1. Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease that causes cognitive impairment and memory loss, characterised by β-amyloid (Aβ) and Phosphorylated-Tau protein (p-Tau) in the cerebral cortex [1]. In 2015, approximately 47 million people were estimated to have dementia, and it is speculated that this population will increase to over 130 million by 2050 [2]. Although AD is a prevalent dementia that is estimated to affect more than 50 million people worldwide, there are no efficient therapeutic drugs for patients.
The detailed mechanisms responsible for AD pathology and progression have yet to be fully understood, mainly due to the multiple risk factors involved, including genetics, age, and environmental influences [1]. Among these environmental risk factors, increasing evidence points to infection, cellular stress, and neuroinflammation as potential triggers for AD. For instance, neurotrophic viruses such as Zika virus (ZIKV), Japanese encephalitis virus (JEV), herpes simplex virus (HSV), and cytomegalovirus (CMV) can infect neurons, potentially damage the central nervous system and ultimately contribute to the development of AD [1]. The precise interaction between viral infections and the risk of developing AD has long been a subject of interest; however, direct causative effects could not be proven. The first and newest review in 2022, “Zika virus infection accelerates Alzheimer’s disease phenotypes in brain organoids”, provided an overview of the molecular mechanisms of ZIKV infection underlying AD. The study pointed out that ZIKV infection causes significant endoplasmic reticulum (ER) stress and perturbs ER-related pathways associated with apoptosis. It induces massive vacuolisation and activates the unfolded protein response (UPR) in infected cells, where studies have detected upregulated ER stress and UPR in ZIKV-infected regions of mouse embryos and human neural stem cells (NSCs) [1].
Further research study also illustrates ER stress has been increasingly linked to the pathogenesis of Alzheimer's disease (AD) [3]. The unfolded protein response (UPR), a cellular stress response related to the endoplasmic reticulum (ER), is crucial in managing protein folding and maintaining cellular homeostasis. When ER stress becomes chronic, it can lead to neurodegenerative conditions such as AD. In Alzheimer's, markers of ER stress and components of the UPR are often found to be upregulated, indicating a disrupted proteostasis network. Specifically, the PERK-eIF2α pathway, one of the significant UPR pathways, becomes hyperactivated under prolonged ER stress, decreasing overall protein synthesis and contributing to neurodegeneration [3,4]. Moreover, studies have shown that the PERK pathway's hyperactivation results in increased phosphorylation of eIF2α, which is observed in the brains of AD patients. This pathway has been implicated in both amyloid-beta (Aβ) production and tau phosphorylation, which are key pathological features of Alzheimer's disease. These findings suggest that targeting ER stress and its associated pathways might offer new therapeutic strategies for treating Alzheimer's disease.
Salubrinal, a selective inhibitor of eIF2α dephosphorylation, reportedly inhibits ER stress-induced apoptosis in neural cells [5]. It prevents the action of protein phosphatase 1 (PP1) on eIF2α, thereby maintaining eIF2α in its phosphorylated state. Phosphorylation of eIF2α is a critical step in the unfolded protein response (UPR) that helps mitigate ER stress. By keeping eIF2α phosphorylated, Salubrinal can reduce ER stress and its associated cellular damage. In new studies, rarely papers discuss the relation between those three components; thus, based on the finding above, I made a hypothesis that inhibiting eIF2α dephosphorylation can possibly alleviate AD pathology by observing any reduction in Aβ and Phosphorylated-Tau levels.
2. Experimental approach
This experiment is conducted at a preclinical level, utilizing animal models (C57BL/6 mice) to investigate the effects of a potential therapeutic intervention (Salubrinal, an eIF2α dephosphorylation inhibitor) on Alzheimer's disease pathology exacerbated by Zika virus infection.
