1. Introduction
Diabetes and heart failure are two common, deadly chronic diseases worldwide, with many links between the two that people with diabetes are at a significantly increased risk of developing heart failure-related conditions. The concept of diabetic cardiomyopathy was initially introduced by Rubler [1]. Diabetic cardiomyopathy is a series of myocardial dysfunctions associated with diabetes, in addition to traditional cardiovascular risk factors such as coronary artery disease and hypertension [2]. By 2019, the global population of individuals with diabetes amounted to 451 million, and diabetes significantly elevates the risk of cardiovascular disease by a factor of 2 to 2.5. An examination of the diabetic patient count reveals that 32.7% of this population experienced cardiovascular disease, with cardiovascular disease accounting for half of all recorded deaths [3]. It is obvious that the resulting complications can worsen the patient's health, such as orthostatic hypotension, syncope, coronary artery diastolic dysfunction, painless myocardial infarction, cardiac arrest, or sudden death. The author will explore the mechanisms involved in this disease, such as hyperoxidation, inflammation, and immune response, along with the available treatments based on literature and data. It is hoped that this article provides people in need with the latest knowledge and tools to help them better recognize, treat and manage diabetic cardiomyopathy. Efforts in research have the potential to discover more efficacious interventions that aid individuals affected by diabetes in controlling their illness, while also preventing or postponing the development of diabetic cardiomyopathy, ultimately improving both the quality of life and lifespan of patients.
2. Celluar and molecular mechanism
2.1. Oxidative stress and reactive oxygen species
According to Cheng et al., “oxidative stress (OS) refers to the imbalance of oxidative metabolism in vivo, mainly due to the enhancement of oxidation, which promotes inflammatory responses and the production of large amounts of oxidative intermediate products” [2]. This dysregulation can cause damage to cells and tissues. It can also destroy proteins, lipids, and DNA. ROS formation primarily occurs in the mitochondria and endoplasmic reticulum of eukaryotic cells. High blood sugar is a major oxidative stress trigger for people with diabetes. In this case, the sources of oxidative stress are manifold.
The first case is glycosylation due to hyperglycemia. In the first instance, hyperglycemia-related glycosylation occurs. Advanced glycation end products, or AGEs, are finally formed via glycosylation, whereas the Maillard process produces AGEs. The Maillard reaction is a browning process that occurs naturally when carbonyl compounds — primarily reducing sugars — combine with other molecules that contain free amino groups, like proteins, amines, and amino acids. The carbonyl group of the reducing sugar interacts with the amino group of the protein's amino acid to form the unstable Schiff base intermediate during this reaction, which is the result of a non-enzymatic interaction between the carbonyl moiety of the sugar and the amino acids of the protein. During this reaction, the carbonyl moiety of the sugar engages in a non-enzymatic interaction with the protein's amino acids, initiating an amino reaction wherein the carbonyl group of the reducing sugar interacts with the amino group of the protein's amino acid, resulting in the formation of the unstable Schiff base intermediate. Subsequently, the Schiff base can be rearranged to form a more stable Amadori product. Finally, the Amoria product undergoes chemical reactions such as oxidation, rearrangement and condensation to form AGEs. In addition, there are other ways to generate AGEs [4]. Eventually, AGEs bind to RAGE resulting in the activation of NADPH oxidase and the production of ROS, which further exacerbates the oxidative stress response and accelerates disease progression [5].
The second condition is mitochondrial dysfunction, leading to a decrease in the rate of ATP synthesis. First, high blood sugar levels increase oxidative stress within the mitochondria, leading to damage to mitochondrial DNA (mtDNA). Secondly, insulin resistance and abnormal fatty acid metabolism play a key role in diabetic cardiomyopathy. In insulin-resistant states, fatty acid oxidation is enhanced while glucose oxidation is weakened, which alters the energy substrate utilization pattern of mitochondria. The overload of the electron transport chain during fatty acid oxidation increases ROS production, which further aggravates oxidative stress. This process damages the mitochondrial membrane and respiratory chain complex, ultimately reducing the efficiency of ATP synthesis. Taken together, these mechanisms work together to exacerbate the energy metabolism disorder of cardiomyocytes, ultimately adversely affecting cardiac function [6].
