1.Introduction
Biomedical metal materials are closely related to human life activities and health needs and have a long history of application as orthopedic implants [1, 2]. Metallic orthopedic implants have been widely used to replace and regenerate damaged hard tissues due to their superior mechanical strength and toughness compared to polymers or polymer-ceramic composites [3, 4]. Metals have long dominated orthopedic surgery, contributing significantly to the majority of orthopedic de-vices, including both temporary ones like bone plates, pins, and screws and permanent ones like complete joint re-placements. Modern biomedical applications require the use of biomedical alloys, which account for nearly 80% of all materials used in bioimplants [5]. As orthopedic implants, medical alloy materials serve in the complex environment of body fluids, so the material properties of implants are very strict. Due to their high specific strength and adequate Young's modulus, natural degradability, good biocompatibility, and osteopromoting qualities, magnesium and its alloys are regarded as a breakthrough biomaterial [6, 7]. At present, Mg-based alloy orthopedic implants can be divided into two categories: (1) Bone fixation devices mainly include bone screws, bone needles, bone plates, etc., which perform a stabilizing effect in the healing of bones; (2) Magnesium and related alloys are also considered viable scaffold materials for bone tissue engineering transplants of autologous osteoblasts, bone marrow stromal cells, or chondrocytes [8, 9].
In addition, the plasticity, stiffness and surface treatment of magnesium alloy are also satisfactory, and the process and sterilization are easy to control [10, 11]. Conventional nondegradable biometals need to be surgically removed a second time, which frequently causes the patient more agony and financial hardship. However, magnesium and its alloys have attractive breakdown characteristics and can totally disintegrate in vivo [12]. The majority of common surgical alloys (such those made of cobalt, chromium, and nickel) include corrosion byproducts that could potentially injure human tissues [13, 14]. Degradation products of Mg-based alloy implants are mainly magnesium ions, which have no obvious toxicity to human tissues [15]. Additionally, Mg transporter 1 (MagT1) and transient receptor potential cation channel subfamily member 7 (TRPM7), which facilitate the release of calcitonin gene-related peptide (CGRP), mediate the release of magnesium ions [16]. When cyclic AMP binds to the response element-binding protein of the released CGRP, osterix substantially rises and new bone formation in the periosteal region is stimulated [17].
Millions of individuals suffer from bone defects brought on by natural illnesses and unintentional traumas, and as the population ages, life expectancy increases, and the urban environment deteriorates, bone defect therapy has become a significant therapeutic endeavor [18]. For age-related bone illnesses, such as osteoporotic fractures, there is an increasing need for cutting-edge clinical orthopedic implants [19]. Meanwhile, patients' expectations for medical prognosis continue to increase, which promotes the continuous development of medical technology and medical consumables. According to a survey by Allied Market Research, the market for orthopedic implants worldwide reached $47.261 billion in 2016 and is projected to reach $74.796 billion by 2023 [20]. On the whole, Mg and its alloys are one of the important development directions of orthopedic implant materials in the future, which is worthy of further research and clinical application and has great market potential.
An increasingly used statistical method known as bibliometrics may track the general direction of research in a certain topic [21]. One of the primary bibliometrics methods is citation analysis, and the quantity of citations to a given work is typically seen as a measure of its impact and worth [22]. Significant new discoveries and trends in a scientific subject can be found in highly cited works [23]. To our current understanding, magnesium alloy orthopedic implants have not been the subject of a thorough biblio-metric analysis. The top 100 highly cited publications on magnesium alloy orthopedic implants that were indexed by the Web of Science (WoS) were analyzed bibliometrically and visually by our team. We wish to provide researchers with research hotspots and to discuss the existing status and new initiatives in magnesium alloy orthopedic implants. These instructions, which are structured in the manner of a submission, outline the ideal Microsoft Word layout for your work. utilize the following page setup measures if you don't want to utilize the provided Word template.
2.Materials and methods
As shown in Figure 1, using the specific search function with the restrictions of "English" and "Articles or Review Articles" as the document type, we were able to obtain pertinent articles from the WoS Core Collection databases on November 20, 2022.The articles were found using the terms TS=(Magnesium or Mg) AND TS=(alloy) AND (TS=(orthopedic) OR TS=(Orthopaedic) OR TS=(bone)) AND TS=(implant). After sorting the search results by the quantity of citations, the top 100 papers were analyzed. The full texts or abstracts were evaluated independently by two reviewers (T.B. and J. W.) to determine the top 100 referenced articles on magnesium alloy orthopedic implants. When the two reviewers couldn't agree, a third reviewer (Y.C.) jumped in and helped forge a consensus.
Bibliometric analysis can swiftly comprehend the fundamental knowledge and research trends in a certain topic in addition to exploring the characteristics, structure, and evolution of academic literature. A bibliometric analysis often includes the evaluation of citations, journals, authors, nations, institutions, and keywords [24]. The journal impact factors (IF) were obtained from the “Journal Citation Reports (JCR)©(2021)”[25]. Moreover, Microsoft Excel and VosViewer were used for data mining, mapping, and visualization of the network analyses [26].
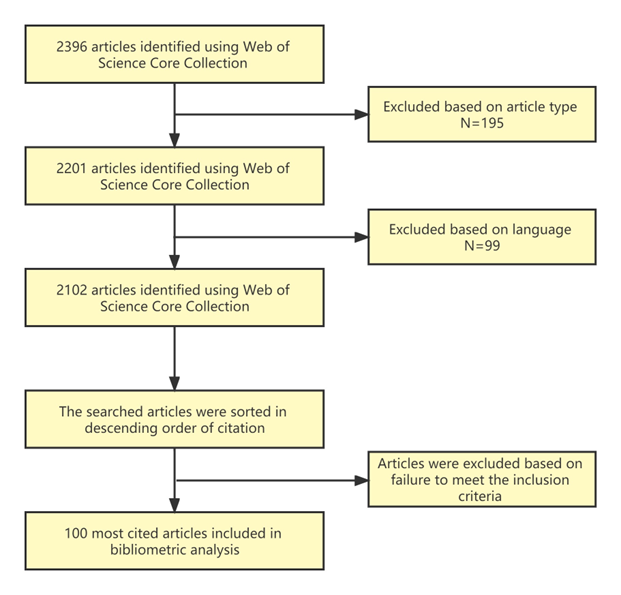
Figure 1. Flowchart of the methodology for identifying the top 100 cited articles.
3.Results and Discussion
Using the aforementioned method, a total of 2396 documents were obtained from the WoS Core Collection database. After narrowing our search to only original research articles and reviews in English, we eventually found 2102 publications. The top 100 articles with the most citations based on the inclusion criteria were our last focus. The top 100 most cited articles on magnesium alloy orthopedic implant research are presented in Table A1.
3.1.Temporal and spatial analysis
The publication year was from 2005 to 2020, and 2013 and 2016 were the most productive, with 12 papers published respectively. The number of published articles in 2013 and 2016 ranked second, with 11 articles each. Figure 2 displays the publishing time patterns in terms of citations and publications. Prior research has shown that it often takes 10 to 20 years for well-known works to achieve their maximum recognition and peak in terms of the number of citations [27, 28]. The findings indicate a general upward trend in research on orthopedic implants made of magnesium alloy.
A total of 26 countries published the 100 most cited articles. Figure 3 shows the geographic distribution of the top 100 literature. The results showed that China started the research on magnesium alloy orthopedic implants early and was in a leading position in this field, with the largest number of publications (n=44). The second place was Germany and USA, with 21 publications each. This was followed by Australia (n=8), Iran (n=4) and South Korea (n=4). Canada, India, Italy and New Zealand published three papers each. The output of China, Germany and USA far ex-ceeded that of other countries during the whole study period. The cooperation between these countries is relatively frequent (Figure 4). Among them, the USA has the most active partnerships, with close cooperation with China, Germany, Japan, Italy, Israel and Canada. China also has close cooperation with the USA, Germany, Australia and Japan. In general, the countries with the highest number of publications are generally rich in magnesium resources or advanced metallurgical technology.
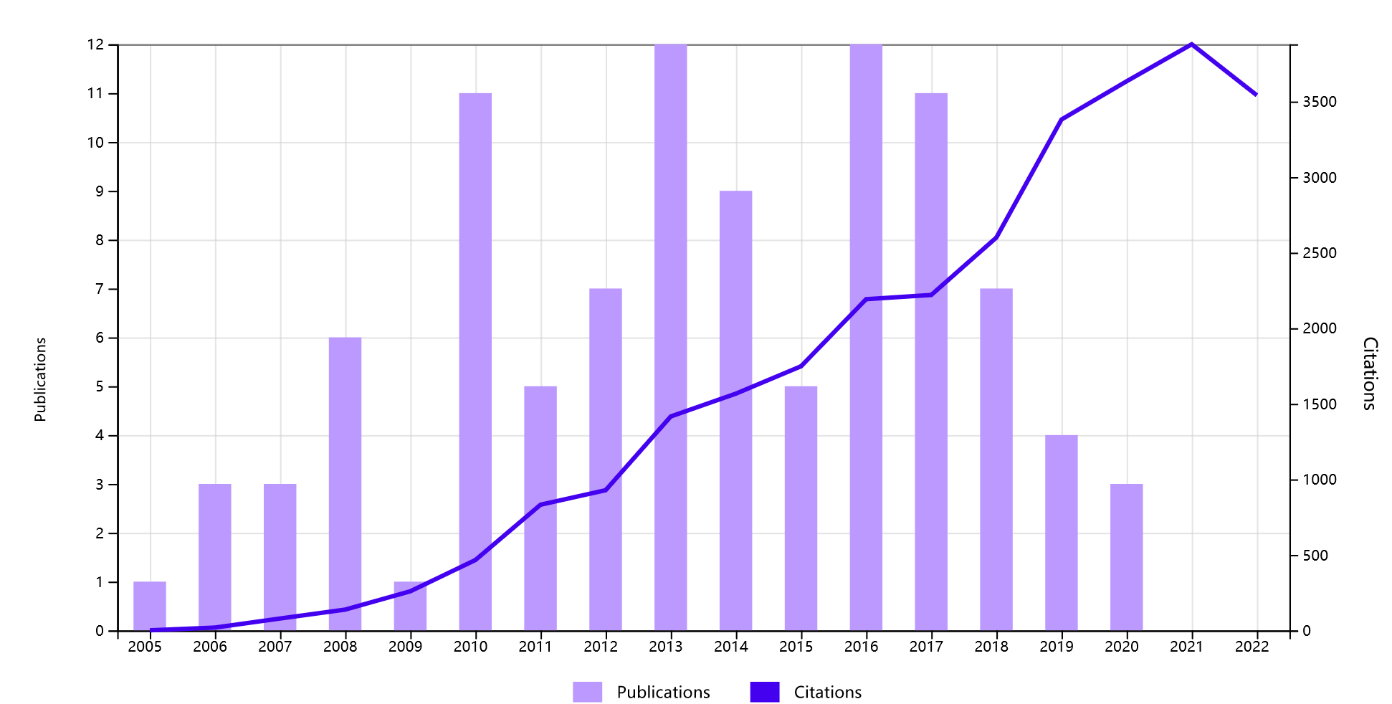
Figure 2. Total publications and citations on magnesium alloy orthopedic implants.