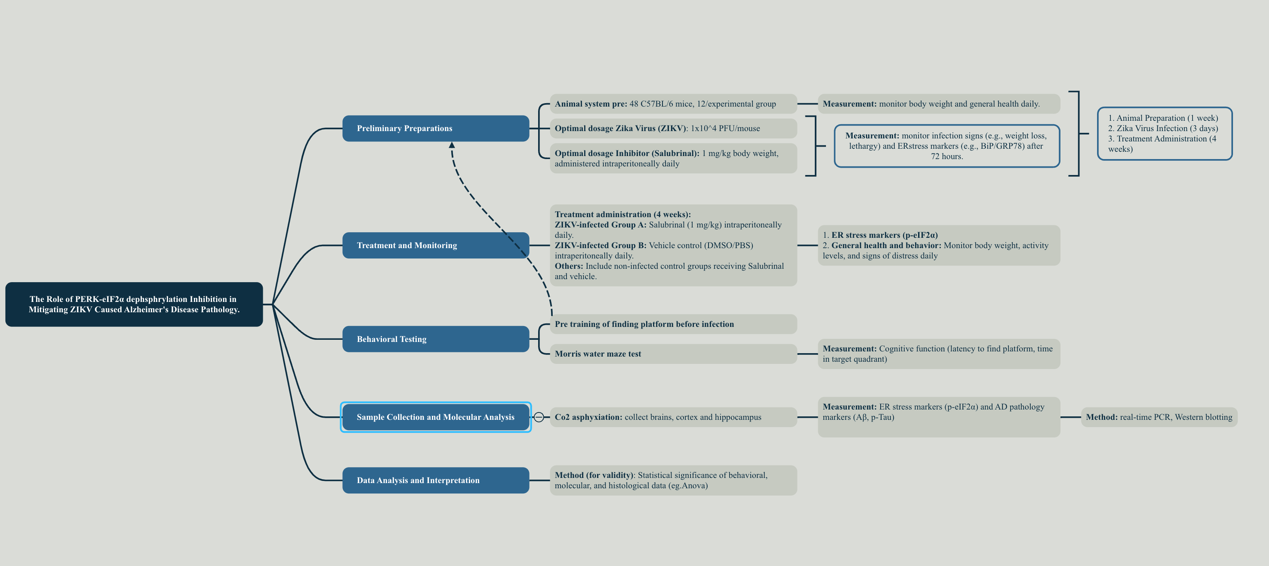
Figure 1. Technology roadmap of the experiment by Jiale Li (2024)
2.1. Experimental preparation for C57BL/6 mice of both gender (8-week-old) model
The choice to use C57BL/6 is because other gene development models for AD, such as APP and PSEN1, have been overexpressed or knocked into human APP genes that carry FAD-related mutations, so they are not good candidates for ZIKV infection [6]. However, C57BL/6, on the one hand, does not appear to have senile plaques or neurofibrillary tangles after ageing; on the other hand, it shows stronger local allergic inflammation responses. Thus, the pathological part will be more prominent after ZIKV infection.
Using both gendered adult mice (8-week-old) is another crucial aspect of the study design. At this age, mice are considered mature adults, which means their cognitive functions are fully developed, providing a stable baseline for assessing the effects of ZIKVinfection and subsequent treatments. Younger or older mice might introduce variability due to developmental changes or age-related decline, respectively. The reason for choosing both genders is to mitigate any potential confounding effects that sex-specific factors might introduce; for instance, hormonal differences between male and female mice could influence the progression of AD, resilience to ER stress and the response to treatment. We can account for these variables by studying both genders, ensuring that the data obtained reflects the broader population.
2.2. Preliminary for determining optimal ZIKV dose and Salubrinal dose
The optimal dosage for Zika virus (ZIKV) infection is typically within the range of 103-105 plaque-forming units (PFU) per mouse. In particular, the dosage of 104 PFU is commonly used in studies to induce significant ER stress and Alzheimer’s-like pathology without causing excessive mortality [7]. Additionally, each mouse would receive a 4-week infection and the ER stress and AD marker expression level should be monitored and recorded for future comparison [7].
For the inhibitor, each C57BL/6 mouse will receive a dose of 1 mg/kg of Salubrinal, which will be administered intraperitoneally daily for four weeks. This dosage is selected based on research demonstrating its efficacy in modulating eIF2α phosphorylation levels and mitigating ER stress without causing significant adverse effects. In studies such as those by Boyce et al. (2005), Salubrinal at this dose has effectively reduced ER stress markers and improved pathological outcomes in mouse models of neurodegenerative diseases, including Alzheimer’s disease [8]. Specifically, this dosage has been observed to lower levels of phosphorylated eIF2α (p-eIF2α) and reduce amyloid-beta (Aβ) and phosphorylated Tau (p-Tau) accumulation, which are critical markers of Alzheimer’s pathology.