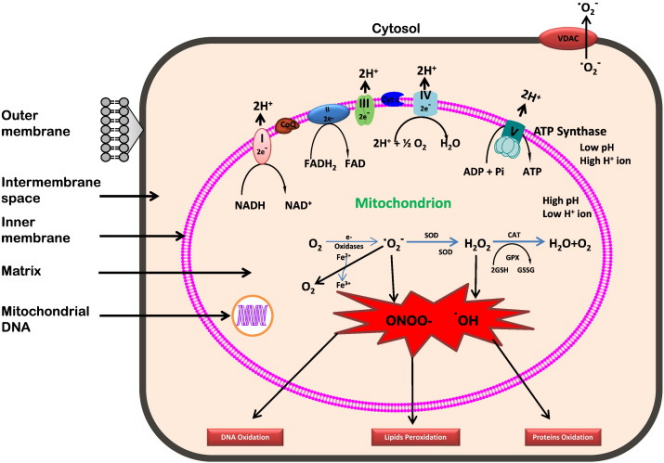
Figure 1. Generation of reactive oxygen species in mitochondrion [PNG][6]
Hyperglycemia results in an elevated production of ROS, ultimately leading to mitochondrial impairment [7]. This condition exacerbates ROS generation, subsequently targeting the cell membrane and inducing lipid peroxidation. Obese individuals are prone not only to diabetes but also to non-alcoholic fatty liver disease (NAFLD). In the progression of NAFLD, the accumulation of fatty acids within liver cells initiates a cascade of reactions, promoting the formation of reactive oxygen species (ROS) and lipid peroxidation. Specifically, fatty acids within hepatocytes undergo β-oxidation, a process primarily occurring in mitochondria. Excessive activation of fatty acid β-oxidation leads to substantial ROS production. While ROS are natural byproducts of cellular metabolism and typically cleared by the antioxidant system, pathological states like NAFLD disrupt this balance, causing ROS accumulation within cells. These accumulated ROS then target unsaturated fatty acids in the cell membrane, initiating a free radical chain reaction. In this reaction, ROS interact with an unsaturated fatty acid, forming a lipid free radical that subsequently reacts with additional molecules, propagating the chain reaction.
Oxidative stress can also lead to autophagy and endoplasmic reticulum stress. "The unfolded protein response (UPR) is activated to resolve this protein-folding defect when there is an accumulation of unfolded and misfolded proteins in the ER lumen, a condition called ER stress," state Cao SS and Kaufman RJ [8]. If endoplasmic reticulum stress is prolonged or too severe, or if UPR damage does not alleviate protein deficiencies, the pro-apoptotic signaling pathway in the cell is activated, leading to autophagic dysfunction. Furthermore, as a response to endoplasmic reticulum stress, the imbalance in the regulation of disulfide bond formation and cleavage, which exhibits heightened sensitivity to redox balance alterations, can result in the accumulation of ROS and subsequently induce oxidative stress [9].
2.2. Inflammation and immune response
Under the environmental conditions of hyperglycemia, the phagocytosis of diabetic patients decreases, which promotes the continuation of the inflammatory response; at the same time, the activity of T and B cells in diabetic patients is also affected, and their adaptive immune response is weakened, leading to an increased risk of infection[10]. The studies focused on the pathological mechanisms of diabetic complications, especially emphasizing the key roles of oxidative stress and inflammatory response. Studies have shown that immune cells secrete more pro-inflammatory cytokines in this setting, which also leads to a persistent increase in the inflammatory response [11].
Increased oxidative stress can also lead to increased inflammation. Neutrophils in diabetes are activated and produce older ROS, which increases the risk of organ damage. Multiple pathways such as oxidative stress or AGEs also activate intracellular inflammatory signaling pathways (e.g., NF-κB, MAPK), which leads to a broad inflammatory response and increases the persistence and severity of chronic inflammatory states [12].
2.3. Pancreatic islet β cell dysfunction
Pancreatic β cells play a key role in maintaining normal blood glucose homeostasis, and their main function is to synthesize and secrete insulin to ensure that blood sugar in the body is maintained within a healthy range.