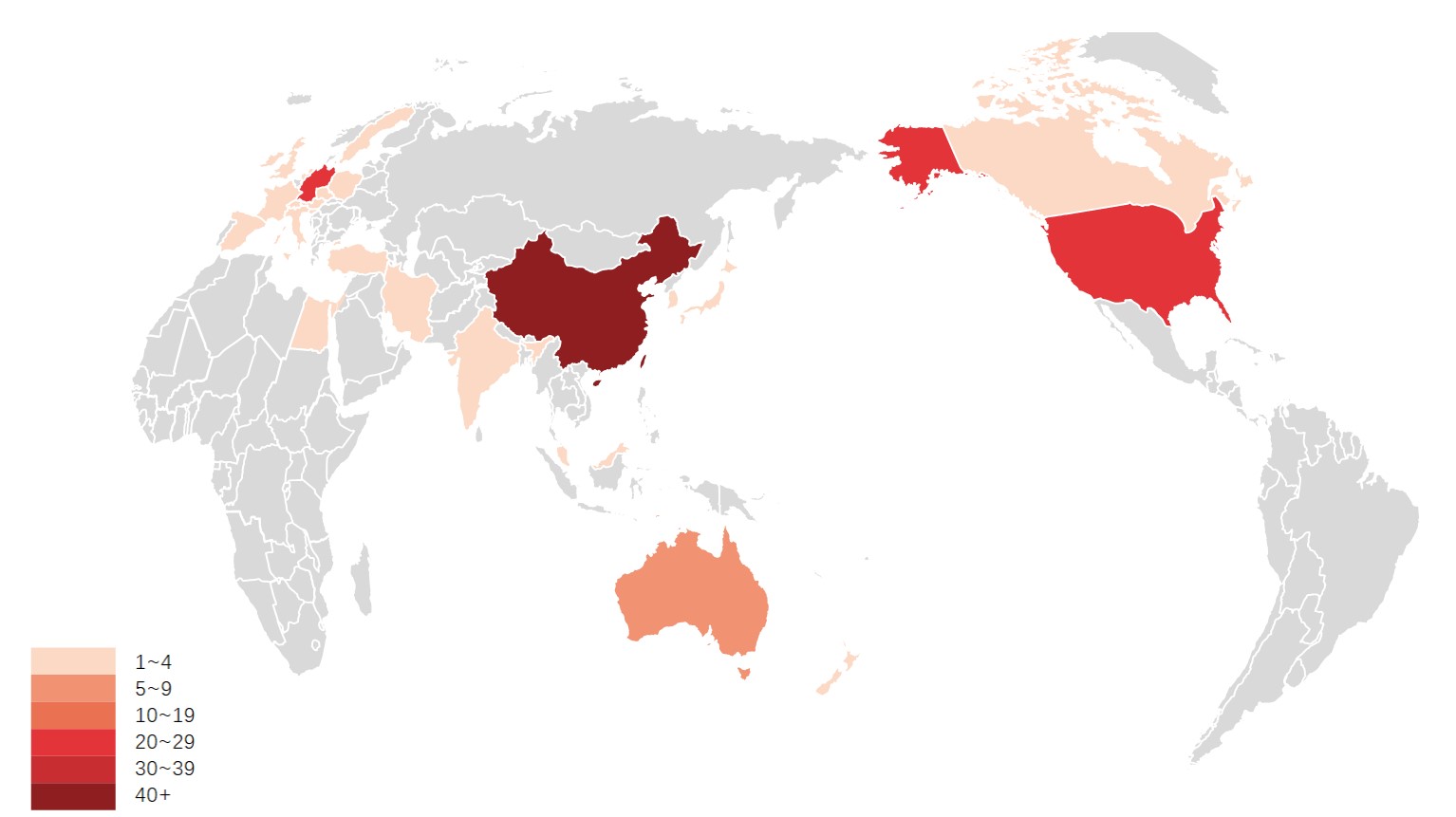
Figure 3. Geographical distributions of publications.
Figure 4. The collaboration network of the most productive countries.
3.2.Analysis of journal, institution and author
42 journals published the 100 selected papers, with only one publication appearing in roughly two-thirds of them. Table 1 lists 14 journals that have published more than 2 articles on magnesium alloy orthopedic implants, accounting for 72% of the total number of publications. Acta Biomaterialial (IF=10.633) had the largest number of publications, with a total of 24 articles. The second was Biomaterials with the highest impact factor (IF=15.304) and a total of 9 articles. Journal of Biomedical Materials Research Part A (IF=4.854) and Journal of Biomedical Materials Research Part B Applied Biomaterials (IF=3.405) each published 5 articles. Among the most influential journals, most were produced in the USA (n=5), followed by the Netherlands (n=4) and China (n=3).
A total of 162 institutions participated in 100 articles. Table 2 reports the top 10 institutions that have published more than 5 articles, of which the institution with the most published articles was the Chinese Academy of Sciences(n=18), which was cited over 3,230 times. In the second place, Hannover Medical School from Germany published 14 articles, which were cited 1,868 times. Peking University from China ranked third with a total of 13 publications and 1,291 citations. Next in line were Leibniz University Hannover (Germany) (n=9), Shanghai Jiao Tong University (China) (n=8) and Helmholtz Association (Germany) (n=7). In the top 10 institutions, six were in China, four were in Germany. A cooperative network composed of the following institutions was created by the leading organizations: Chinese Academy of Sciences, Peking University, Shanghai Jiao Tong University, City University of Hong Kong, Fudan University, Monash University and so on. Figure 5 shows the close cooperation among these institutions. Cooperation between institutions from China is very active, and cooperation with institutions from other countries has also been carried out.
Magnesium alloy orthopedic implants were the subject of research by 444 writers, however only 77% of them had more than one article published. The top 12 authors, each of whom produced at least 4 publications, are listed in Table 3. Witte F and Zheng YF are the most prolific authors with 13 articles each. Yang K from Peking University ranked third with 9 articles. Witte F has published 6 articles as the first author, also ranking first in these highly productive authors. The networks of author partnerships on magnesium alloy orthopedic implant research were evaluated using Vosviewer software in order to examine the interaction and coordination of high-yield authors with other authors (Figure 6).
Based on the above analysis, it can be found that the research and development of magnesium alloy orthopedic implants require extensive cooperation of different countries, institutions and authors at home and abroad.
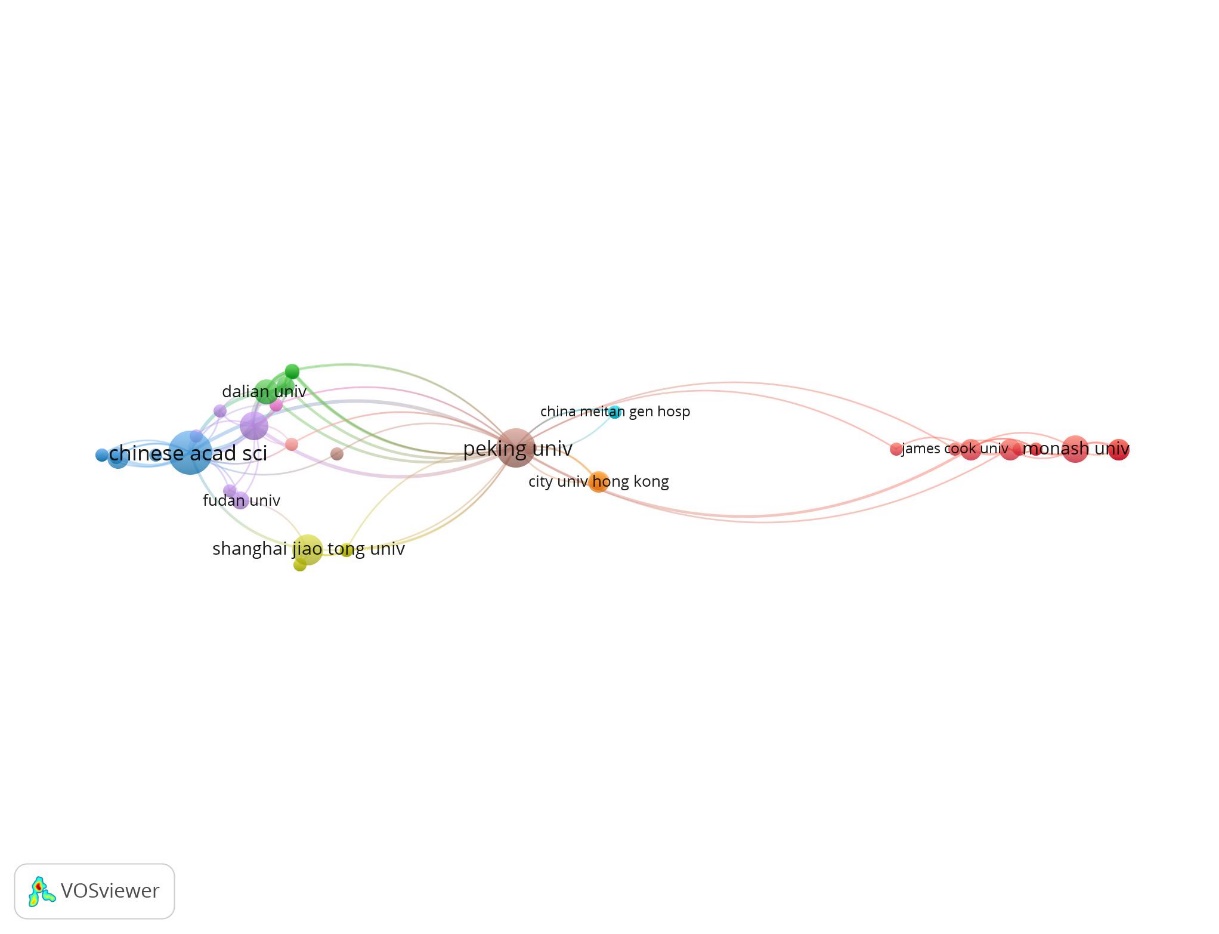
Figure 5. The collaboration network of the most productive institutions.