2.3. Experimental grouping
The 40-48 C57BL/6 mice are divided into potentially 4 groups (ABCD), each containing 10-12 mice with gender proportion 1:1 (E.g.6 male, 6 female). First, Group A: ZIKV + Salubrinal will involve mice infected with Zika virus and treated with Salubrinal (1 mg/kg). This group is the primary experimental group to assess the effect of inhibiting eIF2α dephosphorylation on ER stress and AD pathology. Second, Group C: Salubrinal Only (Non-Infected Control) will consist of non-infected mice treated with Salubrinal (1 mg/kg), which assesses the effects of Salubrinal in the absence of ZIKV infection, ensuring that any observed changes in ER stress markers or AD pathology in Group A are specifically due to the interactions between the two. There could potentially be two more groups, B and D, that are treated by Vehicle paired with A and B (E.g. Group B: ZIKV + Vehicle (Control, PBS/DMSO)), which are used to establish a baseline response in the absence of the active treatment ensuring that any observed effects in the experimental groups (Groups A and B) can be explicitly attributed to the Salubrinal and not to the solvent or carrier substance used to administer the treatment. Thus, enhancing the validity of Data.
2.4. The therapeutic impact of Salubrinal on AD-like symptoms (Behavioral testing)
2.4.1. Apparatus
The Morris Water Maze test apparatus includes a circular tank with a diameter of 1.5 meters and a height of 60 centimeters, constructed from non-reflective, opaque material to prevent visual cues from outside the tank. The tank is filled with water at 24°C, made opaque with non-toxic white paint or powdered milk to minimize stress and consistent testing environment. A hidden platform, 10 centimeters in diameter and submerged 1-2 centimeters below the water surface, is placed in a fixed location within one quadrant of the maze. External visual cues, such as geometric shapes and patterns, are placed around the tank to aid the mice in navigation. An overhead camera connected to a computer with tracking software (e.g., EthoVision or ANY-maze) records and [9]
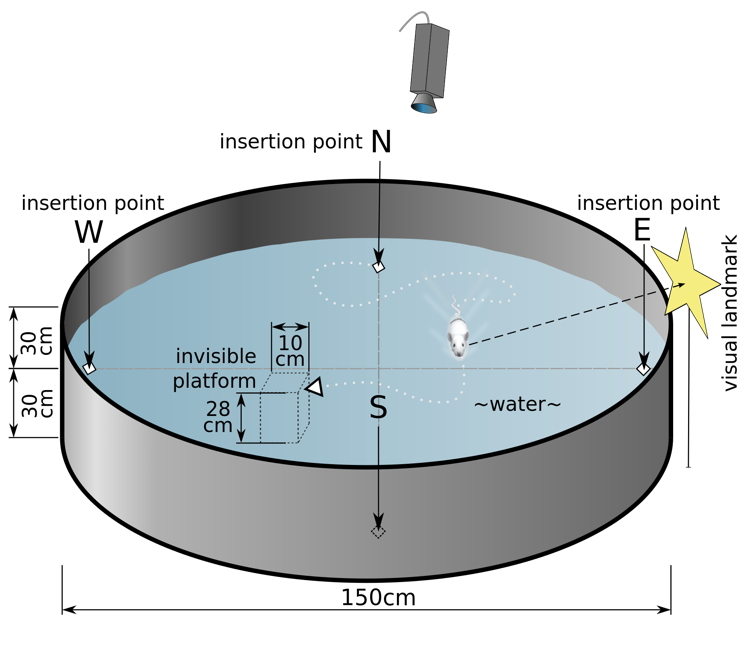
Figure 2. Schematic drawing of the Morris water navigation test for rats. Reproduced from Wikipedia by Samuel John (2010).
2.4.2. Training
The training phase of each mouse is necessary before actual behavioural testing, and the training can also let the mouse get familiar with the water maze apparatus, reducing any anxiety and stress during the experiment that might affect any results. The training procedure is partially adapted from Vorhees & Williams, 2006 [9]. The training phase involves habituation, where mice are placed in the tank without the platform for 1-2 minutes per day for two days to reduce stress. Over 4-5 days, each mouse undergoes 4-6 training trials per day, starting from different points to prevent bias. The mouse swims until it finds the hidden platform or for a maximum of 60 seconds; if unsuccessful, it is guided to the platform to learn its location.
2.4.3. Potential Probe trial measurements
The behavioural experiment would mainly monitor swim path length (cm), representing the distance travelled by moues, latency (s), and the time it takes to look for a hidden platform. Additionally, Qualitative assessment, such as search strategies, can be applied according to experimental demand (Spatial strategy and not spatial strategy).
