1. Introduction
As transportation technology and advanced biotechnology are continually evolving and being applied to extending the time before food spoils, food storage has become the most pressing issue in the food supply chain. Farmers and scientists have already implemented techniques such as gene editing and grafting. However, the unpredictability of the outcomes and the length of time make these methods susceptible to failure. Customers abhorred the practice of harvesting fruits from trees and stems before they are ready since the unripe flavor makes for an unpleasant eating experience, especially for one fruit — the pear.
Pear, the experimental subject of our study, is a globally popular fruit. According to indexbox.io, the global output of pears reaches 29.2 billion dollars and exceeds 25 million tons. The corruption of pears causes a significant drop in the gross domestic product and the product's value [1]. In China, 20 to 40 percent of pears have lost value due to corruption, amounting to more than $3.5 billion. In comparison, the overall economic impact of planting is typically less than 400 billion dollars. Due to the importance of pear production to the economy, it is imperative that this issue be resolved as soon as possible. In addition, we are going to experiment with grape, whose output and profitability are significantly impacted by corruption.
Pear quality is maintained by its own ripening and senescence, as well as by the decay produced by pathogenic bacteria [2]; these factors are among those that contribute to the rot of fruits. Therefore, the in-depth and systematic study on the regulation mechanism of fruit ripening and senescence and the pathogenic mechanism of pathogenic bacteria is not only of great significance to enrich the biological knowledge of fruits after harvest, but also lays the theoretical foundation for the development of new technologies for disease prevention and preservation, reducing fruit loss after harvest, and ensuring the high-quality and safe quality of fruits [3]. Diseases caused by fungi have caused enormous losses in agricultural production throughout the world. There are comparatively few studies on biological illness prevention and control. Use of chemical agents, such as Bordeaux liquid, carbendazim, tobuzin, phenate, etc., constitutes the majority of prevention and control measures in production. Calcium chloride is also an effective preservation reagent. In addition to its own sterilizing function, it can regulate the opening and shutting of pores on the surface of pear fruit and is resistant to the immersion of dangerous microorganisms. Pear storage problems such as anthracnose, penicillium, gray mold, and brown rot have been combated with these methods. Additionally, fungicides are utilized to control pear scab and pear ring rot. However, as dangerous diseases continue to evolve, chemical pesticides are used increasingly frequently. Although they have played a significant role in disease prevention, they have also wreaked havoc on the environment, and pathogenic fungi may evolve antibodies to these pesticides, rendering them incapable of eradicating disease. Among the physical measures used to prevent and control physiological disorders, ozone and radiation can also harm the surface fungi of pears. However, these physical methods are environment-dependent and in some respects dependent on chance.
To prevent and avoid the listed problems, we will use bacillus sp. 22T during our experiment to deactivate or retard penicillium expansum in pear and grape. By doing so, bacillus sp. 22T can successfully suppress penicillium expansum-caused pear disease or mildew.
2. Concept
Bacillus-produced antibacterial compounds can prevent and control a variety of plant diseases [4]. A number of bacillus biocontrol strains have been commercialized or have gained limited commercial manufacturing and application licences. During its creation, Bacillus Thuringiensis can build a parasporal crystal that has become the largest microbial pesticide [5]. Some Bacillus Paraspora strains are also capable of producing crystal proteins and enzymes that are poisonous to invertebrates. The biological activities of Bacillus, such as phosphorus, potassium, and nitrogen fixation, are beneficial to crop production enhancement. Additionally, bacillus has excellent stress resistance. Bacillus Megaterium, Bacillus Gelatinosus, Bacillus Nitrogenus, Bacillus Sphaericus, and Bacillus brevis lateralis are utilized extensively in the production of biological fertilizers. Bacillus produces antibacterial compounds that promote growth, health care, and illness treatment in cattle and poultry. It has no hazardous side effects, leaves no trace, and does not promote bacterial resistance. The bacillus-produced antimicrobial peptides have a particularly distinct bactericidal action. It is difficult for pathogens to develop resistance to antimicrobial peptides [6].
Bacillus produces antibacterial compounds with broad-spectrum bactericidal activity, significant bactericidal effect on a variety of food-related gram-negative and positive bacteria, and excellent thermal stability. They can be utilized to prevent contamination by other bacteria during the heat processing of food, recontamination after pasteurization, and contamination by other bacteria during the fermentation of food [7].