|
Ranking |
Journal |
Country |
IFa |
TPb |
TCc |
|
1 |
Acta Biomaterialia |
USA |
10.633 |
24 |
6589 |
|
2 |
Biomaterials |
Netherlands |
15.304 |
9 |
9930 |
|
3 |
Journal Of Biomedical Materials Research Part A |
USA |
4.854 |
5 |
1090 |
|
4 |
Journal Of Biomedical Materials Research Part B Applied Biomaterials |
USA |
3.405 |
5 |
600 |
|
5 |
Applied Surface Science |
Netherlands |
7.392 |
4 |
366 |
|
6 |
Journal Of Materials Science Technology |
China |
10.32 |
4 |
932 |
|
7 |
Materials Science Engineering C Materials for Biological Applications |
Netherlands |
8.457 |
4 |
793 |
|
8 |
Acs Applied Materials Interfaces |
USA |
10.383 |
3 |
333 |
|
9 |
Journal Of Materials Science Materials in Medicine |
Netherlands |
4.727 |
3 |
433 |
|
10 |
Surface Coatings Technology |
Switzerland |
4.865 |
3 |
375 |
|
11 |
Cirp Annals Manufacturing Technology |
USA |
4.482 |
2 |
214 |
|
12 |
Journal Of Magnesium and Alloys |
China |
11.862 |
2 |
309 |
|
13 |
Materials Design |
England |
9.417 |
2 |
259 |
|
14 |
Regenerative Biomaterials |
China |
5.763 |
2 |
174 |
Table 1. The most productive journals that have published more than 2 articles on magnesium alloy orthopedic implants.
Note: aIF is the impact factor; bTP is the number of total publications; cTC is the number of total citations.
|
No. |
Institute (country) |
TPa |
TCb |
|
1 |
Chinese Academy of Sciences (China) |
18 |
4285 |
|
2 |
Hannover Medical School (Germany) |
14 |
7812 |
|
3 |
Peking University (China) |
12 |
3764 |
|
4 |
Leibniz University Hannover (Germany) |
9 |
5489 |
|
5 |
Shanghai Jiao Tong University (China) |
7 |
953 |
|
6 |
Helmholtz Association (Germany) |
7 |
3683 |
|
7 |
Chinese University of Hong Kong (China) |
6 |
1824 |
|
8 |
Shenzhen Institute of Advanced Technology Cas (China) |
6 |
508 |
|
9 |
Monash University (Australia) |
5 |
2032 |
|
10 |
University of Veterinary Medicine Hannover Foundation (Germany) |
5 |
2532 |
|
11 |
Zhengzhou University (China) |
5 |
653 |
Table 2. The most productive institutions that published more than 5 articles on magnesium alloy orthopedic implants.
Note: aTP is the number of total publications; bTC is the number of total citations.
Table 3. The authors who have published more than 5 articles on magnesium alloy orthopedic implants research.
|
Authors |
Institution |
Position on author list |
TPa |
TCb |
|
Witte F |
Chinese Academy of Sciences |
First author-6 correspond author-4 second-2, third-1 last-5 |
13 |
8361 |
|
Zheng YF |
Hannover Medical School |
First author-0 correspond author-8 second-1, third-0 last-11 |
12 |
3764 |
|
Yang K |
Peking University |
First author-0 correspond author-3 second-0, third-1 last-9 |
9 |
1780 |
|
Qin L |
Institute Of Metal Research Cas |
First author-0 correspond author-3 second-2, third-0 last-6 |
6 |
1824 |
|
Windhagen H |
Leibniz University Hannover |
First author-1 correspond author-0 second-1 third-0 last-5 |
6 |
3818 |
|
Guan SK |
Shanghai Jiao Tong University |
First author-0 correspond author-5 second-4, third-0 last-1 |
5 |
653 |
|
Hort N |
Helmholtz Association |
First author-1 correspond author-0 second-2, third-0 last-4 |
5 |
2484 |
|
Wang LG |
Chinese University of Hong Kong |
First author-0 correspond author-0 second-0, third-1 last-4 |
5 |
653 |
Note: aTP is the number of total publications; bTC is the number of total citations.
3.3.Citation analysis
Citation times is one of the important indexes to measure the influence of publications. Citations may be influ-enced by a variety of elements, including the accessibility and age of IF journals [29]. Due to cumulative effects, older articles are likely to receive more citations. A total of 28,981 times were cited in the top 100 articles on orthopedic implants made of magnesium alloy. The most cited article was Magnesium and its alloys as orthopedic biomaterials: A review, which was cited 3,234 times. Staiger MP was the first author of the article, which was published in Biomaterials in 2006. This review examines the characteristics, biological effectiveness, difficulties, and potential applications of magnesium-based biomaterials [4].
According to earlier research, average citation rates (ACY) are a better indicator of an article's impact and ability to affect future trends [30]. We show the top ten articles with the highest ACY in Table 4. As the article with the highest number of citations, Magnesium and its alloys as orthopedic biomaterials: A review was also the article with the highest ACY, which was 190.24 times. Metallic implant biomaterials ranked second, which was published by Chen QZ et al. in Materials Science & Engineering R-Reports in 2015. This review discusses critical issues with clinical applications of metallic implant biomaterials, including movement-induced wear of joint replacements, fatigue failure of structural components from repeated loading, and systemic toxicity of released metal ions from corrosion [31]. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopedic implants: A review ranked third and was published by Wang XJ et al. in Biomaterials in 2016. This article examines the most recent topological design and manufacturing techniques for various kinds of porous metals [32]. The original works are less frequently cited as concepts from earlier, prominent pieces are incorporated into common understanding. More time must pass before studies with more recent publication dates can amass enough citations to support their significance. Articles with high citation counts but low ACY are probably the product of historical accumulation [30]. From the ACY top ten articles, we can find that the research on magnesium alloy orthopedic implants mainly focuses on degradability, biocompatibility, surface modification and clinical application.
Table 4. The top ten articles with the highest ACY on magnesium alloy orthopedic implants research.
|
Ranking |
Title |
First author |
Journal |
ACYa |
TCb |
|
1 |
Magnesium and its alloys as orthopedic biomaterials: A review |
Staiger, MP |
Biomaterials |
190.24 |
3234 |
|
2 |
Metallic implant biomaterials |
Chen, Qizhi |
Materials Science & Engineering R-Reports |
103.94 |
1223 |
|
3 |
Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review |
Wang, Xiaojian |
Biomaterials |
86.2 |
1022 |
|
4 |
In vivo corrosion of four magnesium alloys and the associated bone response |
Witte, F |
Biomaterials |
152.88 |
1871 |
|
5 |
The history of biodegradable magnesium implants: A review |
Witte, Frank |
Acta Biomaterialia |
93 |
1209 |
|
6 |
Degradable biomaterials based on magnesium corrosion |
Witte, Frank |
Current Opinion in Solid State & Materials Science |
79.6 |
1293 |
|
7 |
Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective |
Zhao, Dewei |
Biomaterials |
64.59 |
507 |
|
8 |
Recent advances on the development of magnesium alloys for biodegradable implants |
Chen, Yongjun |
Acta Biomaterialia |
146 |
737 |
|
9 |
The development of binary Mg-Ca alloys for use as biodegradable materials within bone |
Li, Zijian |
Biomaterials |
81.89 |
1194 |
|
10 |
Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications |
Agarwal, Sankalp |
Materials Science & Engineering C-Materials for Biological Applications |
34.13 |
489 |
Note: aACY, average citations per year; bTC is the number of total citations.
3.4.Keyword analysis and research hotspots
3.4.1.Keyword analysis. A total of 243 keywords from 100 research are sorted and combined according to the gerund's abbreviation, singular form, and plural form. The co-occurrence network based on high-frequency terms (more than 2) is shown in Figure 7. Keywords co-occurrence analysis found that the words “corrosion”, “biocompatibility”, “biodegradable”, “degradation”, “mechanical properties”, “coating”, “corrosion resistance”,” antibacterial activity”,” orthopedic applications” and “surface modification” had the highest frequency of co-occurrence in the research of magnesium alloy orthopedic implants. In addition, the keywords “porous structures”, “nanoparticles” and “bone scaffold” were also worthy of attention in the highly cited articles. Through the analysis of high-cited articles and keywords, our study objectively reflects the research hotspots of magnesium alloy orthopedic implants.