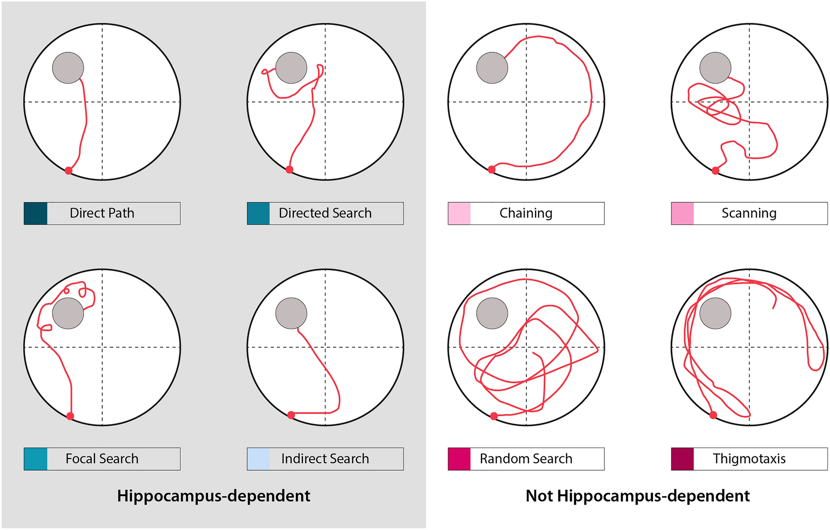
Figure 3. Representative examples of possible search strategies. Reproduced from [10]. Curdt, N., Schmitt, F.W., Bouter, C. et al. Search strategy analysis of Tg4-42 Alzheimer Mice in the Morris Water Maze reveals early spatial navigation deficits. Sci Rep 12, 5451 (2022).
2.5. Analysis in the Molecular Level indicating the effect of Inhibition of eIF2α on Alzheimer’s disease Pathology
Molecular analysis aims to measure the expression levels of ER stress and AD markers (Aβ and p-Tau) by observing changes in both mRNA and protein quantity, which would be extracted from the cortex and hippocampus of the mice using Qiagen RNeasy Kit and RIPA buffer, respectively. Then, real-time PCR will quantify mRNA levels of p-eIF2α, Aβ, and p-Tau using specific primers, while Western blotting will analyze protein levels of these markers with specific primary antibodies and HRP-conjugated secondary antibodies. Furthermore, Commercial ELISA (Enzyme-Linked Immunosorbent Assay) kits would be applied to measure the level of soluble and insoluble forms of Aβ and Tau again ensuring the treatment is effectively reducing the markers of AD.
3. Analysis and Predicted Outcomes
Based on my hypothesis that there may exist some effect that Salubrinal could have on ER stress and Alzheimer’s disease pathology, I made the following analysis and inference.
3.1. Expected result in MWM test
In the behavioural experiment (Morris water maze), Group A (ZIKV + Salubrinal) should exhibit reduced latency, shorter swim path lengths, and more efficient search strategies compared to Group B (ZIKV + Vehicle) because Salubrinal is anticipated to enhance learning and memory by alleviating P-eIF2α Dephosphorylation caused by ZIKV. Group A is also expected to spend significantly more time in the target quadrant during the probe trial, demonstrating better memory retention, with consistent swim speed ensuring cognitive improvements are not due to physical changes. In contrast, Group B should show higher latency, longer swim paths, and less efficient search strategies, reflecting impaired learning and memory due to ZIKV infection, as ER stress is likely to hinder cognitive function. Group C (Salubrinal Only) is anticipated to have similar results to Group D (Vehicle Only), suggesting Salubrinal does not negatively impact learning and memory in non-infected mice, thereby indicating its safety and lack of adverse effects on cognition. Group D is expected to establish a baseline for normal learning and memory capabilities with the lowest latency, shortest swim paths, and highest time in the target quadrant, as these mice are healthy and unaffected by ZIKV or any treatment.
3.2. Expected result in molecular analysis
The molecular analysis is expected to show a significant reduction in the mRNA and protein levels of p-eIF2α, Aβ, and p-Tau, approximately 30% to 50%, which indicates that Salubrinal inhibits ER stress and has a positive effect on AD pathology [3]. Besides, The ELISA test is predicted to show lower soluble and insoluble Aβ and Tau levels in Group A (ZIKV + Salubrinal) compared to Group B (ZIKV + Vehicle). Group C (Salubrinal Only) is expected to exhibit levels like Group D (Vehicle Only), indicating that Salubrinal does not adversely affect Aβ and Tau levels in non-infected mice.
