitochondria during glycolysis when there is hyperglycemia. Oxidative stress (OS) is brought on by the buildup of ROS and damages essential cellular constituents such as proteins, DNA, and lipids [12]. In diabetic hearts, ROS enhances prosurvival signaling pathways including Akt and triggers proinflammatory and cell death pathways. As a result, these pathways control the expression of molecules involved in cell adhesion, proinflammatory cytokines, and inducible nitric oxide synthases (NOS). Scavenging enzymes like glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) can counteract the harmful effects of reactive oxygen species (ROS) in physiological settings. Nonetheless, greater concentrations of enzymes like GSH-Px are needed for the clearance of elevated ROS levels. It has been shown that in the diabetic heart, overexpression of GSH-Px reduces diastolic dysfunction, myocyte hypertrophy, and interstitial fibrosis [13].
4.5. Lipotoxicity to the Myocardium
Lipotoxicity refers to the deposition of excess fat in organs where it should not normally accumulate, leading to toxicity and organ damage, and contributing to serious lipid metabolic disorders [14]. In the case of insufficient insulin secretion or insulin resistance, the body may secrete more insulin in order to maintain the stability of blood sugar, promote the synthesis and storage of fat, and thus increase the free fatty acids of patients. An elevation in circulating free fatty acids results in an augmentation of intracellular fatty acid levels within cardiomyocytes. Concurrently, the levels of diacylglycerol and ceramide, a type of sphingolipid, also exhibit an increase. In scientific investigations, diacylglycerol has been identified as a detrimental lipid intermediate in cardiac tissue. Furthermore, accumulating evidence suggests that ceramide accumulation contributes to the progression of left ventricular hypertrophy and cardiac dysfunction. Consequently, this lipotoxicity predominantly impairs diastolic function [14].
5. Therapy for diabetic heart failure
5.1. Lifestyle modification
Diet and exercise therapy is the basic treatment for diabetes. If hypertension or heart failure is present as a complication, it is recommended to further reduce salt intake. In short-lived model organisms, such as mice, the extension of lifespan has been observed through calorie restriction. Studies conducted on healthy individuals have also demonstrated an enhancement in health-related quality of life and a reduction in oxidative stress among those subjected to a 15% calorie restriction over a period of two years, as compared to the group without any dietary restrictions [14].
5.2. Regulating lipid
First, cut down on saturated fat. Saturated fats are found primarily in animal foods, such as red meat, full-fat dairy products, and certain oils, and reducing intake of these foods can help lower LDL cholesterol levels in the blood. Second, increase dietary fiber. Eating more fiber-rich foods, such as whole grains, and legumes, can help lower cholesterol levels and improve gut health. Third, stop smoking and limit alcohol consumption. Smoking will damage the endothelial cells of blood vessels, resulting in the deposition of blood lipids on the wall of blood vessels, forming atherosclerosis. Quitting smoking is a crucial step in reducing blood lipid levels. Excessive drinking can cause liver damage, affect fat metabolism, and increase blood lipid levels, and alcohol intake should be limited.
5.3. Blood glucose control
Improving blood glucose control is a key factor in preventing DC and reducing morbidity and mortality of cardiovascular diseases.
5.3.1. Insulin
Insulin is essential for patients with T1DM and plays a crucial role in managing hyperglycemia in select individuals with T2DM. Given the enhanced renal sodium retention, it is important to note that fluid retention may be further exacerbated in patients with heart failure.
5.3.2. Dietary patterns
A heart-healthy dietary pattern plays a crucial role in the management of T2DM, and evidence supports the effectiveness of Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and vegetarian diets in promoting weight loss and enhancing glycemic control among individuals with T2DM [15]. The Mediterranean diet emphasizes plant-based foods, seasonal fresh produce, substituting fresh fruit for dessert, using olive oil as the primary cooking oil, and moderate dairy consumption. It is also characterized by a high intake of whole grains, such as rye bread, whole grain crisps, and oats. Much epidemiological evidence consistently supports the protective effect of dietary fiber, particularly cereal fiber, in improving insulin sensitivity and reducing diabetes risk. Whole grain foods have a lower glycemic index, which is conducive to the control of blood sugar after meals. Additionally, the Mediterranean diet advocates eating more fish, poultry and eggs and less red meat (pig, cow, lamb and their products). Fish products, especially seafood, are rich in n-3 polyunsaturated fatty acids, which can regulate insulin secretion through gastrointestinal hormones, and also have anti-inflammatory effects.
5.3.3. Drug therapy
Metformin is first-line therapy as it exerts a suppressive effect on hepatic glucose production and enhances peripheral insulin sensitivity, resulting in amelioration of hyperglycemia in individuals with T2DM. Other drugs, such as sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide-1 receptor agonists, also help in the treatment of diabetic heart failure [15]. Patients should be treated with proven medications such as beta-blockers, angiotensin-converting enzyme inhibitors (ACEI), and angiotensin receptor blockers (ARBs) as soon as symptoms of heart failure with reduced ejection fraction (HFrEF) appear.