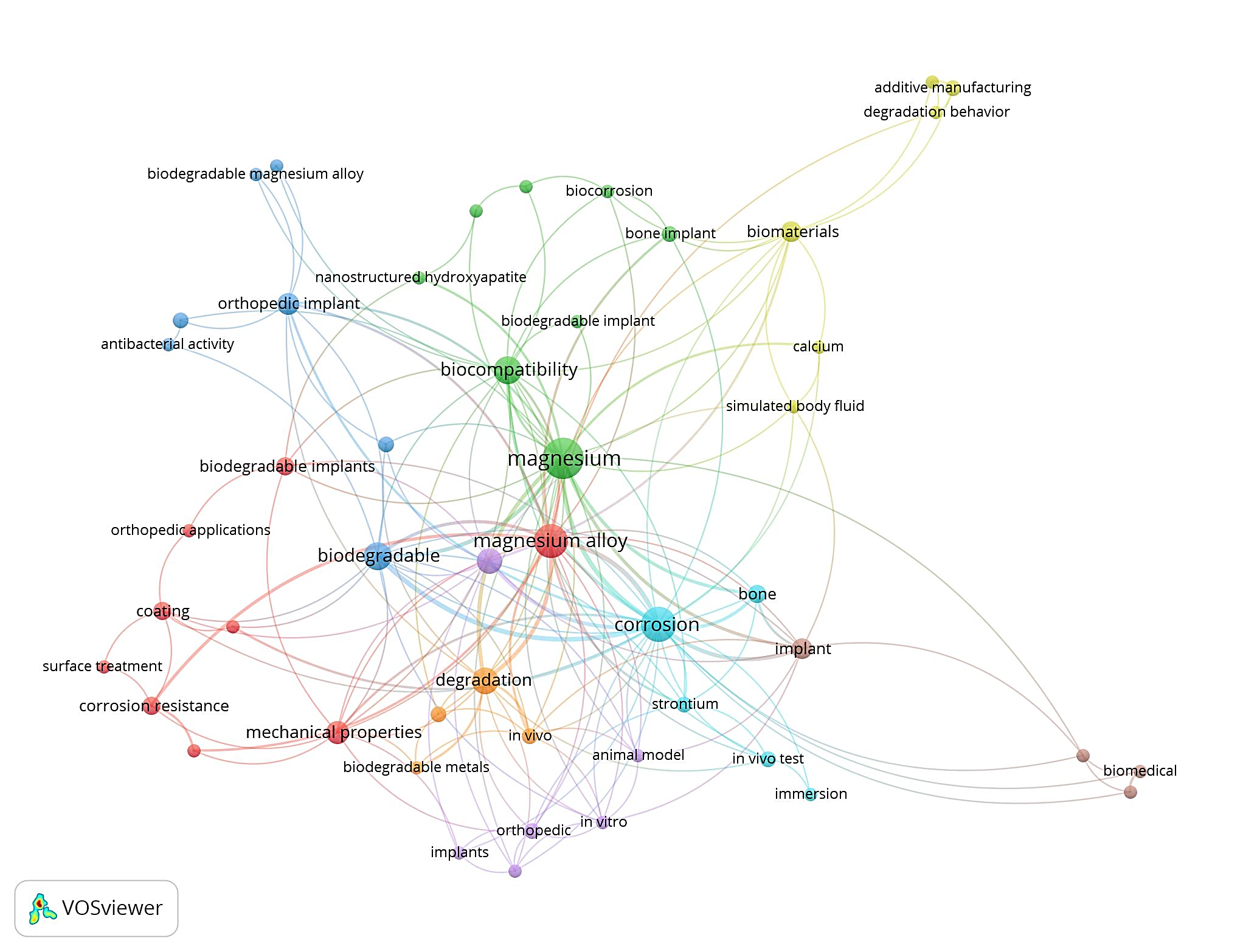
Figure 7. The co-occurrence network of magnesium alloy orthopedic implants keywords.
3.4.2.Clinical application status of magnesium alloy orthopedic implants. An increasing number of surgeons are reevaluating the clinical application potential of biodegradable metals as a result of the rapid advancement in metallurgy in recent years, which has made it possible for engineers and scientists to create magnesium or magnesium alloys with improved mechanical qualities and increased corrosion resistance [6]. In general, there are two types of magnesium alloy orthopedic implants: scaffolds for bone tissue engineering and bone fixation devices [33].
The basic components of a bone fixation device, which is used to mend broken bones, are a bone screw, bone pin, bone plate, etc. Since the first clinical test employing Mg materials as orthopedic implants, more than a century has passed [34]. In Magnesium alloys were originally used as bone nails and bone plates for the fixing of injured bone in 1900, according to Payr et al. [4]. Mg-Al-Mn alloy screws, nuts, and plates were used in 20 fracture treatments carried out by Mcbridge et al. in 1938 [33]. Plates and screws constructed of Mg-Cd alloy were used to treat 34 cases of pseudoarthrosis successfully in 1948, according to Troitskii et al. [34]. Surgeons in Korea recently used Mg-Ca-Zn screws to fix two radial fractures, while Germany was the first country to report using MgYReZr alloy screws to treat hallux valgus [35].
Magnesium and related alloys may be suitable materials for tissue engineering bone scaffolds due to its good complete mechanical characteristics, biodegradability, and osteogenesis [36]. Autologous osteoblasts, bone marrow stromal cells, or chondrocytes are placed onto biocompatible synthetic scaffolds for bone tissue engineering before eventually degrading and being absorbed by the body [8, 37]. Recently, a bone defect repair scaffold with anti-infective potential was created, and it has also been extensively proposed that porous magnesium containing biological growth factors or cells might serve as an ideal scaffold for bone tissue creation [38, 39]. A magnesium scaffold was created by Čapek et al. using powder metallurgy and pore-forming chemicals [40]. Mechanical testing revealed that the scaffolds' flexural strength, which ranged from 12 to 38 percent porosity, was comparable to that of human bone. Using the template replication technique, Jia et al. successfully obtained Mg scaffolds and assessed their degrading behavior [41]. There has recently been some work that discusses the laser additive fabrication of magnesium bone scaffolds [42, 43].
3.4.3.Advantages of magnesium alloy orthopedic implants. Magnesium and its alloys have a density of about 1.74 g/cm3, which is comparable to the density of human compact bone (1.75 g/cm3), and their Young's modulus (about 45 GPa) is comparable to that of human bone (15-30 GPa), which can offers good mechanical compatibility and reduces stress shielding during load transfer at the bone-implant interface [44, 45]. Magnesium has a higher modulus than permanent metals, but a lower mechanical strength than biodegradable polymers. The toughness of magnesium is higher than that of bioceramics. In order to prevent mechanical failure imposed on implants and to address potential stress-shielding effects, these point to magnesium and its alloys as being ideal for development as fixators for low load-bearing sites in orthopedics.
Mg and its alloys have favorable biocompatibility as biomaterials. The degradation products of magnesium alloy orthopedic implants are mainly Mg ions, which have no obvious toxicity to human tissues [7]. Mg and its alloys can totally decay in vivo, displaying attractive degradation characteristics and distinctive osteopromotive effects [46]. In order to fully accomplish their therapeutic goal as a temporary alternative, researchers are hoping that magnesium alloy orthopedic implants will gradually disintegrate over time as the bone tissue is healed in addition to initially providing stable mechanical support. Without the need for a second operation to remove this temporarily implanted device, the implants can gradually disintegrate and be absorbed by the body or eliminated from the body, which significantly lowers the expense of surgery and the agony associated with the second operation. Magnesium transporter 1 (MagT1) and transient receptor potential cation channel sub-family member 7 (TRPM7), which can facilitate the release of calcitonin gene-related peptide (CGRP), mediate the release of magnesium ions following the implantation of magnesium alloy implants in vivo [17]. Cyclic AMP can attach to its response element-binding protein in reaction to the released CGRP. Osterix is consequently markedly increased, promoting the production of new bone in the periosteal area.
3.4.4.Techniques to improve corrosion resistance. Despite the many advantages of Mg-based alloys, their main limitation as biomedical materials is the high corrosion rate [47]. We found that there are four typical methods to improve corrosion resistance, including purification, alloying treatment, surface coating and magnesium-based composite (MMC):
• Purification. Researchers have suggested using purification techniques to increase the corrosion resistance of magnesium alloys because electrochemical kinetics shows that lowering impurities and the second phase in the Mg matrix can effectively reduce the incidence of galvanic corrosion [48].
•Alloying treatment. To enhance the mechanical characteristics and corrosion resistance of magnesium, alloying is a necessary step [49, 50]. A workable strategy is to utilize alloying elements to either generate a second phase that is more favorable or minimize the volume and size of the second phase in the magnesium matrix. This will lessen the galvanic corrosion that the second phase causes. Utilizing alloying components to increase the inertness or structural integrity of the oxides or hydroxides on the surface of the magnesium matrix would be another viable strategy, creating a more protective surface film to halt corrosion expansion [51].
•Surface coating. Surface modification of the Mg matrix, as opposed to alloying, can effectively isolate or limit the surface area of interaction between the matrix and body fluids, which may assist retain the mechanical integrity of the Mg-based im-plant until the fracture is fully healed [52, 53].
•Magnesium based composite (MMC). Another possible method to control the pace of deterioration is to produce magnesium-based MMC with reinforcing particles [54-56]. Currently, several biologically active biological cancers, including HAp, TCP, and bioglass, are used as enhancing particles of magnesium-based MMC [57-59]. As a low-dimensional nano-material, Graphene oxide (GO) has also been reported to be used as the reinforcement phase of magnesium-based materials [60]. In some research, mesoporous silica (MS) has also been used as reinforcing particles in magnesium-based MMC [61].
4.Conclusions
Mg and Mg alloys have good biocompatibility, degradability and biosafety. In addition, Mg is abundant in re-sources, low in price, suitable for large-scale industrial production, and can be degraded by corrosion in the physio-logical electrolyte environment, which shows its great potential application prospects in the field of orthopedics, and its application effect in orthopedics is remarkable. Although biodegradable magnesium alloys have a lot of potential for orthopedic applications, the primary issue is their high rate of corrosion, which makes the surrounding tissues bioincompatible. There are many animal experiments and basic researches on magnesium alloy implants, and re-searchers are focusing on improving the corrosion resistance of magnesium alloy orthopedic implants and exploring more advanced manufacturing techniques.