Antibacterial substances produced by bacillus typically have a broad antibacterial spectrum and can kill bacteria including drug-resistant strains, fungi, parasites, viruses, tumor cells, etc., in addition to being able to bind lipopolysaccharide and neutralize endotoxin, etc., which has attracted the attention of scientists and medical professionals. Bacillus-produced microecological agents have a crucial role in the treatment of intestinal flora abnormalities, treatment of candida infection, and avoidance of sore face infection, among other medical processes [8].
3. Methodology
3.1. Gram staining
Gram staining, a form of differential staining, is commonly employed in bacteriology [9]. A water-immiscible crystal violet and iodine complex would form in the cell wall of Gram-Positive bacteria following crystal violet primary dyeing and iodine solution mordant dyeing. Due to its thick cell wall, several layers of peptidoglycan nets, and dense cross-linking peptidoglycan nets, it would shrink due to water loss when decolorized with ethanol or acetone [10]. In addition, it would remain purple when the crystal violet and iodine combination is almost entirely retained in the cell wall due to the absence of lipids and the absence of fissures during the ethanol treatment. Gram-Negative bacteria have a thin cell wall, a high concentration of lipids in the outer membrane layer, a thin peptidoglycan layer, and a low degree of crosslinking, which causes the outer membrane layer to disintegrate fast in the presence of a decolorizer. Under these conditions, the loose peptidoglycan network is unable to block the breakdown of the crystal violet and iodine complex, hence Gram-negative bacteria remain colorless after ethanol decolorization. After being restained with red dyes such as sand yellow, Gram-negative bacteria would turn red.
In general, Gram staining involves four steps: primary dyeing, mordant dyeing, decolorization, and re-dyeing [10]. In our experiment, we adjusted timing in order to achieve our objectives. We added crystal violet solution, iodine solution, 95% ethanol, and safranine liquid in chronological order. With them, we dye or decolorize bacillus sp. 22T for one minute, with the exception of 95% ethanol, which takes only thirty seconds. After drying, we visually inspected the color of the bacteria.
Gram staining requires tight control over the degree of decolorization. Gram-Positive bacteria may be misidentified as Gram-Negative bacteria if an excessive amount of decolorization is performed. Gram-Negative bacteria may be mistaken for Gram-Positive bacteria if a negligible amount of decolorization is employed. In addition, the age of bacteria influences the staining outcome. Gram-Positive bacteria would yield a Gram-Negative result if they were cultured for too long, if they partially self-disintegrated, or if they died.
3.2. Design and testing
Slicing observation of penicillium expansum. To observe the cultured penicillium expansum, we devised a series of protocols to examine the degree of cultivation in greater detail. We placed a drop of emulsifiable phenol oil on a clean microscope slide. We suspend a tiny number of bacteria in emulsifiable phenol oil using bits of culture material placed between the colony's center and periphery. We next cover the cover glass with care and remove any surplus emulsifiable phenol oil using a rag. We observed the thickness of mycelium and the shape of the conidial head of bacteria using an optical microscope. With a high-powered microscope, the morphology, size, and surface properties of conidia were also studied.
