1. Introduction
At present, with the aging of the population and the change in people’s lifestyles, more and more patients suffer from acute ischemic stroke, which constantly jeopardizes human health. It is a disease with a high incidence rate, mortality and disability rate. According to the statistics of the World Health Organization, approximately 26 million people around the world die of thrombotic diseases each year. In China, the number of deaths from cardiovascular and cerebrovascular diseases accounts for 40.7% of the total number of deaths due to diseases, ranking first among the causes of death [1]. Thus, there is an increasing demand for thrombolytic enzymes in society, and research on the treatment of thrombotic diseases has become a hot topic in the field of medicine. Thrombolytic drugs are widely utilized in thrombotic treatment, and various thrombolytic enzymes also have different roles and differences. This paper mainly introduces the treatment of this disease with alteplase, and refers to multiple papers to study the clinical efficacy and safety analysis of alteplase. In addition, this paper analyzes the safety of alteplase, allowing an understanding of the advantages and disadvantages of this enzyme, which is safe and highly effective. This paper helps to elucidate the clinical efficacy of alteplase, gives people a better understanding of this thrombolytic enzyme, and promotes its wider application..
2. Overview of Acute Ischemic Stroke
2.1. Etiology and Pathogenesis
Ischemic stroke, also known as cerebral infarction or ischemic stroke, refers to a clinical syndrome in which various cerebrovascular diseases cause blood supply disorders in the brain, leading to local brain tissue ischemia and hypoxic necrosis, and rapidly resulting in corresponding neurological deficits [2]. Moreover, ischemic stroke is the most common type of cerebrovascular disease, accounting for about 70% to 80%. The patient mainly presents with focal neurological deficits, such as hemiplegia, sensory disorders, aphasia, ataxia, etc., and may also have headaches, vomiting, coma, etc. Mild cases have a good prognosis, while severe cases can be life-threatening. The condition is a medical emergency caused by reduced blood flow to the brain, resulting in damage to brain cells. The signs and symptoms of stroke may include sudden numbness or weakness in the arms or legs, facial sagging, difficulty speaking or understanding language, blurred consciousness, difficulty balancing or coordinating, and loss of vision. Transient ischemic attack (TIA) can sometimes occur before acute ischemic stroke, which is a temporary onset of brain dysfunction caused by reduced blood flow. In addition, acute ischemic stroke is clinically found to be very common. According to statistics, acute ischemic stroke is the leading cause of death and disability in the United States, affecting approximately 700,000 people annually. The main risk factor of acute ischemic stroke is hypertension. Other diseases related to the increased risk of stroke include TIA history, smoking, high cholesterol, diabetes, obesity, end-stage renal disease and arrhythmia called atrial fibrillation, which may be the disease inducement of acute ischemic stroke [3-5].
2.2. Clinical Manifestations
Acute ischemic stroke is mainly manifested by atherosclerosis of the large arteries, and the main mechanisms by which atherosclerosis leads to ischemic stroke include thrombosis, atheroembolism, carrier arterial lesions blocking perforating arteries, and hypoperfusion [6]. Atherosclerosis of large arteries results in significant narrowing of intracranial and extracranial large arteries or their cortical branches due to atherosclerosis, or clinical or imaging manifestations of vascular obstruction. In addition, cardiac embolism is manifested in clinical diseases. This disease causes cardiogenic embolism, mainly due to causes including atrial fibrillation, atrial flutter, valvular heart disease, prosthetic heart valves, infective endocarditis, myocardial infarction, cardiomyopathy, heart failure, and cardiac myxoma [7]. Foramen ovale failure (PFO) has gained attention in recent years and it is also an undeniable cause of cardiac stroke. Its clinical and imaging manifestations are identical to those of atherosclerosis. The diagnosis of cardiogenic embolism is supported if the patient has had a TIA or stroke in one or more vascular-dominated areas or has had systemic emboli prior to the onset of the disease, and it should be possible to identify at least one embolism of cardiac origin.
In addition, hypertension represents the primary etiology in small artery occlusive disease, including cerebral artery capillary hyalinization, atherosclerosis, and fibrinoid necrosis; a small number of them are caused by microangiopathy due to diabetes mellitus; atherosclerosis, vasculitis, and hereditary disorders of small arterial perforation can also lead to small arterial perforation occlusion. The main cause of cerebral watershed infarction is ischemia of the small arteries in the marginal zone, which commonly occurs due to various reasons such as shock, anesthetic overdose, inappropriate use of antihypertensive drugs, cardiac surgery combined with hypotension, and severe dehydration. This subtype is referred to as lacunar infarction in other classification methods [8]. The clinical manifestations of lacunar syndrome include pure motor stroke, pure sensory stroke, sensory-motor stroke, and ataxic hemiplegic syndrome, wherein there is no dysarthric clumsiness syndrome without any cortical involvement.