Despite numerous reports using magnesium and its alloys as bone-conducting, biodegradable orthopedic im-plants, considerable research is still needed to properly evaluate the potential of magnesium alloys. In order to achieve the wide clinical application of magnesium alloy orthopedic implants, there are still some challenges to be faced, and we look forward to the future research direction in this field. The durability of magnesium alloy orthopedic implants is the primary issue of patient safety. To reliably maintain damaged tissues during service, the mechanical characteristics of magnesium alloys must be improved. Although local sight alkalinity generated by Mg breakdown can limit bacterial adhesion, it is commonly acknowledged that bacterial infection usually occurs in orthopedic treatment, which results in bone healing failure. Therefore, it is crucial to design magnesium implants with anti-bacterial qualities for bone healing. Additionally, the development of orthopedic implants made of magnesium alloy with sustained release mechanisms appears promising. The demand for customized bone implants will rise in the future, so it's important to pay attention to the additive fabrication of magnesium bone implants. Magnesium items produced by laser additive manufacturing can have tiny and consistent microstructures, which should result in enhanced mechanical and corrosion properties. Another pressing issue is the creation of specialized magnesium-based material systems for laser additive manufacturing procedures. However, hybrid materials have a lot of potential for the development of bone implant materials since they combine the benefits of several different materials. In addition, there has to be more research done on the impact of dynamic degradation on the morphology and mechanical characteristics of bone implants. Finally, the difficulties of accurately characterizing the local characteristics of magnesium alloy implants in vivo has drawn research attention to the simulation of thermochemical-mechanical coupling behavior in recent years.
Based on the bibliometric and visual analysis of the top 100 cited articles on magnesium alloy orthopedic implants, we found that more and more attention has been paid to this field since 2005, and the performance of magnesium alloy as an orthopedic implant has also made considerable development. Currently, medical alloy materials are undergoing changes. Researchers have developed new medical alloy materials represented by degradable metals, nano-crystalline metals, and bulk amorphous alloys. Material properties are developing from biological inertia to biological activity and biological function (antibacterial, anti-proliferation, antitumor). At the same time, advanced manufacturing technologies represented by 3D printing technology, thin film technology and composite technology have also been tried. The study of magnesium alloy bone implants will take time because the human physiological environment is a complex system. Increased interdisciplinary research is urgently required to hasten the clinical adoption of magnesium alloy orthopedic implants.
References
[1]. M. A. H. Gepreel and M. Niinomi, "Biocompatibility of Ti-alloys for long-term implantation," (in English), Journal of the Mechanical Behavior of Biomedical Materials, Article vol. 20, pp. 407-415, Apr 2013, doi: 10.1016/j.jmbbm.2012.11.014.
[2]. X. Y. Liu, P. K. Chu, and C. X. Ding, "Surface modification of titanium, titanium alloys, and related materials for biomedical applications," (in English), Mater. Sci. Eng. R-Rep., Review vol. 47, no. 3-4, pp. 49-121, Dec 2004, doi: 10.1016/j.mser.2004.11.001.
[3]. E. Poinern, S. Brundavanam, and D. Fawcett, "Biomedical Magnesium Alloys: A Review of Material Properties, Surface Modifications and Potential as a Biodegradable Orthopaedic Implant," American Journal of Biomedical Engineering, vol. 2, pp. 218-240, 01/01 2012, doi: 10.5923/j.ajbe.20120206.02.
[4]. M. P. Staiger, A. M. Pietak, J. Huadmai, and G. Dias, "Magnesium and its alloys as orthopedic biomaterials: A review," (in English), Biomaterials, Review vol. 27, no. 9, pp. 1728-1734, Mar 2006, doi: 10.1016/j.biomaterials.2005.10.003.
[5]. X. X. Yan, W. Cao, and H. H. Li, "Biomedical Alloys and Physical Surface Modifications: A Mini-Review," (in English), Materials, Review vol. 15, no. 1, p. 16, Jan 2022, Art no. 66, doi: 10.3390/ma15010066.
[6]. Y. F. Zheng, X. N. Gu, and F. Witte, "Biodegradable metals," Materials Science and Engineering: R: Reports, vol. 77, pp. 1-34, 2014/03/01/ 2014, doi: https://doi.org/10.1016/j.mser. 2014.01.001.
[7]. A. H. Martinez Sanchez, B. J. Luthringer, F. Feyerabend, and R. Willumeit, "Mg and Mg alloys: how comparable are in vitro and in vivo corrosion rates? A review," Acta Biomater, vol. 13, pp. 16-31, Feb 2015, doi: 10.1016/j.actbio.2014.11.048.
[8]. P. Gupta, M. Adhikary, M. J. Christakiran, M. Kumar, N. Bhardwaj, and B. B. Mandal, "Biomimetic, Osteoconductive Non-mulberry Silk Fiber Reinforced Tricomposite Scaffolds for Bone Tissue Engineering," (in English), ACS Appl. Mater. Interfaces, Article vol. 8, no. 45, pp. 30797-30810, Nov 2016, doi: 10.1021/acsami.6b11366.
[9]. M. Yazdimamaghani, M. Razavi, D. Vashaee, and L. Tayebi, "Development and degradation behavior of magnesium scaffolds coated with polycaprolactone for bone tissue engineering," (in English), Materials Letters, Article vol. 132, pp. 106-110, Oct 2014, doi: 10.1016/j.matlet.2014.06.036.
[10]. L. Y. Chen et al., "Processing and properties of magnesium containing a dense uniform dispersion of nanoparticles," (in English), Nature, Article vol. 528, no. 7583, pp. 539-+, Dec 2015, doi: 10.1038/nature16445.
[11]. X. J. Wang et al., "What is going on in magnesium alloys?," (in English), Journal of Materials Science & Technology, Article vol. 34, no. 2, pp. 245-247, Feb 2018, doi: 10.1016/j.jmst.2017.07.019.
[12]. S. X. Zhang et al., "Research on an Mg-Zn alloy as a degradable biomaterial," (in English), Acta Biomaterialia, Article vol. 6, no. 2, pp. 626-640, Feb 2010, doi: 10.1016/j.actbio.2009.06.028.
[13]. P. A. Dearnley, C. G. F. Pina, and J. Fisher, "Assessment of S-phase coated medical grade stainless steel (Ortron 90) for use in the human joint replacement corrosion-wear environment," (in English), J. Phys. D-Appl. Phys., Article vol. 41, no. 10, p. 9, May 2008, Art no. 105305, doi: 10.1088/0022-3727/41/10/105305.
[14]. D. Upadhyay, M. A. Panchal, R. S. Dubey, and V. K. Srivastava, "Corrosion of alloys used in dentistry: A review," (in English), Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process., Review vol. 432, no. 1-2, pp. 1-11, Sep 2006, doi: 10.1016/j.msea.2006.05.003.
[15]. A. Myrissa et al., "In vitro and in vivo comparison of binary Mg alloys and pure Mg," (in English), Mater. Sci. Eng. C-Mater. Biol. Appl., Article vol. 61, pp. 865-874, Apr 2016, doi: 10.1016/j.msec.2015.12.064.
[16]. S. C. Chen et al., "Tuning surface properties of bone biomaterials to manipulate osteoblastic cell adhesion and the signaling pathways for the enhancement of early osseointegration," (in English), Colloid Surf. B-Biointerfaces, Article vol. 164, pp. 58-69, Apr 2018, doi: 10.1016/j.colsurfb.2018.01.022.
[17]. Y. F. Zhang et al., "Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats," (in English), Nature Medicine, Article vol. 22, no. 10, pp. 1160-1169, Oct 2016, doi: 10.1038/nm.4162.
[18]. C. Gao, S. Peng, P. Feng, and C. Shuai, "Bone biomaterials and interactions with stem cells," (in English), Bone Research, Article vol. 5, no. 4, pp. 253-285, 2017, Art no. 2095-4700(2017)5:4<253:Bbaiws>2.0.Tx;2-9. [Online]. Available: <Go to ISI>://CSCD:6151121.
[19]. J. L. Wang, J. K. Xu, C. Hopkins, D. H. K. Chow, and L. Qin, "Biodegradable Magnesium-Based Implants in Orthopedics-A General Review and Perspectives," (in English), Advanced Science, Review vol. 7, no. 8, p. 19, Apr 2020, Art no. 1902443, doi: 10.1002/advs.201902443.
[20]. "Bio-implants Market by Product Types (Cardiovascular (Stents, Pacing plants Market devices), Dental and Prosthetic bio-implants, Orthopedic/joint reconstruction and replacement bio-implants (Orthobiologics, Trauma Implants, Sport Medicines), Spinal bio-implants, Ophthalmology bio-implants (Glaucoma Implants, Intraocular Implants)) - Global Opportunity Analysis and Industry Forecast, 2013 - 2020." https://www. alliedmarketresearch.com/bio-implants-market (accessed November 25, 2022).
[21]. F. A. Ponce and A. M. Lozano, "Highly cited works in neurosurgery. Part II: the citation classics A review (vol 112, pg 233, 2010)," (in English), Journal of neurosurgery, Correction vol. 120, no. 5, pp. 1252-1257, May 2014, doi: 10.3171/2014.2.JNS14358a.
[22]. J. S. Kreutzer et al., "The top 100 cited neurorehabilitation papers," (in English), NeuroRehabilitation, Article vol. 40, no. 2, pp. 163-174, 2017, doi: 10.3233/nre-161415.
[23]. A. Berlinberg, J. Bilal, I. B. Riaz, and D. J. B. Kurtzman, "The 100 top-cited publications in psoriatic arthritis: a bibliometric analysis," (in English), International journal of dermatology, Review vol. 58, no. 9, pp. 1023-1034, Sep 2019, doi: 10.1111/ijd.14261.