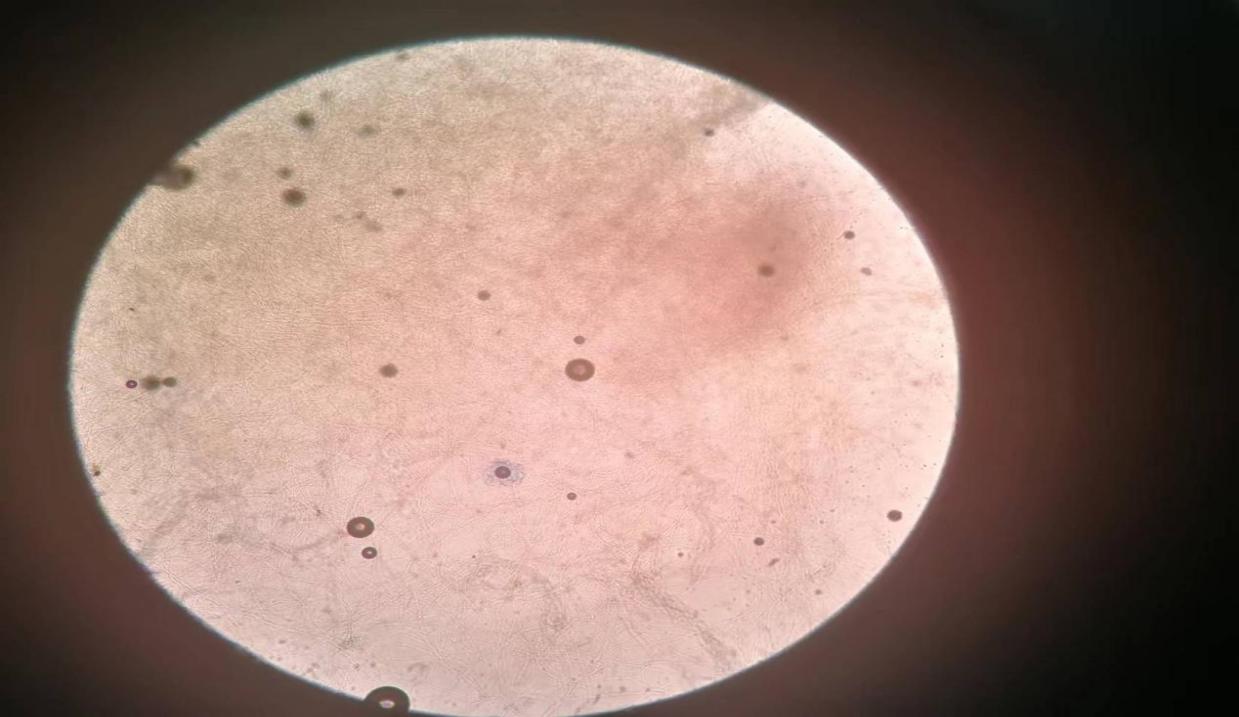
Figure 1. Penicillium expansum under 10x eye lens and 10x objective lens (original).
Gram staining testing. Gram Staining was performed on the bacteria to determine whether bacillus sp. 22T is Gram-Positive or Gram-Negative. Gram staining typically consists of four steps: primary dyeing, mordant dyeing, decolorization, and re-dying.
The specific ways of operation are as follows: (1) Slicing: spread, dry, and fix the bacterial culture. (2) Primary dyeing: dribble crystal violet over the mycoderm for 1 minute and then rinse the slice with ddH2O. (3) Mordant dyeing: remove any remaining water with iodine solution, cover the fabric with drops of iodine solution for approximately one minute, and then rinse with ddH2O; (4) Decolorization: remove any remaining water on the glass slide with filter paper, tilt the glass slide and decolorize it with 95% ethanol dripping from a dropper under a white background until the effluent ethanol is no longer visibly purple, and then quickly wash it with ddH2O. The duration of decolorization is 30 seconds. (5) Re-dyeing: re-dye the slice with a safranine solution for approximately one minute and rinse with ddH2O. (6) Microscopic examination: see with an oil lens after drying. Between blue and purple-colored bacteria should be Gram-positive bacteria, while red-colored bacteria should be Gram-negative bacteria.
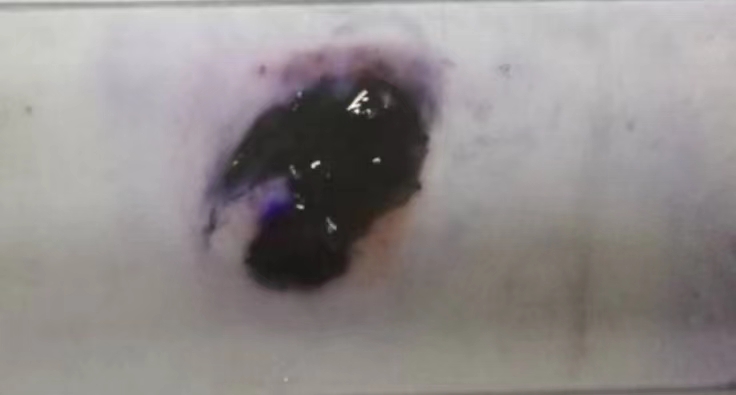
Figure 2. Bacillus sp. 22T after Gram Staining (original).