3. The Action Mechanism and Clinical Efficacy of Alteplase
3.1. Mechanism of Action
rt-PA is a pharmaceutical agent that exhibits high cost, high affinity, non-immunogenic properties, which is metabolized by the liver with a half-life of 5 minutes. Alteplase is a glycoprotein that has the capacity to activate plasminogen, thereby converting it into plasmin. The intravenous administration of this pharmaceutical agent within the circulatory system demonstrates activity exclusively subsequent to its binding with fibrin, which exhibits a high degree of affinity for fibrin. When combined with fibrin, the drug is activated, inducing plasminogen to become plasmin, thus dissolving blood clots. Alteplase binds to plasminogen and converts it into an active fibrinolytic enzyme, thereby dissolving blood clots. The mechanism of action of this drug includes the promotion of the conversion of plasminogen to a fibrinolytic enzyme, a reduction in the concentration of fibrinogen in plasma, and the inhibition of the formation of new blood clots. Additionally, it stimulates endothelial cells to produce a series of thrombolytic reactions, which promote the dissolution of blood clots. However, the systemic effect on all components of the coagulation system is mild, and therefore there is no tendency for bleeding. Moreover, the drug is non-antigenic and can be reused. The clinical mechanism of action of the alteplase thrombolysis system is illustrated in Figure 1 [9].
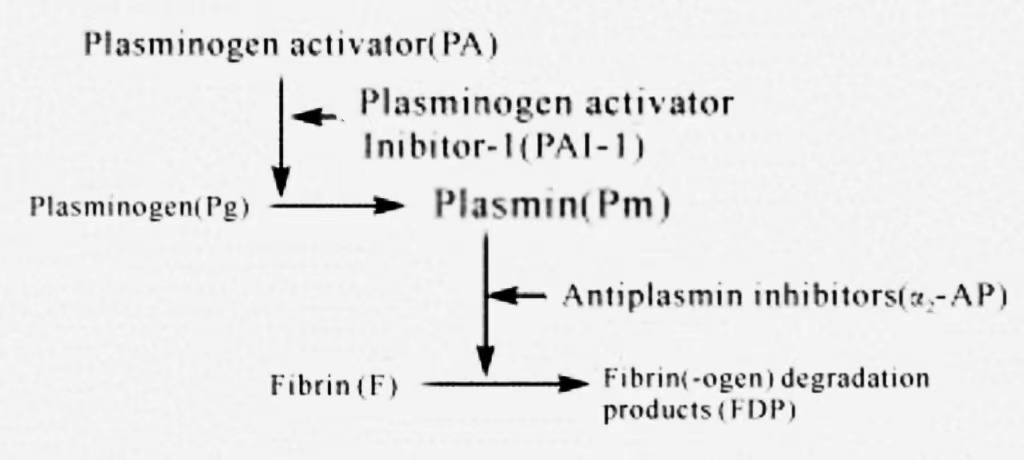
Figure 1. Thrombolytic System [9]
3.2. Clinical Efficacy
Alteplase can specifically activate plasminogen in thrombi, converting it into plasmin and dissolving thrombi, making it particularly suitable for acute thrombotic diseases. And it has a fast onset of action and can rapidly exert its effects in the body after intravenous administration, especially in the early treatment of acute myocardial infarction and stroke, which shows good efficacy. The following is a specific analysis of the clinical effects of alteplase.