[24]. Y. M. Wei, Z. F. Mi, and Z. M. Huang, "Climate policy modeling: An online SCI-E and SSCI based literature review," (in English), Omega-Int. J. Manage. Sci., Review vol. 57, pp. 70-84, Dec 2015, doi: 10.1016/j.omega.2014.10.011.
[25]. "Clarivate Releases Journal Citation Reports 2022(Impact factor & Ranking of 2021)." https://jcr.clarivate.com/jcr/browse-journals (accessed February 1, 2023).
[26]. L. Waltman, N. J. van Eck, and E. C. M. Noyons, "A unified approach to mapping and clustering of bibliometric networks," (in English), Journal of Informetrics, Article vol. 4, no. 4, pp. 629-635, Oct 2010, doi: 10.1016/j.joi.2010.07.002.
[27]. E. Garfield, "Citation analysis as a tool in journal evaluation," (in eng), Science (New York, N.Y.), vol. 178, no. 4060, pp. 471-9, Nov 3 1972, doi: 10.1126/science.178.4060.471.
[28]. G. M. Hall, "BJA citation classics 1945-1992," (in English), British journal of anaesthesia, Editorial Material vol. 80, no. 1, pp. 4-6, Jan 1998, doi: 10.1093/bja/80.1.4.
[29]. Q. Gao et al., "The top 100 highly cited articles on osteoporosis from 1990 to 2019: a bibliometric and visualized analysis," (in English), Archives of osteoporosis, Article vol. 15, no. 1, p. 11, Sep 2020, Art no. 144, doi: 10.1007/s11657-020-0705-z.
[30]. N. Tang, W. C. Zhang, D. M. George, Y. Su, and T. L. Huang, "The Top 100 Most Cited Articles on Anterior Cruciate Ligament Reconstruction: A Bibliometric Analysis," (in English), Orthopaedic journal of sports medicine, Article vol. 9, no. 2, p. 16, Feb 2021, Art no. 2325967120976372, doi: 10.1177/2325967120976372.
[31]. Q. Chen and G. A. Thouas, "Metallic implant biomaterials," Materials Science and Engineering: R: Reports, vol. 87, pp. 1-57, 2015/01/01/ 2015, doi: https://doi.org/10.1016/ j.mser.2014.10.001.
[32]. X. J. Wang et al., "Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review," (in English), Biomaterials, Review vol. 83, pp. 127-141, Mar 2016, doi: 10.1016/j.biomaterials.2016.01.012.
[33]. A. Yy et al., "Mg bone implant: Features, developments and perspectives," Materials & Design, vol. 185, 2020, doi: 10.1016/j.matdes.2019.108259
[34]. D. W. Zhao, F. Witte, F. Q. Lu, J. L. Wang, J. L. Li, and L. Qin, "Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective," (in English), Biomaterials, Review vol. 112, pp. 287-302, Jan 2017, doi: 10.1016/j.biomaterials.2016.10.017.
[35]. J. W. Lee et al., "Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy," (in English), Proceedings of the National Academy of Sciences of the United States of America, Article vol. 113, no. 3, pp. 716-721, Jan 2016, doi: 10.1073/pnas.1518238113.
[36]. A. H. Yusop, A. A. Bakir, N. A. Shaharom, M. R. Abdul Kadir, and H. Hermawan, "Porous biodegradable metals for hard tissue scaffolds: a review," (in English), International journal of biomaterials, vol. 2012, p. 641430, 2012 (Epub 2012 Jul 2012, doi: 10.1155/2012/641430.
[37]. M. M. Stevens, "Biomaterials for bone tissue engineering," Materials Today, vol. 11, no. 5, pp. 18-25, 2008/05/01/ 2008, doi: https://doi.org/10.1016/S1369-7021(08)70086-5.
[38]. S. Wu, X. Liu, K. Yeung, C. Liu, and X. Yang, "Biomimetic porous scaffolds for bone tissue engineering," Materials Science and Engineering R Reports, vol. 80, pp. 1–36, 2014, doi: 10.1016/j.mser.2014.04.001
[39]. Y. X. L. R. Ma, L. Li, H.L. Tan, J.L. Wang, Y. Li, T.T. Tang, L. Qin,, "Use of a three-dimensional printed polylactide-coglycolide/tricalcium phosphate composite scaffold incorporating magnesium powder to enhance bone defect repair in rabbits," Journal of Orthopaedic Translation, vol. 16, pp. 62-70, 2019/01/01/ 2019, doi: 10.1016/j.jot.2018.07.007.
[40]. J. Čapek and D. Vojtěch, "Properties of porous magnesium prepared by powder metallurgy," (in eng), Mater Sci Eng C Mater Biol Appl, vol. 33, no. 1, pp. 564-9, Jan 1 2013, doi: 10.1016/j.msec.2012.10.002.
[41]. C. C. G. Jia, J. Zhang, Y. Wang, R. Yue, B.J. Luthringer-Feyerabend, R. Willumeit-Roemer, H. Zhang, M. Xiong, H. Huang, "In vitro degradation behavior of Mg scaffolds with three-dimensional interconnected porous structures for bone tissue engineering," Corrosion Science, vol. 144, pp. 301-312, 2018/11/01/ 2018, doi: 10.1016/j.corsci.2018.09.001.
[42]. Y. Li et al., "Additively manufactured biodegradable porous iron," (in English), Acta Biomaterialia, Article vol. 77, pp. 380-393, Sep 2018, doi: 10.1016/j.actbio.2018.07.011.
[43]. A. Kopp et al., "Influence of design and postprocessing parameters on the degradation behavior and mechanical properties of additively manufactured magnesium scaffolds," (in eng), Acta Biomater, vol. 98, pp. 23-35, Oct 15 2019, doi: 10.1016/j.actbio.2019.04.012.
[44]. Y. W. Yang et al., "System development, formability quality and microstructure evolution of selective laser-melted magnesium," (in English), Virtual and Physical Prototyping, Article vol. 11, no. 3, pp. 173-181, 2016, doi: 10.1080/17452759.2016.1210522.
[45]. T. B. A. H.Q. Ang, S. Zhu, C. Gu, M.A. Easton, "Proof stress measurement of die-cast magnesium alloys," Materials & Design, vol. 112, pp. 402-409, 2016, doi: 10.1016/j.matdes.2016.09.088.
[46]. S. Zhang et al., "Research on an Mg-Zn alloy as a degradable biomaterial," Acta Biomater, vol. 6, no. 2, pp. 626-40, Feb 2010, doi: 10.1016/j.actbio.2009.06.028.
[47]. Z. Ying, G. Wu, J. Jiang, H. M. Wong, and P. K. Chu, "Improved corrosion resistance and cytocompatibility of magnesium alloy by two-stage cooling in thermal treatment," Corrosion Science, vol. 59, no. 6, pp. 360–365, 2012, doi: 10.1016/j.corsci.2012.03.020
[48]. L. Zhang et al., "Effects of Different Concentrations of BSA on In Vitro Corrosion Behavior of Pure Zinc in Artificial Plasma," (in eng), ACS biomaterials science & engineering, vol. 8, no. 10, pp. 4365-4376, Oct 10 2022, doi: 10.1021/acsbiomaterials.2c00894.
[49]. P. L. Miller, B. A. Shaw, R. G. Wendt, and W. C. Moshier, "Assessing the Corrosion Resistance of Nonequilibrium Magnesium-Yttrium Alloys," Corrosion -Houston Tx-, vol. 51, no. 12, pp. 922-931, 1995, doi: 10.5006/1.3293568
[50]. V. Kaesel, P.-T. Tai, F.-W. Bach, H. Haferkamp, F. Witte, and H. Windhagen, "Approach to Control the Corrosion of Magnesium by Alloying," in Magnesium, 2003, pp. 534-539.
[51]. E. Willbold et al., "Effect of the addition of low rare earth elements (lanthanum, neodymium, cerium) on the biodegradation and biocompatibility of magnesium," (in eng), Acta Biomater, vol. 11, pp. 554-62, Jan 2015, doi: 10.1016/j.actbio.2014.09.041.
[52]. J. Wang et al., "Surface modification of magnesium alloys developed for bioabsorbable orthopedic implants: a general review," (in eng), Journal of biomedical materials research. Part B, Applied biomaterials, vol. 100, no. 6, pp. 1691-701, Aug 2012, doi: 10.1002/jbm.b.32707.
[53]. J. Tang et al., "Surface coating reduces degradation rate of magnesium alloy developed for orthopaedic applications," Journal of Orthopaedic Translation, vol. 1, no. 1, pp. 41-48, 2013.
[54]. Z. Q. Cui, W. J. Li, L. X. Cheng, D. Q. Gong, W. L. Cheng, and W. X. Wang, "Effect of nano-HA content on the mechanical properties, degradation and biocompatible behavior of Mg-Zn/HA composite prepared by spark plasma sintering," (in English), Materials Characterization, Article vol. 151, pp. 620-631, May 2019, doi: 10.1016/ j.matchar.2019.03.048.
[55]. Y. Deng et al., "Mechanism for corrosion protection of β-TCP reinforced ZK60 via laser rapid solidification," International journal of bioprinting, vol. 4, 11/21 2017, doi: 10.18063/ijb.v4i1.124.
[56]. A. M. Kumar, S. F. Hassan, A. A. Sorour, M. Paramsothy, and M. Gupta, "Investigation on the Controlled Degradation and Invitro Mineralization of Carbon Nanotube Reinforced AZ31 Nanocomposite in Simulated Body Fluid," Met. Mater. Int., vol. 25, pp. 105–116, 2019, doi: 10.1007/s12540-018-0161-0.
[57]. C. Shuai et al., "Biodegradation Resistance and Bioactivity of Hydroxyapatite Enhanced Mg-Zn Composites via Selective Laser Melting," (in eng), Materials (Basel), vol. 10, no. 3, Mar 17 2017, doi: 10.3390/ma10030307.