Under the adverse conditions of metabolic syndrome, which include elevated blood sugar, increased free fatty acids, and inflammation, β cells develop a compensatory response that is used to improve the functional capacity of β cells and insulin secretion in order to meet increased metabolic demands. Subsequently, the quality or function of the cell is damaged, resulting in the loss of its metabolic function and the abnormality of the cell's defense mechanism. Because of endoplasmic reticulum stress, mitochondrial dysfunction, oxidative stress and inflammatory beta cell dysfunction. Eventually, stressed β cells undergo phenotypic changes, including cell death, dedifferentiation, transdifferentiation, or impaired function. β cells also enhance β cell performance through intrinsic mechanisms in order to adapt to a highly nutrient environment. However, long-term elevated insulin can affect insulin sensing and may lead to metabolic syndrome [13].
In a hyperglycemic environment, β cells need to increase insulin synthesis and secretion to accommodate higher insulin demand, which stems from insulin resistance, a major feature of type 2 diabetes. To meet this need, β cells increase cell quality and insulin secretion. However, persistently high insulin secretion requirements and excess nutrients such as high glucose and free fatty acids can lead to the accumulation of unfolded proteins in the endoplasmic reticulum. When these unfolded proteins are not sufficiently processed by molecular chaperones in the endoplasmic reticulum, endoplasmic reticulum stress and UPR are triggered, potentially worsening β cellular performance.
In addition, due to this condition, β cells often experience a condition of endoplasmic reticulum overload, which leads to endoplasmic reticulum stress. Endoplasmic reticulum stress may be exacerbated by pro-inflammatory cytokines and oxidative stress, and the combination of these factors can lead to more severe damage to β cells, ultimately affecting normal insulin secretion and aggravating type 2 diabetes [14].
Cells initiate the UPR in response to endoplasmic reticulum stress, Which is a biochemical program designed to restore endoplasmic reticulum function and protein homeostasis. UPR activates genes involved in protein folding and degradation through transcription, as well as temporarily attenuates mRNA translation to reduce endoplasmic reticulum burden. And UPR senses and responds to the endoplasmic reticulum stress through PERK, IRE1, and ATF6. The process is that PERK and IRE1 regulate protein synthesis and degradation by phosphorylating eIF2α and activating XBP-1, respectively. ATF6 activates genes such as XBP-1 and is involved in UPR regulation. However, excessive UPR signaling leads to CHOP activation, which promotes apoptosis in β cells [14].
2.4. Insulin resistance
Insulin resistance affects insulin signaling in the liver, skeletal muscle, and adipose tissue, leading to impaired blood glucose management, which can lead to hyperglycemia. To compensate, pancreatic β cells need to increase insulin synthesis and secretion, but long-term insulin resistance exposes β cells to high levels of glucose and lipids, which may eventually lead to β cell dysfunction, failure, and even death.
A major factor in the onset and course of diabetic cardiomyopathy is the activation of many inflammatory responses, which is linked to insulin resistance. In insulin resistance, the following aspects lead to increased inflammation:
The first is related to high free fatty acid (FFA) levels. During insulin resistance, increased FFA levels in the body occur alongside impaired insulin metabolic signaling and elevated blood sugar. NLRP3 (NACHT, LRR, and PYD structural domain-containing protein 3) is a novel molecular marker closely associated with the development of diabetic cardiomyopathy, and high levels of FFA activate the NLRP3 (NACHT, LRR, and PYD structural domain-containing protein 3) inflammatory vesicle [14].
Second, activation of the NLRP3 inflammasome may also lead to an increased proinflammatory response. Activation of the NLRP3 inflammasome involves separation and oligomerization with cytoplasmic chaperone proteins, followed by recruitment and activation of procaspase-1. Activated caspase-1 not only processes the precursors interleukin-1β (IL-1β) and interleukin-18 (IL-18), but also acts as an enhancer of multiple pro-inflammatory pathways involved in NFκB, chemokines, and ROS. In addition, the activation of NFκB creates a positive feedback loop that further increases the assembly of the NLRP3 inflammasome, the activation of procaspase-1, and the processing and maturation of the precursor IL-1β [14].