4. Results and analysis
4.1. In vitro bacteriostasis test
We conducted a bacteriostasis test to determine the degree of our bacillus sp. 22T's bacteriostasis capacity. Under sterile conditions, a 100 ul suspension of penicillium expansum mold spores is uniformly distributed across nine PDA culture media. Using pre-sterilized tweezers, we placed four 5 mm-diameter filter papers on each culture medium. We deposited 3 ul of fermentation broth of bacillus sp. 22T, 3 ul of supernatant of bacillus sp. 22T, 3 ul of bacteria suspension of bacillus sp. 22T, and 3 ul of stroke-physiological saline solution onto filter paper in one culture medium. After two hours, we used parafilm to seal the containers. After 48 hours, the diameters of the bacteriostasis circles are measured. Figure 3 is displayed below. The upper left filter contains supernatant; the upper right one contains bacteria suspension; the lower left one contains stroke-physiological saline solution; the lower right one contains fermentation broth.
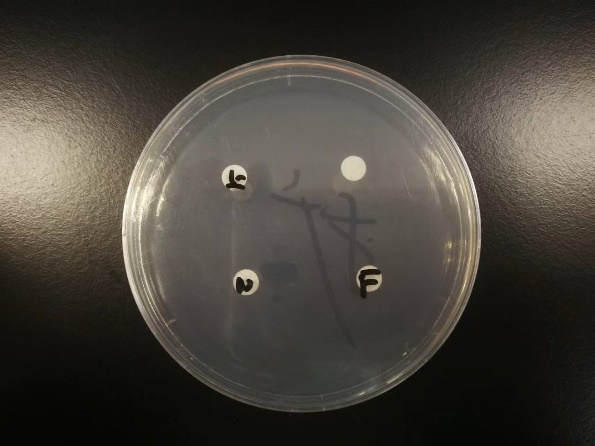
Figure 3. Bacteriostasis circle test (original).
With the data collected, we recorded them in the table 1:
Table 1. Diameter of bacteriostasis circle at different time (original).
Bacteriostasis circle of samples (cm) | Stroke-physiological saline solution | 22T fermentation broth | 22T supernatant | 22T bacteria suspension |
Sample 1 | 0.50 | 2.20 | 1.00 | 2.50 |
Sample 2 | 0.50 | 2.00 | 1.00 | 2.00 |
Sample 3 | 0.50 | 2.30 | 1.90 | 2.10 |
Sample 4 | 0.50 | 2.30 | 2.00 | 2.40 |
Mean | 0.50 | 2.25 | 1.48 | 2.25 |
Standard Deviation | 0.00 | 0.21 | 0.55 | 0.24 |
4.2. In vivo bacteriostasis test
In order to evaluate the bacteriostasis capacity of bacillus sp. 22T, we conducted an in vivo bacteriostasis test against penicillium expansum on pear and grape, which allowed us to compare the antibacterial potential of various fruits. 18 pears and 12 grapes are tested in the experiment.
After cleaning and sterilizing the pears and grapes with 0.2% sodium chlorite concentration, we utilized a surface-sterilized puncher to create holes in the fruits. For pears, we drilled four 3 mm by 1.5 mm holes. For grapes, the stems were carefully removed and the stem holes were used to implant the bacteria. To assess their bacteriostasis ability, we inoculated 20 ul of LB liquid culture medium, 20 ul of bacillus sp. 22T fermentation broth, 20 ul of bacillus sp. 22T supernatant, and 20 ul of bacillus sp. 22T suspension into different pear holes. After 2 hours, 20 ul of a penicillium expansum mold spore suspension was applied to the holes. In these grape holes, we added identical solutions, but with a volume of 10 ul. The fruit was then held at ambient temperature after being sealed with PE plastic film for a moisturizing treatment (relative humidity of about 90%). After 48 hours, the morbidity rate of fruit holes and the width of disease spots were regularly inspected and measured every 24 hours. With the data collected, we recorded them in table 2-6.
Table 2. Morbidity rate of in vivo bacteriostasis test of pear (original).
Morbidity Rate (%) | 0h | 48h | 72h | 96h |
Stroke-physiological saline solution | 0% | 50% | 83.33% | 100% |
22T fermentation broth | 0% | 0% | 33.33% | 66.67% |
22T supernatant | 0% | 50% | 66.67% | 83.33% |
22T bacteria suspension | 0% | 33.33% | 50% | 100% |
Table 3. Morbidity number of in vivo bacteriostasis test of pear (original).