[58]. H. Shao et al., "3D printing magnesium-doped wollastonite/β-TCP bioceramics scaffolds with high strength and adjustable degradation," Journal of the European Ceramic Society, pp. 1495-1503, 2016, doi: 10.1016/j.jeurceramsoc.2016.01.010
[59]. Y. Wan et al., "Mechanical and biological properties of bioglass/magnesium composites prepared via microwave sintering route," Materials & Design, vol. 99, no. jun., pp. 521-527, 2016, doi: 10.1016/j.matdes.2016.03.096
[60]. B. W. C. Shuai, Y. Yang, S. Peng, C. Gao, "3D honeycomb nanostructure-encapsulated magnesium alloys with superior corrosion resistance and mechanical properties," Composites Part B: Engineering, vol. 162, pp. 611-620, 2019/04/01/ 2019, doi: 10.1016/j.compositesb.2019.01.031.
[61]. Y. Yang, X. Guo, C. He, C. Gao, and C. Shuai, "Regulating Degradation Behavior by Incorporating Mesoporous Silica for Mg Bone Implants," (in eng), ACS biomaterials science & engineering, vol. 4, no. 3, pp. 1046-1054, Mar 12 2018, doi: 10.1021/acsbiomaterials.8b00020.
Cite this article
Bao,T.;Wang,J.;Chen,Y.;Xu,F.;Qiao,G.;Li,F. (2023). The top 100 most cited articles on magnesium alloy orthopedic implants: A bibliometric and visualized analysis. Theoretical and Natural Science,15,201-224.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. M. A. H. Gepreel and M. Niinomi, "Biocompatibility of Ti-alloys for long-term implantation," (in English), Journal of the Mechanical Behavior of Biomedical Materials, Article vol. 20, pp. 407-415, Apr 2013, doi: 10.1016/j.jmbbm.2012.11.014.
[2]. X. Y. Liu, P. K. Chu, and C. X. Ding, "Surface modification of titanium, titanium alloys, and related materials for biomedical applications," (in English), Mater. Sci. Eng. R-Rep., Review vol. 47, no. 3-4, pp. 49-121, Dec 2004, doi: 10.1016/j.mser.2004.11.001.
[3]. E. Poinern, S. Brundavanam, and D. Fawcett, "Biomedical Magnesium Alloys: A Review of Material Properties, Surface Modifications and Potential as a Biodegradable Orthopaedic Implant," American Journal of Biomedical Engineering, vol. 2, pp. 218-240, 01/01 2012, doi: 10.5923/j.ajbe.20120206.02.
[4]. M. P. Staiger, A. M. Pietak, J. Huadmai, and G. Dias, "Magnesium and its alloys as orthopedic biomaterials: A review," (in English), Biomaterials, Review vol. 27, no. 9, pp. 1728-1734, Mar 2006, doi: 10.1016/j.biomaterials.2005.10.003.
[5]. X. X. Yan, W. Cao, and H. H. Li, "Biomedical Alloys and Physical Surface Modifications: A Mini-Review," (in English), Materials, Review vol. 15, no. 1, p. 16, Jan 2022, Art no. 66, doi: 10.3390/ma15010066.
[6]. Y. F. Zheng, X. N. Gu, and F. Witte, "Biodegradable metals," Materials Science and Engineering: R: Reports, vol. 77, pp. 1-34, 2014/03/01/ 2014, doi: https://doi.org/10.1016/j.mser. 2014.01.001.
[7]. A. H. Martinez Sanchez, B. J. Luthringer, F. Feyerabend, and R. Willumeit, "Mg and Mg alloys: how comparable are in vitro and in vivo corrosion rates? A review," Acta Biomater, vol. 13, pp. 16-31, Feb 2015, doi: 10.1016/j.actbio.2014.11.048.
[8]. P. Gupta, M. Adhikary, M. J. Christakiran, M. Kumar, N. Bhardwaj, and B. B. Mandal, "Biomimetic, Osteoconductive Non-mulberry Silk Fiber Reinforced Tricomposite Scaffolds for Bone Tissue Engineering," (in English), ACS Appl. Mater. Interfaces, Article vol. 8, no. 45, pp. 30797-30810, Nov 2016, doi: 10.1021/acsami.6b11366.
[9]. M. Yazdimamaghani, M. Razavi, D. Vashaee, and L. Tayebi, "Development and degradation behavior of magnesium scaffolds coated with polycaprolactone for bone tissue engineering," (in English), Materials Letters, Article vol. 132, pp. 106-110, Oct 2014, doi: 10.1016/j.matlet.2014.06.036.
[10]. L. Y. Chen et al., "Processing and properties of magnesium containing a dense uniform dispersion of nanoparticles," (in English), Nature, Article vol. 528, no. 7583, pp. 539-+, Dec 2015, doi: 10.1038/nature16445.
[11]. X. J. Wang et al., "What is going on in magnesium alloys?," (in English), Journal of Materials Science & Technology, Article vol. 34, no. 2, pp. 245-247, Feb 2018, doi: 10.1016/j.jmst.2017.07.019.
[12]. S. X. Zhang et al., "Research on an Mg-Zn alloy as a degradable biomaterial," (in English), Acta Biomaterialia, Article vol. 6, no. 2, pp. 626-640, Feb 2010, doi: 10.1016/j.actbio.2009.06.028.
[13]. P. A. Dearnley, C. G. F. Pina, and J. Fisher, "Assessment of S-phase coated medical grade stainless steel (Ortron 90) for use in the human joint replacement corrosion-wear environment," (in English), J. Phys. D-Appl. Phys., Article vol. 41, no. 10, p. 9, May 2008, Art no. 105305, doi: 10.1088/0022-3727/41/10/105305.
[14]. D. Upadhyay, M. A. Panchal, R. S. Dubey, and V. K. Srivastava, "Corrosion of alloys used in dentistry: A review," (in English), Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process., Review vol. 432, no. 1-2, pp. 1-11, Sep 2006, doi: 10.1016/j.msea.2006.05.003.
[15]. A. Myrissa et al., "In vitro and in vivo comparison of binary Mg alloys and pure Mg," (in English), Mater. Sci. Eng. C-Mater. Biol. Appl., Article vol. 61, pp. 865-874, Apr 2016, doi: 10.1016/j.msec.2015.12.064.
[16]. S. C. Chen et al., "Tuning surface properties of bone biomaterials to manipulate osteoblastic cell adhesion and the signaling pathways for the enhancement of early osseointegration," (in English), Colloid Surf. B-Biointerfaces, Article vol. 164, pp. 58-69, Apr 2018, doi: 10.1016/j.colsurfb.2018.01.022.
[17]. Y. F. Zhang et al., "Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats," (in English), Nature Medicine, Article vol. 22, no. 10, pp. 1160-1169, Oct 2016, doi: 10.1038/nm.4162.
[18]. C. Gao, S. Peng, P. Feng, and C. Shuai, "Bone biomaterials and interactions with stem cells," (in English), Bone Research, Article vol. 5, no. 4, pp. 253-285, 2017, Art no. 2095-4700(2017)5:4<253:Bbaiws>2.0.Tx;2-9. [Online]. Available: <Go to ISI>://CSCD:6151121.
[19]. J. L. Wang, J. K. Xu, C. Hopkins, D. H. K. Chow, and L. Qin, "Biodegradable Magnesium-Based Implants in Orthopedics-A General Review and Perspectives," (in English), Advanced Science, Review vol. 7, no. 8, p. 19, Apr 2020, Art no. 1902443, doi: 10.1002/advs.201902443.
[20]. "Bio-implants Market by Product Types (Cardiovascular (Stents, Pacing plants Market devices), Dental and Prosthetic bio-implants, Orthopedic/joint reconstruction and replacement bio-implants (Orthobiologics, Trauma Implants, Sport Medicines), Spinal bio-implants, Ophthalmology bio-implants (Glaucoma Implants, Intraocular Implants)) - Global Opportunity Analysis and Industry Forecast, 2013 - 2020." https://www. alliedmarketresearch.com/bio-implants-market (accessed November 25, 2022).
[21]. F. A. Ponce and A. M. Lozano, "Highly cited works in neurosurgery. Part II: the citation classics A review (vol 112, pg 233, 2010)," (in English), Journal of neurosurgery, Correction vol. 120, no. 5, pp. 1252-1257, May 2014, doi: 10.3171/2014.2.JNS14358a.
[22]. J. S. Kreutzer et al., "The top 100 cited neurorehabilitation papers," (in English), NeuroRehabilitation, Article vol. 40, no. 2, pp. 163-174, 2017, doi: 10.3233/nre-161415.
[23]. A. Berlinberg, J. Bilal, I. B. Riaz, and D. J. B. Kurtzman, "The 100 top-cited publications in psoriatic arthritis: a bibliometric analysis," (in English), International journal of dermatology, Review vol. 58, no. 9, pp. 1023-1034, Sep 2019, doi: 10.1111/ijd.14261.
[24]. Y. M. Wei, Z. F. Mi, and Z. M. Huang, "Climate policy modeling: An online SCI-E and SSCI based literature review," (in English), Omega-Int. J. Manage. Sci., Review vol. 57, pp. 70-84, Dec 2015, doi: 10.1016/j.omega.2014.10.011.
[25]. "Clarivate Releases Journal Citation Reports 2022(Impact factor & Ranking of 2021)." https://jcr.clarivate.com/jcr/browse-journals (accessed February 1, 2023).
[26]. L. Waltman, N. J. van Eck, and E. C. M. Noyons, "A unified approach to mapping and clustering of bibliometric networks," (in English), Journal of Informetrics, Article vol. 4, no. 4, pp. 629-635, Oct 2010, doi: 10.1016/j.joi.2010.07.002.
[27]. E. Garfield, "Citation analysis as a tool in journal evaluation," (in eng), Science (New York, N.Y.), vol. 178, no. 4060, pp. 471-9, Nov 3 1972, doi: 10.1126/science.178.4060.471.
[28]. G. M. Hall, "BJA citation classics 1945-1992," (in English), British journal of anaesthesia, Editorial Material vol. 80, no. 1, pp. 4-6, Jan 1998, doi: 10.1093/bja/80.1.4.