Within the coronary arteries, monocyte/macrophage migration increases due to the phenomenon of elevated ROS levels and a decrease in bioavailable nitric oxide (NO), which leads to an increase in the number of macrophages in the heart. These macrophages can be polarized into a pro-inflammatory M1 phenotype in the presence of increased ROS, and in diabetic heart tissue, this M1 polarization is upregulated while the anti-inflammatory M2 response is suppressed [14].
3. Clinical presentation and diagnosis
3.1. Symptoms and signs
In the first stage, patients with diabetic cardiomyopathy are clinically asymptomatic, but are characterized by increased fibrosis and stiffness; decreased early diastolic filling, increased atrial filling and enlargement, and increased left ventricular end-diastolic pressure. In the second stage, it is characterized by left ventricular hypertrophy, cardiac remodeling, and worsening diastolic dysfunction [14].
3.2. Current therapeutic strategies and management
The techniques used in clinical diagnosis of diabetic cardiomyopathy include Echocardiography, Magnetic Resonance Imaging (MRI) and Transmitral Doppler and Tissue Doppler Imaging, as well as Positron Emission Tomography (PET). Echocardiography is a non-invasive imaging technique that provides detailed information about the shape and function of the heart. With this technique, structural changes in the heart, such as the degree of left ventricular (LV) hypertrophy and fibrosis, can be observed, as well as the systolic and diastolic function of the heart can be assessed. MRI is more accurate than echocardiography in detecting abnormal heart morphology. It is able to provide information about myocardial fibrosis, fat deposition, left ventricular quality, and diastolic function. MRI is valuable for early detection of subtle changes in diabetic cardiomyopathy. Transmitral Doppler and Tissue Doppler Imaging are used to quantify abnormalities in myocardial function. In particular, the E/e' ratio, that is, the ratio of the early passive mitral valve inflow velocity (E) to the medial mitral annular velocity (e'), is a surrogate measure of left ventricular filling pressure, which is of great significance for predicting the prognosis of diabetic patients. PET technology is used to evaluate abnormal myocardial metabolism. Although currently mainly used for research purposes, it has not yet been widely used in clinical diagnosis due to its high cost, time requirements, and expertise required to interpret results [15].
In terms of treatment strategies, traditional antihyperglycemic drugs focus on glycemic control to reduce the risk of microvascular complications such as renal failure, retinopathy, and nerve damage. However, these drugs are less effective in preventing or treating diabetic cardiomyopathy, and more targeted therapies are needed. At the same time, patients also need to make changes to their lifestyle, including a healthy diet and long-term exercise. In addition to glucose-lowering drugs, cardioprotective drugs such as ACE inhibitors, ARBs, β blockers, and aldosterone antagonists are also needed. Some novel drugs such as GLP-1 receptor agonists and SGLT2 inhibitors are not only effective in controlling blood glucose, but also show direct benefits to the cardiovascular system. For example, GLP-1 receptor agonists may reduce myocardial infarction and improve cardiac function through cardioprotective effects, while SGLT2 inhibitors have shown the potential to reduce cardiovascular events and all-cause mortality. In the future, the treatment of diabetic heart disease may lead to a new breakthrough. As an emerging therapeutic approach, gene modulation therapy is being studied to regulate the expression of key genes or non-coding RNAs to treat diabetic cardiomyopathy. Additionally, the potential of non-coding RNAs as biomarkers is being explored, and they may play an important role in the development of early diagnosis and treatment strategies [15].
The first new technology that could be developed in the future is gene therapy. It treats diabetic cardiomyopathy by regulating the expression of certain heart genes in the body. For example, inhibition of the expression of the ubiquitin ligase E3 mitsugomin 3 improves insulin resistance by inhibiting the breakdown of the insulin receptor and IRS-1. In addition, the absence of heart-specific FoxO1 can rescue high-fat diet-induced cardiac dysfunction and insulin sensitivity [15]. New technologies for regulating oxidative stress involve substances such as sulforaphane(a compound that activates the Nrf2 transcription factor) to reduce ROS production in the blood vessels of diabetic mice, thereby alleviating cardiac remodeling and dysfunction. In addition, Coenzyme Q10 supplementation has also been shown to reduce heart inflammation, fibrosis and hypertrophy in diabetic patients [15].