Morbidity number | 0h | 48h | 72h | 96h |
Stroke-physiological saline solution | 0 | 9 | 15 | 18 |
22T fermentation broth | 0 | 0 | 6 | 12 |
22T supernatant | 0 | 9 | 12 | 15 |
22T bacteria suspension | 0 | 6 | 9 | 18 |
Table 4. Diameter of disease spots of in vivo bacteriostasis test of pear (original).
Diameter of Disease Spots (cm) | 0h | 48h | 72h | 96h |
Stroke-physiological saline solution | 0 | 1.44 | 1.61 | 2.09 |
22T fermentation broth | 0 | 0.77 | 0.91 | 1.21 |
22T supernatant | 0 | 1.23 | 1.34 | 1.54 |
22T bacteria suspension | 0 | 1.12 | 1.18 | 1.30 |
Table 5. Morbidity rate of in vivo bacteriostasis test of grape (original).
Morbidity Rate (%) | 0h | 48h | 72h | 96h |
Stroke-physiological saline solution | 0% | 66.67% | 100% | 100% |
22T fermentation broth | 0% | 33.33% | 50% | 83.33% |
22T supernatant | 0% | 66.67% | 83.33% | 100% |
22T bacteria suspension | 0% | 50% | 66.67% | 100% |
Table 6. Morbidity number of in vivo bacteriostasis test of grape (original).
Morbidity Number | 0h | 48h | 72h | 96h |
Stroke-physiological saline solution | 0 | 8 | 12 | 12 |
22T fermentation broth | 0 | 4 | 6 | 10 |
22T supernatant | 0 | 8 | 10 | 12 |
22T bacteria suspension | 0 | 6 | 8 | 12 |
Upon examination of the results after 48 hours, neither pears nor grapes exhibited morbidity in any of the samples examined. After 72 hours, the morbidity rate of pears infected with 22T fermentation broth and 22T bacteria suspension was no larger than 50%. Even after 96 hours, or four days, 33.3% and 16.6% of pear and grape samples inoculated with bacillus 22T fermentation broth remained unaffected by penicillium expansum.
5. Conclusion
By introducing bacillus sp. 22T to inhibit the growth and expansion of penicillium expansum, its bacteriostasis capacity shown a significant favorable effect. According to the results and data, bacillus sp. 22T fermentation broth has a powerful ability to inhibit the growth of penicillium expansum. Pears and grapes inoculated with bacillus sp. 22T fermentation broth were roughly fifty percent resistant to penicillium expansum after forty-eight hours, as shown in the tables. In this instance, it may be determined that bacillus sp. 22T fermentation broth is a successful way of bacteriostasis. In addition, the in vitro bacteriostasis tests yielded satisfactory findings, allowing the experiment to be deemed a success.
Due to the universality of bacillus sp. 22T's bacteriostasis capacity, we anticipate introducing it into grapes in the future to tackle the botrytis cinerea problem. By doing so, it is probable that we will find that bacillus sp. 22T is most successful in suppressing certain bacteria that are crucial factors in lowering the value of fruits, so that ripening of fruits would no longer be a significant problem affecting the industrial chain of fruits.
In contrast, the findings of the bacteriostasis test suggested that bacillus sp. 22T was more effective on pear than on grape. Only 16.6 percent of grapes, compared to 33.33 percent of pears, were not impacted by penicillium expansum after 96 hours. Bacillus sp. 22T has not only showed an enormous future contribution, but also a stronger ability to combat bacteria on pear than on grape.
References
[1]. Zhang, S; Ma, M; Zhang, H; Wang, L; et al. Genome-wide analysis of polygalacturonase gene family from pear genome and identification of the member involved in pear softening. BMC Plant Biol. 2019-12-27;19(1):587.
[2]. Cao, GT; Zhan, XA; Zhang, LL; Yang, CM; et al. Modulation of broilers' caecal microflora and metabolites in response to a potential probiotic Bacillus amyloliquefaciens. J ANIM PHYSIOL AN N. 2018-04-01;102(2):e909-e917.
[3]. Matichenkov, V; Bocharnikova, E; Romanova, A; Doullet, P. Growth of Bacillus amyloliquefaciens as influence by Si nutrition. ARCH MICROBIOL. 2021-09-01;203(7):4329-4336.
[4]. Evrendilek, GA; Tok, FM; Soylu, EM; Soylu, S; Inactivation of Penicillum expansum in sour cherry juice, peach and apricot nectars by pulsed electric fields. FOOD MICROBIOL. 2008-08-01;25(5):662-7.