6. Conclusion
Persistent high blood sugar can lead to inflammation, which contributes to the progression of atherosclerosis and eventually heart failure. For patients and high-risk groups, this research outlines various treatments and prevention strategies, with lifestyle adjustments being the top priority. In diabetes, both T1DM and T2DM are related to diabetic heart disease. Early intervention of blood glucose can reduce the incidence of complications. Screening out related diseases as soon as possible and giving patients intervention can improve the prognosis of patients. The present study is based on the existing research results. Future studies can be combined with specific experiments to analyze the relationship between type 1 diabetes and type 2 diabetes and heart failure, respectively, from the data level.
Acknowledgment
Firstly, I want to show my deepest gratitude to my teachers and professors in this program and my university, who have provided invaluable guidance throughout the writing of this thesis. Furthermore, I express my heartfelt gratitude to all my friends and parents for their unwavering encouragement and unwavering support. Without their invaluable guidance and remarkable kindness, the completion of my thesis would not have been possible.
References
[1]. Jia, G., DeMarco, V. G., & Sowers, J. R. (2016). Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nature Reviews Endocrinology, 12(3), 144–153. https://doi.org/10.1038/nrendo.2015.216
[2]. Kenny, H. C., & Abel, E. D. (2020). Heart Failure in Type 2 Diabetes Mellitus: Impact of Glucose Lowering Agents, Heart Failure Therapies and Novel Therapeutic Strategies.
[3]. Skyler, J. S., Bakris, G. L., Bonifacio, E., Darsow, T., Eckel, R. H., Groop, L., Groop, P.-H., Handelsman, Y., Insel, R. A., Mathieu, C., McElvaine, A. T., Palmer, J. P., Pugliese, A., Schatz, D. A., Sosenko, J. M., Wilding, J. P. H., & Ratner, R. E. (2017). Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis. Diabetes, 66(2), 241–255. https://doi.org/10.2337/db16-0806
[4]. Schwinger, R. H. G. (2021). Pathophysiology of heart failure. Cardiovascular Diagnosis and Therapy, 11(1), 263–276. https://doi.org/10.21037/cdt-20-302
[5]. Kenny, H. C., & Abel, E. D. (2020). Heart Failure in Type 2 Diabetes Mellitus: Impact of Glucose Lowering Agents, Heart Failure Therapies and Novel Therapeutic Strategies.
[6]. Junlin Qiu, Shuoming Luo&Zhiguang Zhou. (2020). Research progress of diabetic heart disease. Chinese Journal of Arteriosclerosis(08), 679-687
[7]. Ritchie, R. H., & Abel, E. D. (2020). Basic Mechanisms of Diabetic Heart Disease. Circulation Research, 126(11), 1501–1525. https://doi.org/10.1161/CIRCRESAHA.120.315913
[8]. Jia, G., DeMarco, V. G., & Sowers, J. R. (2016). Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nature Reviews Endocrinology, 12(3), 144–153. https://doi.org/10.1038/nrendo.2015.216
[9]. Jankauskas, S. S., Kansakar, U., Varzideh, F., Wilson, S., Mone, P., Lombardi, A., Gambardella, J., & Santulli, G. (2021). Heart failure in diabetes. Metabolism, 125, 154910. https://doi.org/10.1016/j.metabol.2021.154910
[10]. Wang, L., Cai, Y., Jian, L., Cheung, C. W., Zhang, L., & Xia, Z. (2021). Impact of peroxisome proliferator-activated receptor-α on diabetic cardiomyopathy. Cardiovascular Diabetology, 20(1), 2. https://doi.org/10.1186/s12933-020-01188-0
[11]. Rohm, T. V., Meier, D. T., Olefsky, J. M., & Donath, M. Y. (2022). Inflammation in obesity, diabetes, and related disorders. Immunity, 55(1), 31–55. https://doi.org/10.1016/j.immuni.2021.12.013
[12]. Darenskaya, M. A., Kolesnikova, L. I., & Kolesnikov, S. I. (2021). Oxidative Stress: Pathogenetic Role in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bulletin of Experimental Biology and Medicine, 171(2), 179–189. https://doi.org/10.1007/s10517-021-05191-7
[13]. Tsutsui, H., Kinugawa, S., & Matsushima, S. (2011). Oxidative stress and heart failure. 301.
[14]. Nakamura, K., Miyoshi, T., Yoshida, M., Akagi, S., Saito, Y., Ejiri, K., Matsuo, N., Ichikawa, K., Iwasaki, K., Naito, T., Namba, Y., Yoshida, M., Sugiyama, H., & Ito, H. (2022). Pathophysiology and Treatment of Diabetic Cardiomyopathy and Heart Failure in Patients with Diabetes Mellitus. Int. J. Mol. Sci.