4. Discussion
This study illustrated an experimental design in investigating that inhibiting eIF2α dephosphorylation with Salubrinal can mitigate Alzheimer's disease (AD) pathology exacerbated by Zika virus (ZIKV) infection. The behavioural results from the MWM test are expected to have significant cognitive improvements in the ZIKV + Salubrinal group compared to the ZIKV + Vehicle group, aligning with the hypothesis that Salubrinal alleviates P-eIF2α Dephosphorylation. Furthermore, on a molecular level, analysis is predicted to illustrate reduced levels of about 30%-50% of protein and mRNA in the markers Aβ, Tau and P-eIF2α, as well as levels of soluble and insoluble Aβ and Tau in Salubrinal-treated mice.
4.1. Limitations
However, the study did not explore the long-term effects of Salubrinal treatment. Chronic diseases like AD require long-term therapeutic strategies, and the short-term benefits observed in this study might not translate into sustained improvements over extended periods. Another critical aspect that was not addressed is the potential side effects of chronic Salubrinal treatment [11]. While the study did not observe any immediate adverse effects, longer exposure to the drug could have unforeseen consequences on C57BL/6. Salubrinal's mechanism of action involves the modulation of ER stress pathways, which are implicated in various cellular processes [12]. Therefore, Prolonged inhibition of eIF2α dephosphorylation could disrupt normal cellular functions and lead to adverse outcomes; in particular, it could cause an increased mortality rate during the preliminary stage. This means that comprehensive toxicological studies are needed to evaluate the safety profile of Salubrinal over extended periods and in various biological contexts. Additionally, the study should explore other potential impacts on the overall health and behaviour of the mice. For instance, changes in metabolism, immune response, or other physiological systems might occur with Salubrinal treatment, affecting the overall interpretation of its therapeutic potential. Moreover, the interaction between Salubrinal and other potential treatments or medications was not investigated. AD and its exacerbation by ZIKV are complex conditions that might require multi-faceted therapeutic approaches.
4.2. Implications
The findings of this study have significant implications for developing new therapeutic strategies for Alzheimer's disease (AD), particularly in cases where viral infections like Zika virus (ZIKV) exacerbate the disease. By demonstrating that Salubrinal can reduce both behavioural deficits and biochemical markers of AD, this research supports the targeting of ER stress pathways as a viable approach to mitigate neurodegeneration. Recent studies have shown that ER stress and the unfolded protein response (UPR) are crucial in the pathogenesis of neurodegenerative diseases, including AD [3,13]. These results suggest that therapies that maintain eIF2α phosphorylation may offer dual benefits of alleviating ER stress and reducing AD pathology [3,13], thus providing a novel direction for future AD treatments.
5. Conclusion
This study explores the therapeutic potential of inhibiting eIF2α dephosphorylation using Salubrinal to alleviate Alzheimer's disease (AD) pathology induced by Zika virus (ZIKV) infection. Through a combination of behavioural assessments using the MWM test and biochemical analyses via ELISA, it is expected to demonstrate that Salubrinal effectively mitigates cognitive impairments and reduces biochemical markers associated with AD. Specifically, treated mice showed improved learning and memory and decreased levels of soluble and insoluble Aβ and Tau proteins. These findings suggest that targeting the eIF2α pathway can counteract ER stress and neurodegenerative processes exacerbated by viral infections, offering a promising therapeutic strategy for conditions like AD exacerbated by ZIKV.
References
[1]. Lee, SE., Choi, H., Shin, N. et al. Zika virus infection accelerates Alzheimer’s disease phenotypes in brain organoids. Cell Death Discov. 8, 153 (2022). https://doi.org/10.1038/s41420-022-00958-x
[2]. Hashimoto, Shoko, and Takaomi C Saido. “Critical review: involvement of endoplasmic reticulum stress in the aetiology of Alzheimer's disease.” Open biology vol. 8, 4 (2018): 180024. doi:10.1098/rsob.180024
[3]. Hetz, C., Saxena, S. ER stress and the unfolded protein response in neurodegeneration. Nat Rev Neurol 13, 477–491 (2017). https://doi.org/10.1038/nrneurol.2017.99
[4]. Hetz, C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13, 89–102 (2012). https://doi.org/10.1038/nrm3270
[5]. Teng, Y., Gao, M., Wang, J. et al. Inhibition of eIF2α dephosphorylation enhances TRAIL-induced apoptosis in hepatoma cells. Cell Death Dis 5, e1060 (2014). https://doi.org/10.1038/cddis.2014.24
[6]. Yokoyama, Miyabishara et al. “Mouse Models of Alzheimer's Disease.” Frontiers in molecular neuroscience vol. 15 912995. 21 Jun. 2022, doi:10.3389/fnmol.2022.912995
[7]. Lazear, Helen M et al. “A Mouse Model of Zika Virus Pathogenesis.” Cell host & microbe vol. 19, 5 (2016): 720-30. doi:10.1016/j.chom.2016.03.010
[8]. Boyce, Michael et al. “A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress.” Science (New York, N.Y.) vol. 307, 5711 (2005): 935-9. doi:10.1126/science.1101902
[9]. Vorhees, C. V., & Williams, M. T. (2006). Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nature Protocols, 1(2), 848-858.