Firstly, alteplase is a tissue-type plasminogen activator (t-PA), which promotes the conversion of plasminogen to plasmin by highly specific binding to fibrin, thus directly acting on thrombus tissue and dissolving thrombus. Its efficient thrombolytic ability makes it widely used in the clinical treatment of acute thrombotic diseases, especially in the early intervention of acute myocardial infarction and acute ischemic stroke, which can significantly improve the prognosis of patients and reduce the disability and mortality rates. Secondly, alteplase has a good safety profile. When used in the acute phase, there is a certain risk of bleeding, but the thrombolytic effect and the risk of bleeding complications can be effectively balanced by strict selection of indications and rational management of the time window of administration. Existing studies have shown that the use of alteplase within 3 to 4.5 hours after onset of the disease significantly improves the success rate of thrombolysis and reduces long-term neurologic impairment. The metabolism of alteplase is primarily dependent on the liver, undergoing uptake and degradation by hepatocytes to produce inactive metabolites. Accordingly, monitoring of liver function is imperative for patients undergoing long-term alteplase therapy, particularly those with concomitant hepatic insufficiency. The half-life of the drug in the body is relatively brief, typically ranging from five to ten minutes. However, its pharmacological effects can persist for several hours, particularly in the context of acute-phase thrombophilia, where timely adjustments to dosage and dosing regimens are essential, contingent on the patient's condition. Additionally, alteplase has been demonstrated to be efficacious in the treatment of deep vein thrombosis and pulmonary embolism, extending the scope of its applications beyond the treatment of acute cardiovascular and cerebrovascular events. As research progresses, the potential applications of alteplase in other fields, such as peripheral arterial embolism, thromboembolism of artificial blood vessels and valves, and so forth, have also been increasingly highlighted. It is likely that further research and clinical application scenarios will emerge in the future.
4. Side Effects and Safety of Alteplase
4.1. Side Effects and Safety of Alteplase
The administration of alteplase has been associated with a number of adverse effects, including bleeding and cerebral hemorrhage, which are commonly observed in clinical settings. The primary adverse effect associated with alteplase is bleeding, which can manifest as serious conditions such as cerebral hemorrhage. Furthermore, the administration of alteplase may result in coagulation disorders and bleeding, as well as a reduction in hematocrit and hemoglobin levels, and the occurrence of bleeding at the injection site. On occasion, cardiac arrhythmias and elevated body temperature have been observed. Rare adverse effects include a reduction in blood pressure, intracranial hemorrhage, retroperitoneal hemorrhage, rectal bleeding, and hematuria. Additionally, there are numerous contraindications to alteplase, including bleeding disorders, hepatic dysfunction, hypertension, patients over the age of 75, and patients taking oral anticoagulants, who should be advised to avoid this medication.
The use of alteplase during thrombolytic therapy requires close monitoring to ensure safety and efficacy. Patients should be strictly monitored in the hospital during the use of alteplase, especially for changes in vital signs such as blood pressure and heart rate, as well as dynamic assessment of coagulation function. Monitoring of coagulation indices (e.g., prothrombin time, activated partial thromboplastin time, etc.) is essential for the prevention and timely detection of possible bleeding complications. Meanwhile, precise control of blood pressure plays an important role in reducing the risk of complications such as intracranial hemorrhage. In addition, the use of alteplase should take into account the patient's past medical history. In patients with a history of cerebrovascular disease, such as previous cerebral hemorrhage, intracranial aneurysm, or arteriovenous malformation, the use of alteplase carries a higher risk of bleeding and is therefore usually considered a contraindication. Similarly, patients who have recently undergone severe trauma, surgery, or have active bleeding are not candidates for alteplase thrombolysis. In these cases, physicians should weigh the pros and cons of the treatment and consider other alternative treatment options.
4.2. Safety Studies on Alteplase
The advantages of alteplase in thrombolytic therapy are significant, and the safety of its clinical application is supported by several studies. For example, one study conducted a randomized controlled trial of 86 patients with acute ischemic stroke (AIS) admitted to the First Hospital of Wuhan City from January 2017 to December 2021, dividing the patients into an observation group (n=50) and a control group (n=36). The observation group received low-dose (0.6 mg/kg) alteplase treatment, and the control group received conventional dose (0.9 mg/kg) treatment. A comparison of the neurological function, life self-care ability, short-term efficacy, and incidence of adverse reactions between the two groups revealed no statistically significant difference in the total effective rate of thrombolytic therapy between the two groups (P > 0.05). Similarly, no statistically significant difference was observed in the scores of neurological function and life self-care ability before and after treatment (P > 0.05). However, the incidence rate of adverse reactions in the observation group was significantly lower than that in the control group (P=0.046), suggesting that small-dose alteplase can reduce the risk of adverse reactions while maintaining the efficacy of treatment, especially in elderly patients [10].
In addition, another study further verified the safety of alteplase, the incidence of adverse reactions in the observation group (alteplase treatment) was 5.00%, while the incidence of adverse reactions in the control group (conventional drug treatment) was 17.65%, which was statistically significant (P<0.05). This study concluded that alteplase can significantly reduce the incidence of adverse reactions and improve the safety and efficacy of thrombolytic therapy [11]. As a tissue-type fibrinogen activator, alteplase is mainly used in thrombolytic therapy for acute myocardial infarction, pulmonary embolism, cerebral infarction and other thrombotic diseases. Its drug half-life is about 5 minutes, and it is mainly metabolized by the liver, with high safety. In clinical application, alteplase has fewer adverse reactions, further establishing its wide application value in the treatment of acute thrombotic diseases.