[29]. Q. Gao et al., "The top 100 highly cited articles on osteoporosis from 1990 to 2019: a bibliometric and visualized analysis," (in English), Archives of osteoporosis, Article vol. 15, no. 1, p. 11, Sep 2020, Art no. 144, doi: 10.1007/s11657-020-0705-z.
[30]. N. Tang, W. C. Zhang, D. M. George, Y. Su, and T. L. Huang, "The Top 100 Most Cited Articles on Anterior Cruciate Ligament Reconstruction: A Bibliometric Analysis," (in English), Orthopaedic journal of sports medicine, Article vol. 9, no. 2, p. 16, Feb 2021, Art no. 2325967120976372, doi: 10.1177/2325967120976372.
[31]. Q. Chen and G. A. Thouas, "Metallic implant biomaterials," Materials Science and Engineering: R: Reports, vol. 87, pp. 1-57, 2015/01/01/ 2015, doi: https://doi.org/10.1016/ j.mser.2014.10.001.
[32]. X. J. Wang et al., "Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review," (in English), Biomaterials, Review vol. 83, pp. 127-141, Mar 2016, doi: 10.1016/j.biomaterials.2016.01.012.
[33]. A. Yy et al., "Mg bone implant: Features, developments and perspectives," Materials & Design, vol. 185, 2020, doi: 10.1016/j.matdes.2019.108259
[34]. D. W. Zhao, F. Witte, F. Q. Lu, J. L. Wang, J. L. Li, and L. Qin, "Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective," (in English), Biomaterials, Review vol. 112, pp. 287-302, Jan 2017, doi: 10.1016/j.biomaterials.2016.10.017.
[35]. J. W. Lee et al., "Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy," (in English), Proceedings of the National Academy of Sciences of the United States of America, Article vol. 113, no. 3, pp. 716-721, Jan 2016, doi: 10.1073/pnas.1518238113.
[36]. A. H. Yusop, A. A. Bakir, N. A. Shaharom, M. R. Abdul Kadir, and H. Hermawan, "Porous biodegradable metals for hard tissue scaffolds: a review," (in English), International journal of biomaterials, vol. 2012, p. 641430, 2012 (Epub 2012 Jul 2012, doi: 10.1155/2012/641430.
[37]. M. M. Stevens, "Biomaterials for bone tissue engineering," Materials Today, vol. 11, no. 5, pp. 18-25, 2008/05/01/ 2008, doi: https://doi.org/10.1016/S1369-7021(08)70086-5.
[38]. S. Wu, X. Liu, K. Yeung, C. Liu, and X. Yang, "Biomimetic porous scaffolds for bone tissue engineering," Materials Science and Engineering R Reports, vol. 80, pp. 1–36, 2014, doi: 10.1016/j.mser.2014.04.001
[39]. Y. X. L. R. Ma, L. Li, H.L. Tan, J.L. Wang, Y. Li, T.T. Tang, L. Qin,, "Use of a three-dimensional printed polylactide-coglycolide/tricalcium phosphate composite scaffold incorporating magnesium powder to enhance bone defect repair in rabbits," Journal of Orthopaedic Translation, vol. 16, pp. 62-70, 2019/01/01/ 2019, doi: 10.1016/j.jot.2018.07.007.
[40]. J. Čapek and D. Vojtěch, "Properties of porous magnesium prepared by powder metallurgy," (in eng), Mater Sci Eng C Mater Biol Appl, vol. 33, no. 1, pp. 564-9, Jan 1 2013, doi: 10.1016/j.msec.2012.10.002.
[41]. C. C. G. Jia, J. Zhang, Y. Wang, R. Yue, B.J. Luthringer-Feyerabend, R. Willumeit-Roemer, H. Zhang, M. Xiong, H. Huang, "In vitro degradation behavior of Mg scaffolds with three-dimensional interconnected porous structures for bone tissue engineering," Corrosion Science, vol. 144, pp. 301-312, 2018/11/01/ 2018, doi: 10.1016/j.corsci.2018.09.001.
[42]. Y. Li et al., "Additively manufactured biodegradable porous iron," (in English), Acta Biomaterialia, Article vol. 77, pp. 380-393, Sep 2018, doi: 10.1016/j.actbio.2018.07.011.
[43]. A. Kopp et al., "Influence of design and postprocessing parameters on the degradation behavior and mechanical properties of additively manufactured magnesium scaffolds," (in eng), Acta Biomater, vol. 98, pp. 23-35, Oct 15 2019, doi: 10.1016/j.actbio.2019.04.012.
[44]. Y. W. Yang et al., "System development, formability quality and microstructure evolution of selective laser-melted magnesium," (in English), Virtual and Physical Prototyping, Article vol. 11, no. 3, pp. 173-181, 2016, doi: 10.1080/17452759.2016.1210522.
[45]. T. B. A. H.Q. Ang, S. Zhu, C. Gu, M.A. Easton, "Proof stress measurement of die-cast magnesium alloys," Materials & Design, vol. 112, pp. 402-409, 2016, doi: 10.1016/j.matdes.2016.09.088.
[46]. S. Zhang et al., "Research on an Mg-Zn alloy as a degradable biomaterial," Acta Biomater, vol. 6, no. 2, pp. 626-40, Feb 2010, doi: 10.1016/j.actbio.2009.06.028.
[47]. Z. Ying, G. Wu, J. Jiang, H. M. Wong, and P. K. Chu, "Improved corrosion resistance and cytocompatibility of magnesium alloy by two-stage cooling in thermal treatment," Corrosion Science, vol. 59, no. 6, pp. 360–365, 2012, doi: 10.1016/j.corsci.2012.03.020
[48]. L. Zhang et al., "Effects of Different Concentrations of BSA on In Vitro Corrosion Behavior of Pure Zinc in Artificial Plasma," (in eng), ACS biomaterials science & engineering, vol. 8, no. 10, pp. 4365-4376, Oct 10 2022, doi: 10.1021/acsbiomaterials.2c00894.
[49]. P. L. Miller, B. A. Shaw, R. G. Wendt, and W. C. Moshier, "Assessing the Corrosion Resistance of Nonequilibrium Magnesium-Yttrium Alloys," Corrosion -Houston Tx-, vol. 51, no. 12, pp. 922-931, 1995, doi: 10.5006/1.3293568
[50]. V. Kaesel, P.-T. Tai, F.-W. Bach, H. Haferkamp, F. Witte, and H. Windhagen, "Approach to Control the Corrosion of Magnesium by Alloying," in Magnesium, 2003, pp. 534-539.
[51]. E. Willbold et al., "Effect of the addition of low rare earth elements (lanthanum, neodymium, cerium) on the biodegradation and biocompatibility of magnesium," (in eng), Acta Biomater, vol. 11, pp. 554-62, Jan 2015, doi: 10.1016/j.actbio.2014.09.041.
[52]. J. Wang et al., "Surface modification of magnesium alloys developed for bioabsorbable orthopedic implants: a general review," (in eng), Journal of biomedical materials research. Part B, Applied biomaterials, vol. 100, no. 6, pp. 1691-701, Aug 2012, doi: 10.1002/jbm.b.32707.
[53]. J. Tang et al., "Surface coating reduces degradation rate of magnesium alloy developed for orthopaedic applications," Journal of Orthopaedic Translation, vol. 1, no. 1, pp. 41-48, 2013.
[54]. Z. Q. Cui, W. J. Li, L. X. Cheng, D. Q. Gong, W. L. Cheng, and W. X. Wang, "Effect of nano-HA content on the mechanical properties, degradation and biocompatible behavior of Mg-Zn/HA composite prepared by spark plasma sintering," (in English), Materials Characterization, Article vol. 151, pp. 620-631, May 2019, doi: 10.1016/ j.matchar.2019.03.048.
[55]. Y. Deng et al., "Mechanism for corrosion protection of β-TCP reinforced ZK60 via laser rapid solidification," International journal of bioprinting, vol. 4, 11/21 2017, doi: 10.18063/ijb.v4i1.124.
[56]. A. M. Kumar, S. F. Hassan, A. A. Sorour, M. Paramsothy, and M. Gupta, "Investigation on the Controlled Degradation and Invitro Mineralization of Carbon Nanotube Reinforced AZ31 Nanocomposite in Simulated Body Fluid," Met. Mater. Int., vol. 25, pp. 105–116, 2019, doi: 10.1007/s12540-018-0161-0.
[57]. C. Shuai et al., "Biodegradation Resistance and Bioactivity of Hydroxyapatite Enhanced Mg-Zn Composites via Selective Laser Melting," (in eng), Materials (Basel), vol. 10, no. 3, Mar 17 2017, doi: 10.3390/ma10030307.
[58]. H. Shao et al., "3D printing magnesium-doped wollastonite/β-TCP bioceramics scaffolds with high strength and adjustable degradation," Journal of the European Ceramic Society, pp. 1495-1503, 2016, doi: 10.1016/j.jeurceramsoc.2016.01.010
[59]. Y. Wan et al., "Mechanical and biological properties of bioglass/magnesium composites prepared via microwave sintering route," Materials & Design, vol. 99, no. jun., pp. 521-527, 2016, doi: 10.1016/j.matdes.2016.03.096
[60]. B. W. C. Shuai, Y. Yang, S. Peng, C. Gao, "3D honeycomb nanostructure-encapsulated magnesium alloys with superior corrosion resistance and mechanical properties," Composites Part B: Engineering, vol. 162, pp. 611-620, 2019/04/01/ 2019, doi: 10.1016/j.compositesb.2019.01.031.
[61]. Y. Yang, X. Guo, C. He, C. Gao, and C. Shuai, "Regulating Degradation Behavior by Incorporating Mesoporous Silica for Mg Bone Implants," (in eng), ACS biomaterials science & engineering, vol. 4, no. 3, pp. 1046-1054, Mar 12 2018, doi: 10.1021/acsbiomaterials.8b00020.