[15]. Arnett, D. K., Blumenthal, R. S., Albert, M. A., Buroker, A. B., Goldberger, Z. D., Hahn, E. J., Himmelfarb, C. D., Khera, A., Lloyd-Jones, D., McEvoy, J. W., Michos, E. D., Miedema, M. D., Muñoz, D., Smith, S. C., Virani, S. S., Williams, K. A., Yeboah, J., & Ziaeian, B. (2019). 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. Journal of the American College of Cardiology, 74(10), e177–e232. https://doi.org/10.1016/j.jacc.2019.03.010
Cite this article
Xu,Y. (2024). Heart Failure in Diabetes Mellitus: the Pathogenesis and Treatment. Theoretical and Natural Science,64,81-86.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Jia, G., DeMarco, V. G., & Sowers, J. R. (2016). Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nature Reviews Endocrinology, 12(3), 144–153. https://doi.org/10.1038/nrendo.2015.216
[2]. Kenny, H. C., & Abel, E. D. (2020). Heart Failure in Type 2 Diabetes Mellitus: Impact of Glucose Lowering Agents, Heart Failure Therapies and Novel Therapeutic Strategies.
[3]. Skyler, J. S., Bakris, G. L., Bonifacio, E., Darsow, T., Eckel, R. H., Groop, L., Groop, P.-H., Handelsman, Y., Insel, R. A., Mathieu, C., McElvaine, A. T., Palmer, J. P., Pugliese, A., Schatz, D. A., Sosenko, J. M., Wilding, J. P. H., & Ratner, R. E. (2017). Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis. Diabetes, 66(2), 241–255. https://doi.org/10.2337/db16-0806
[4]. Schwinger, R. H. G. (2021). Pathophysiology of heart failure. Cardiovascular Diagnosis and Therapy, 11(1), 263–276. https://doi.org/10.21037/cdt-20-302
[5]. Kenny, H. C., & Abel, E. D. (2020). Heart Failure in Type 2 Diabetes Mellitus: Impact of Glucose Lowering Agents, Heart Failure Therapies and Novel Therapeutic Strategies.
[6]. Junlin Qiu, Shuoming Luo&Zhiguang Zhou. (2020). Research progress of diabetic heart disease. Chinese Journal of Arteriosclerosis(08), 679-687
[7]. Ritchie, R. H., & Abel, E. D. (2020). Basic Mechanisms of Diabetic Heart Disease. Circulation Research, 126(11), 1501–1525. https://doi.org/10.1161/CIRCRESAHA.120.315913
[8]. Jia, G., DeMarco, V. G., & Sowers, J. R. (2016). Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nature Reviews Endocrinology, 12(3), 144–153. https://doi.org/10.1038/nrendo.2015.216
[9]. Jankauskas, S. S., Kansakar, U., Varzideh, F., Wilson, S., Mone, P., Lombardi, A., Gambardella, J., & Santulli, G. (2021). Heart failure in diabetes. Metabolism, 125, 154910. https://doi.org/10.1016/j.metabol.2021.154910
[10]. Wang, L., Cai, Y., Jian, L., Cheung, C. W., Zhang, L., & Xia, Z. (2021). Impact of peroxisome proliferator-activated receptor-α on diabetic cardiomyopathy. Cardiovascular Diabetology, 20(1), 2. https://doi.org/10.1186/s12933-020-01188-0
[11]. Rohm, T. V., Meier, D. T., Olefsky, J. M., & Donath, M. Y. (2022). Inflammation in obesity, diabetes, and related disorders. Immunity, 55(1), 31–55. https://doi.org/10.1016/j.immuni.2021.12.013
[12]. Darenskaya, M. A., Kolesnikova, L. I., & Kolesnikov, S. I. (2021). Oxidative Stress: Pathogenetic Role in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bulletin of Experimental Biology and Medicine, 171(2), 179–189. https://doi.org/10.1007/s10517-021-05191-7
[13]. Tsutsui, H., Kinugawa, S., & Matsushima, S. (2011). Oxidative stress and heart failure. 301.
[14]. Nakamura, K., Miyoshi, T., Yoshida, M., Akagi, S., Saito, Y., Ejiri, K., Matsuo, N., Ichikawa, K., Iwasaki, K., Naito, T., Namba, Y., Yoshida, M., Sugiyama, H., & Ito, H. (2022). Pathophysiology and Treatment of Diabetic Cardiomyopathy and Heart Failure in Patients with Diabetes Mellitus. Int. J. Mol. Sci.
[15]. Arnett, D. K., Blumenthal, R. S., Albert, M. A., Buroker, A. B., Goldberger, Z. D., Hahn, E. J., Himmelfarb, C. D., Khera, A., Lloyd-Jones, D., McEvoy, J. W., Michos, E. D., Miedema, M. D., Muñoz, D., Smith, S. C., Virani, S. S., Williams, K. A., Yeboah, J., & Ziaeian, B. (2019). 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. Journal of the American College of Cardiology, 74(10), e177–e232. https://doi.org/10.1016/j.jacc.2019.03.010