[5]. Stöcklein, W; Sztajer, H; Menge, U; Schmid, RD; Purification and properties of a lipase from Penicillium expansum. BIOCHIM BIOPHYS ACTA. 1993-06-12;1168(2):181-9.
[6]. Zhang, Z; Yang, J; Xu, L; Yan, Y; et al. Cloning, codon optimization and expression of mature lipase gene Penicillium expansum. Wei Sheng Wu Xue Bao. 2010-02-01;50(2):228-35.
[7]. Liu, T; Zhu, L; Zhang, Z; Huang, H; et al. Draft genome sequence of Bacillus sp. M13(2017), a multidrug-resistant subclass B1 blaNDM-producing, spore-forming bacterium isolated from China. J GLOB ANTIMICROB RE. 2018-09-01;14:152-153.
[8]. Sivaramaiah, N; Malik, DK; Sindhu, SS; Improvement in symbiotic efficiency of chickpea (Cicer arietinum) by coinoculation of Bacillus strains with Mesorhizobium sp. Cicer. INDIAN J MICROBIOL. 2007-03-01;47(1):51-6.
[9]. Coico, R; Gram staining. Curr Protoc Microbiol. 2005-10-01;Appendix 3:Appendix 3C.
[10]. Wouthuyzen-Bakker, M; Shohat, N; Sebillotte, M; Soriano, A; et al. Is Gram staining still useful in prosthetic joint infections? J Bone Jt Infect. 2019-01-29;4(2):56-59.
Cite this article
Su,Z. (2023). Bacteriostasis of bacillus sp. 22T against penicillium expansum and comparison between its ability on pear and grape. Theoretical and Natural Science,6,21-28.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the International Conference on Modern Medicine and Global Health (ICMMGH 2023)
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Zhang, S; Ma, M; Zhang, H; Wang, L; et al. Genome-wide analysis of polygalacturonase gene family from pear genome and identification of the member involved in pear softening. BMC Plant Biol. 2019-12-27;19(1):587.
[2]. Cao, GT; Zhan, XA; Zhang, LL; Yang, CM; et al. Modulation of broilers' caecal microflora and metabolites in response to a potential probiotic Bacillus amyloliquefaciens. J ANIM PHYSIOL AN N. 2018-04-01;102(2):e909-e917.
[3]. Matichenkov, V; Bocharnikova, E; Romanova, A; Doullet, P. Growth of Bacillus amyloliquefaciens as influence by Si nutrition. ARCH MICROBIOL. 2021-09-01;203(7):4329-4336.
[4]. Evrendilek, GA; Tok, FM; Soylu, EM; Soylu, S; Inactivation of Penicillum expansum in sour cherry juice, peach and apricot nectars by pulsed electric fields. FOOD MICROBIOL. 2008-08-01;25(5):662-7.
[5]. Stöcklein, W; Sztajer, H; Menge, U; Schmid, RD; Purification and properties of a lipase from Penicillium expansum. BIOCHIM BIOPHYS ACTA. 1993-06-12;1168(2):181-9.
[6]. Zhang, Z; Yang, J; Xu, L; Yan, Y; et al. Cloning, codon optimization and expression of mature lipase gene Penicillium expansum. Wei Sheng Wu Xue Bao. 2010-02-01;50(2):228-35.
[7]. Liu, T; Zhu, L; Zhang, Z; Huang, H; et al. Draft genome sequence of Bacillus sp. M13(2017), a multidrug-resistant subclass B1 blaNDM-producing, spore-forming bacterium isolated from China. J GLOB ANTIMICROB RE. 2018-09-01;14:152-153.
[8]. Sivaramaiah, N; Malik, DK; Sindhu, SS; Improvement in symbiotic efficiency of chickpea (Cicer arietinum) by coinoculation of Bacillus strains with Mesorhizobium sp. Cicer. INDIAN J MICROBIOL. 2007-03-01;47(1):51-6.
[9]. Coico, R; Gram staining. Curr Protoc Microbiol. 2005-10-01;Appendix 3:Appendix 3C.
[10]. Wouthuyzen-Bakker, M; Shohat, N; Sebillotte, M; Soriano, A; et al. Is Gram staining still useful in prosthetic joint infections? J Bone Jt Infect. 2019-01-29;4(2):56-59.