[10]. Curdt, N., Schmitt, F.W., Bouter, C. et al. Search strategy analysis of Tg4-42 Alzheimer Mice in the Morris Water Maze reveals early spatial navigation deficits. Sci Rep 12, 5451 (2022). https://doi.org/10.1038/s41598-022-09270-1
[11]. Moreno, J. A., et al. (2012). Sustained translational repression by eIF2α-P mediates prion neurodegeneration. Nature, 485(7399), 507-511.
[12]. Scheper, W., & Hoozemans, J. J. M. (2015). The unfolded protein response in neurodegenerative diseases: a neuropathological perspective. Acta Neuropathologica, 130(3), 315-331.
[13]. Salminen, A., Kauppinen, A., & Kaarniranta, K. (2013). ER stress in Alzheimer's disease: a novel neuronal trigger for inflammation and Alzheimer's pathology. Journal of Neuroinflammation, 10, 51.
Cite this article
Li,J. (2025). The Role of Endoplasmic Reticulum Stress Inhibition in Mitigating Zika Virus Caused Alzheimer's Disease Pathology. Theoretical and Natural Science,72,177-183.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Lee, SE., Choi, H., Shin, N. et al. Zika virus infection accelerates Alzheimer’s disease phenotypes in brain organoids. Cell Death Discov. 8, 153 (2022). https://doi.org/10.1038/s41420-022-00958-x
[2]. Hashimoto, Shoko, and Takaomi C Saido. “Critical review: involvement of endoplasmic reticulum stress in the aetiology of Alzheimer's disease.” Open biology vol. 8, 4 (2018): 180024. doi:10.1098/rsob.180024
[3]. Hetz, C., Saxena, S. ER stress and the unfolded protein response in neurodegeneration. Nat Rev Neurol 13, 477–491 (2017). https://doi.org/10.1038/nrneurol.2017.99
[4]. Hetz, C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13, 89–102 (2012). https://doi.org/10.1038/nrm3270
[5]. Teng, Y., Gao, M., Wang, J. et al. Inhibition of eIF2α dephosphorylation enhances TRAIL-induced apoptosis in hepatoma cells. Cell Death Dis 5, e1060 (2014). https://doi.org/10.1038/cddis.2014.24
[6]. Yokoyama, Miyabishara et al. “Mouse Models of Alzheimer's Disease.” Frontiers in molecular neuroscience vol. 15 912995. 21 Jun. 2022, doi:10.3389/fnmol.2022.912995
[7]. Lazear, Helen M et al. “A Mouse Model of Zika Virus Pathogenesis.” Cell host & microbe vol. 19, 5 (2016): 720-30. doi:10.1016/j.chom.2016.03.010
[8]. Boyce, Michael et al. “A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress.” Science (New York, N.Y.) vol. 307, 5711 (2005): 935-9. doi:10.1126/science.1101902
[9]. Vorhees, C. V., & Williams, M. T. (2006). Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nature Protocols, 1(2), 848-858.
[10]. Curdt, N., Schmitt, F.W., Bouter, C. et al. Search strategy analysis of Tg4-42 Alzheimer Mice in the Morris Water Maze reveals early spatial navigation deficits. Sci Rep 12, 5451 (2022). https://doi.org/10.1038/s41598-022-09270-1
[11]. Moreno, J. A., et al. (2012). Sustained translational repression by eIF2α-P mediates prion neurodegeneration. Nature, 485(7399), 507-511.
[12]. Scheper, W., & Hoozemans, J. J. M. (2015). The unfolded protein response in neurodegenerative diseases: a neuropathological perspective. Acta Neuropathologica, 130(3), 315-331.
[13]. Salminen, A., Kauppinen, A., & Kaarniranta, K. (2013). ER stress in Alzheimer's disease: a novel neuronal trigger for inflammation and Alzheimer's pathology. Journal of Neuroinflammation, 10, 51.