5. Comparison of multiple thrombolytic enzymes
5.1. Major Thrombolytic Enzymes
The development of thrombolytic drugs has evolved over three generations, with each generation showing significant improvements in thrombolytic mechanism, fibrin specificity and safety. The first generation of thrombolytic drugs, such as urokinase (UK) and streptokinase (SK) [12], dissolve thrombus by activating the conversion of fibrinogen (Pg) to fibrinolysis (Pm), but due to the lack of fibrin specificity, they degrade fibrin while also leading to the non-selective degradation of fibrinogen, which increases the risk of systemic fibrinolysis and hemorrhage, and has a relatively low safety profile. Second-generation thrombolytic drugs such as tissue-type plasminogen activator (t-PA) and single-chain urokinase-type plasminogen activator (scu-PA) have higher fibrin specificity, which enhances the effect of thrombolysis and significantly reduces the incidence of adverse effects such as bleeding. The third generation of thrombolytic drugs has been optimized through genetic engineering technology, and representative drugs such as recombinant tissue plasminogen activator (rt-PA) and Lanteplase, which have faster onset of action, stronger fibrin specificity and higher safety, have become the mainstream choice for clinical thrombolytic therapy.
5.2. Clinical Comparison of Alteplase and Other Drugs
In clinical thrombolytic therapy, alteplase has significant advantages over other drugs. Although the first-generation thrombolytic drug urokinase (UK) has the advantages of being non-antigenic and non-pyrogenic and can be used continuously for more than half a month, its half-life is relatively short (less than 20 minutes), and its long-term use in large doses is likely to cause systemic bleeding. In addition, urokinase has poor selectivity, relatively low thrombolytic efficiency and greater side effects. Compared with alteplase, the thrombolytic effect of urokinase is significantly less effective. According to a randomized trial of alteplase, which was conducted in more than 1,600 patients with acute myocardial infarction (AMI), the results showed that compared with the urokinase-treated group, the alteplase-treated group showed better clinical efficacy with lower incidence of adverse effects, mortality, and large myocardial infarction.
In addition, reteplase, as another thrombolytic drug with structural similarity to alteplase, is able to dissolve a wider range of thrombi, and thus has an important role in the treatment of acute myocardial infarction [13]. Compared to alteplase, reteplase has a longer half-life and restores blood flow through rapid, sustained action. However, studies have shown that alteplase is more effective in restoring blood flow and is more widely recognized for its overall safety and efficacy [14]. And urokinase was once widely used in the treatment of AMI, but its efficacy and safety are less favorable when compared to alteplase and reteplase. However, although alteplase and reteplase are the drugs of choice for the treatment of acute myocardial infarction as they have demonstrated better thrombolytic efficacy and lower risk of side effects in the clinic, the optimal therapeutic regimen needs to be determined in the context of an individualized clinical assessment and the specific condition of the patient due to the complexity and variability of the patient's condition.
6. Conclusion
Based on the comprehensive analysis, it can be seen that alteplase is a thrombolytic agent used to treat acute ischemic stroke, which can effectively dissolve local thrombi and promote cerebral vascular recanalization. The results show that the total effective rate of patients receiving alteplase treatment was high, but its possible adverse reactions, such as nerve damage and cerebral edema, should also be noted. For the safety of alteplase treatment, patients treated with alteplase have a relatively low probability of experiencing adverse reactions. alteplase has significant clinical efficacy in treating patients with acute ischemic stroke, especially in improving treatment outcomes and alleviating patient symptoms. And it has high safety and effectiveness. alteplase has shown good clinical efficacy in the treatment of acute ischemic stroke, and there are also studies supporting its safety. However, it should be noted that adverse reactions may occur with any treatment, and the patient's condition should be closely observed during clinical use to ensure the safety of the treatment. In addition, this paper may focus on the comparison of the efficacy of alteplase with other thrombolytic enzymes in the future.
References
[1]. Yuan, G.Q. (2004) Thrombotic Diseases: Important Diseases Threatening Human Health and Life..Chinese Journal of Laboratory Medicine, 27(8).
[2]. Herpich, F. and Rincon, F. (2020) Management of Acute Ischemic Stroke BMJ., 48(11): 1654-1663.