MiRNA and lncRNA-based therapeutics may be used in future clinical applications, leveraging miRNAs’ multi-target nature to regulate key pathways. For example, changes in miR-1 and miR-133a in diabetic cardiomyopathy and their impact on cardiac function suggest that they might be potential targets for the treatment of this disease. In addition, upregulation of long non-coding RNAs (lncRNAs) such as MIAT is also related to the development of diabetes cardiomyopathy, and its downregulation may improve cardiac function [15].
4. Conclusion
This article reviews the mechanism, diagnosis and treatment of diabetic cardiomyopathy. It is an independent risk factor for heart failure in diabetic patients, and its mechanisms involve oxidative stress, mitochondrial dysfunction, inflammation, and immune response. Clinical manifestations include cardiac fibrosis and left ventricular remodeling, and diagnosis is based on clinical symptoms, laboratory tests, and imaging techniques. In terms of treatment, comprehensive management strategies are emphasized, including lifestyle modifications, the use of traditional cardiovascular drugs, and the potential benefits of newer glucose-lowering drugs, such as GLP-1 receptor agonists and SGLT2 inhibitors, in treating diabetic cardiomyopathy. Future biomarker research may open up new avenues for early diagnosis and treatment of the disease. At the same time, it provides a comprehensive overview of the research progress of diabetic cardiomyopathy, which offers guidance for clinical practice and future research directions.
Acknowledgment
Firstly, I want to sincerely express my gratitude to all the teachers and professors who participated in the nutrition themed group. They deeply helped me learn the basic knowledge about this paper, taught me how to write a paper, and provided detailed answers to my questions at every stage. I am really grateful to my family and friends for their support and encouragement. Without their support, I cannot complete this paper.
References
[1]. Rubler, S. , Dlugash, J. , Yuceoglu, Y. Z. , Kumral, T. , & Grishman, A. (1972). New type of cardiomyopathy associated with diabetic glomerulosclerosis. American Journal of Cardiology, 30(6), 595-602.
[2]. Cheng, X. M. , Hu, Y. Y. , Yang, T. , Wu, N. , & Wang, X. N. (2022). Reactive oxygen species and oxidative stress in vascular-related diseases. Oxidative medicine and cellular longevity, 2022, 7906091.
[3]. Gulsin, G. S. , Athithan, L. , McCann, G. P. (2019). Diabetic cardiomyopathy: prevalence, determinants and potential treatments. Ther Adv Endocrinol Metab. Mar, 10, 1-21.
[4]. Peng, H. , Gao, Y. Q. , Zeng, C. Y. , Hua, R. , Guo, Y. N. , Wang, Y. D. , Wang, Z. (2024). Effects of Maillard reaction and its product AGEs on aging and age-related diseases. Food Science and Human Wellness, 13(3), 1118-1134.
[5]. Sies, & Helmut. (2015). Oxidative stress: a concept in redox biology and medicine. Redox Biology, 4(C), 180-183.
[6]. Bhatti, J. S. , Bhatti, J. K. , & Reddy, P. H. (2017). Mitochondrial dysfunction and oxidative stress in metabolic disorders — A step towards mitochondria based therapeutic strategies. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1863(5), 1066-1077.
[7]. Betteridge, D. J. (2000). What is oxidative stress?. Metabolism-clinical & Experimental, 49(2), 3-8.
[8]. Cao, S. S. , & Kaufman, R. J. (2014). Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxidants & Redox Signaling, 21(3), 396-413.
[9]. Geerlings, S. E. , & Hoepelman, A. I. M. (1999). Immune dysfunction in patients with diabetes mellitus (dm). Fems Immunology & Medical Microbiology, 26(3-4), 259.
[10]. Donath, M. , & Shoelson, S. (2011). Type 2 diabetes as an inflammatory disease. Nature reviews. Immunology, 11(2), 98-107.
[11]. Daryabor, G. , Atashzar, M. R. , Kabelitz, D. , Kalantar, M. S. , & Kalantar, K. (2020). The effects of type 2 diabetes mellitus on organ metabolism and the immune system. Frontiers in Immunology, 11(July), 1582.
[12]. Hudish, L. I. , Reusch, J. E. B. , & Sussel, L. (2019). β cell dysfunction during progression of metabolic syndrome to type 2 diabetes. Journal of Clinical Investigation, 129(12).