[3]. Panuganti, K.K., Tadi, P. and Lui, F. (2023) Transient Ischemic Attack. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
[4]. Sossalla, S. and Vollmann D. (2018) Arrhythmia-Induced cardiomyopathy. Dtsch Arztebl Int. 115(19): 335-341
[5]. Falk, E.(2006) Pathogenesis of atherosclerosis. J.Sci.Commun., 47(8): C7-12.
[6]. Hogan, D.F. and Brainard, B.M. (2015) Cardiogenic Embolism in the Cat. J Vet Cardiol. 1: S202-14.
[7]. Longa, E.Z., Weinstein, P.R., Carlson, S. and Cummins, R. (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke, 20(1): 84-91.
[8]. Du, P.Z. (1998) Research progress of thrombolytic drugs. Foreign Medicine and Pharmacy, 19(2): 67-71.
[9]. Yu, X. (2023) Short term efficacy and safety analysis of low-dose alteplase in the treatment of elderly patients with acute ischemic stroke.Chinese Community Physicians, 39(31).
[10]. Geng, T. (2018) Clinical efficacy and safety analysis of alteplase in the treatment of acute cerebral infarction. Practical Medicine in China, 15: 105-107.
[11]. Kadir, R.R.A. and Bayraktutan, U. (2020) Urokinase Plasminogen Activator: A Potential Thrombolytic Agent for Ischaemic Stroke. Cell Mol Neurobiol, 40(3): 347-355.
[12]. Karaa, A. and Goldstein, A. (2015) The Spectrum of Clinical Presentation, Diagnosis, and Management of Mitochondrial Forms of Diabetes. Pediatr Diabetes, 16(1): 1-9.
[13]. Miller, S. and Warach, S. (2023) Evolving Thrombolytics: from Alteplase to Tenecteplase. Neurotherapeutics, 20(3): 664-678.
[14]. Noble, S. and McTavish, D .(1996) Reteplase. A review of its pharmacological properties and clinical efficacy in the management of acute myocardial infarction. Drugs, 52(4): 589-605
Cite this article
Sha,J. (2024). Clinical Efficacy and Safety Analysis of Alteplase in the Treatment of Acute Ischemic Stroke Patients. Theoretical and Natural Science,67,1-6.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Yuan, G.Q. (2004) Thrombotic Diseases: Important Diseases Threatening Human Health and Life..Chinese Journal of Laboratory Medicine, 27(8).
[2]. Herpich, F. and Rincon, F. (2020) Management of Acute Ischemic Stroke BMJ., 48(11): 1654-1663.
[3]. Panuganti, K.K., Tadi, P. and Lui, F. (2023) Transient Ischemic Attack. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
[4]. Sossalla, S. and Vollmann D. (2018) Arrhythmia-Induced cardiomyopathy. Dtsch Arztebl Int. 115(19): 335-341
[5]. Falk, E.(2006) Pathogenesis of atherosclerosis. J.Sci.Commun., 47(8): C7-12.
[6]. Hogan, D.F. and Brainard, B.M. (2015) Cardiogenic Embolism in the Cat. J Vet Cardiol. 1: S202-14.
[7]. Longa, E.Z., Weinstein, P.R., Carlson, S. and Cummins, R. (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke, 20(1): 84-91.
[8]. Du, P.Z. (1998) Research progress of thrombolytic drugs. Foreign Medicine and Pharmacy, 19(2): 67-71.
[9]. Yu, X. (2023) Short term efficacy and safety analysis of low-dose alteplase in the treatment of elderly patients with acute ischemic stroke.Chinese Community Physicians, 39(31).
[10]. Geng, T. (2018) Clinical efficacy and safety analysis of alteplase in the treatment of acute cerebral infarction. Practical Medicine in China, 15: 105-107.
[11]. Kadir, R.R.A. and Bayraktutan, U. (2020) Urokinase Plasminogen Activator: A Potential Thrombolytic Agent for Ischaemic Stroke. Cell Mol Neurobiol, 40(3): 347-355.
[12]. Karaa, A. and Goldstein, A. (2015) The Spectrum of Clinical Presentation, Diagnosis, and Management of Mitochondrial Forms of Diabetes. Pediatr Diabetes, 16(1): 1-9.
[13]. Miller, S. and Warach, S. (2023) Evolving Thrombolytics: from Alteplase to Tenecteplase. Neurotherapeutics, 20(3): 664-678.
[14]. Noble, S. and McTavish, D .(1996) Reteplase. A review of its pharmacological properties and clinical efficacy in the management of acute myocardial infarction. Drugs, 52(4): 589-605