[13]. Keane, K. N. , Cruzat, V. F. , Carlessi, R. , de Bittencourt PI Jr, Newsholme, P. (2015). Molecular events linking oxidative stress and inflammation to insulin resistance and β-cell dysfunction. Oxidative Medicine & Cellular Longevity, 2015, 181643.
[14]. Jia, G. , Hill, M. A. , & Sowers, J. R. (2018). Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circulation Research, 122(4), 624-638.
[15]. Borghetti, G, Dirk, V. L., Deborah, M.E., Harald, S, Steven, R. H., Markus, W., (2018). Diabetic cardiomyopathy: current and future therapies. Beyond glycemic control. Frontiers in physiology, 9, 1514.
Cite this article
You,L. (2024). Pathogenesis and Clinical Management of Diabetic Cardiomyopathy: a Review from Basic Research to Clinical Practice. Theoretical and Natural Science,72,1-7.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Rubler, S. , Dlugash, J. , Yuceoglu, Y. Z. , Kumral, T. , & Grishman, A. (1972). New type of cardiomyopathy associated with diabetic glomerulosclerosis. American Journal of Cardiology, 30(6), 595-602.
[2]. Cheng, X. M. , Hu, Y. Y. , Yang, T. , Wu, N. , & Wang, X. N. (2022). Reactive oxygen species and oxidative stress in vascular-related diseases. Oxidative medicine and cellular longevity, 2022, 7906091.
[3]. Gulsin, G. S. , Athithan, L. , McCann, G. P. (2019). Diabetic cardiomyopathy: prevalence, determinants and potential treatments. Ther Adv Endocrinol Metab. Mar, 10, 1-21.
[4]. Peng, H. , Gao, Y. Q. , Zeng, C. Y. , Hua, R. , Guo, Y. N. , Wang, Y. D. , Wang, Z. (2024). Effects of Maillard reaction and its product AGEs on aging and age-related diseases. Food Science and Human Wellness, 13(3), 1118-1134.
[5]. Sies, & Helmut. (2015). Oxidative stress: a concept in redox biology and medicine. Redox Biology, 4(C), 180-183.
[6]. Bhatti, J. S. , Bhatti, J. K. , & Reddy, P. H. (2017). Mitochondrial dysfunction and oxidative stress in metabolic disorders — A step towards mitochondria based therapeutic strategies. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1863(5), 1066-1077.
[7]. Betteridge, D. J. (2000). What is oxidative stress?. Metabolism-clinical & Experimental, 49(2), 3-8.
[8]. Cao, S. S. , & Kaufman, R. J. (2014). Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxidants & Redox Signaling, 21(3), 396-413.
[9]. Geerlings, S. E. , & Hoepelman, A. I. M. (1999). Immune dysfunction in patients with diabetes mellitus (dm). Fems Immunology & Medical Microbiology, 26(3-4), 259.
[10]. Donath, M. , & Shoelson, S. (2011). Type 2 diabetes as an inflammatory disease. Nature reviews. Immunology, 11(2), 98-107.
[11]. Daryabor, G. , Atashzar, M. R. , Kabelitz, D. , Kalantar, M. S. , & Kalantar, K. (2020). The effects of type 2 diabetes mellitus on organ metabolism and the immune system. Frontiers in Immunology, 11(July), 1582.
[12]. Hudish, L. I. , Reusch, J. E. B. , & Sussel, L. (2019). β cell dysfunction during progression of metabolic syndrome to type 2 diabetes. Journal of Clinical Investigation, 129(12).
[13]. Keane, K. N. , Cruzat, V. F. , Carlessi, R. , de Bittencourt PI Jr, Newsholme, P. (2015). Molecular events linking oxidative stress and inflammation to insulin resistance and β-cell dysfunction. Oxidative Medicine & Cellular Longevity, 2015, 181643.
[14]. Jia, G. , Hill, M. A. , & Sowers, J. R. (2018). Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circulation Research, 122(4), 624-638.
[15]. Borghetti, G, Dirk, V. L., Deborah, M.E., Harald, S, Steven, R. H., Markus, W., (2018). Diabetic cardiomyopathy: current and future therapies. Beyond glycemic control. Frontiers in physiology, 9, 1514.









