1. Introduction
The innate oscillations governing various physiological processes with a roughly 24-hour cycle are orchestrated by the suprachiasmatic nucleus (SCN) situated in the anterior hypothalamus. These rhythms encompass vital aspects of life, including sleep-wake patterns, hormone secretion, thermoregulation, metabolism, and behavioral cycles. Their influence extends across numerous biological domains, with an emerging emphasis on cardiovascular health. Previous research has shed light on the intricate interplay between circadian rhythms and cardiovascular dynamics. However, our investigation advances this field by offering a holistic perspective, encompassing multifaceted facets of circadian influence and focusing on their implications for cardiovascular well-being.
In a modern world characterized by altered lifestyles, increased stressors, and irregular work patterns, circadian rhythms are not as stable as they used to be. These disruptions have raised concerns about their impact on cardiovascular health. While existing literature has uncovered some facets of this relationship, gaps in understanding remain, necessitating a more comprehensive exploration. The paramount concern centers on how disturbances in circadian rhythms might manifest in cardiovascular pathophysiology, potentially elevating the risk of cardiovascular diseases.
This paper delves into the nuanced interdependence between circadian rhythms and cardiovascular dynamics. It spans a broad spectrum of investigations, including the modulation of essential cardiovascular parameters, the consequences of circadian arrhythmias, and the role of the autonomic nervous system. Furthermore, it examines how peripheral clocks, peripheral tissues’ biological clocks, contribute to this intricate dance between circadian rhythms and cardiovascular health. Our research posits that unraveling these complexities holds the key to devising effective strategies for cardiovascular disease prevention and management. In addition, it unravels the intricate connection between circadian rhythms and cardiovascular dynamics. To do so, it draws from a broad spectrum of fields, including physiology, neurobiology, and cardiology. Data analysis and literature reviews supplement clinical assessments of individuals with circadian abnormalities and studies on animal models with altered circadian rhythms. Furthermore, our study incorporates findings from investigations into how the central circadian rhythm is synchronized with the autonomic nervous system, hormonal regulation in cardiovascular tissue, and peripheral clocks. Even more, the paper offers substantial significance for both clinical practice and public health policy. Understanding the multifaceted relationship between circadian rhythms and cardiovascular dynamics can inform the development of targeted interventions for cardiovascular disease prevention and management. It may also guide recommendations for individuals prone to circadian disruptions, such as shift workers. Additionally, the insights gained from this study may illuminate broader implications for circadian health in a society facing increasing circadian challenges due to modern lifestyles. By exploring these intricate interactions, we anticipate contributing to a better understanding of cardiovascular health and strategies for its preservation in the face of circadian disturbances
2. Circadian Arrhythmicity impacts cardiovascular parameters
One salient facet entails the modulation of cardinal cardiovascular parameters, notably blood pressure (BP) and heart rate (HR), by circadian rhythmicity. The diurnal fluctuation in BP, is characterized by higher values during light time and lower values during night hours.
While the exact mechanism remains a mystery, the research examined the genes responsible for the diurnal rhythmicity of cardiovascular parameters and observed that the genes BMAL1 and CLOCK coordinate circadian oscillations in blood pressure.
2.1. Master Clock Gene
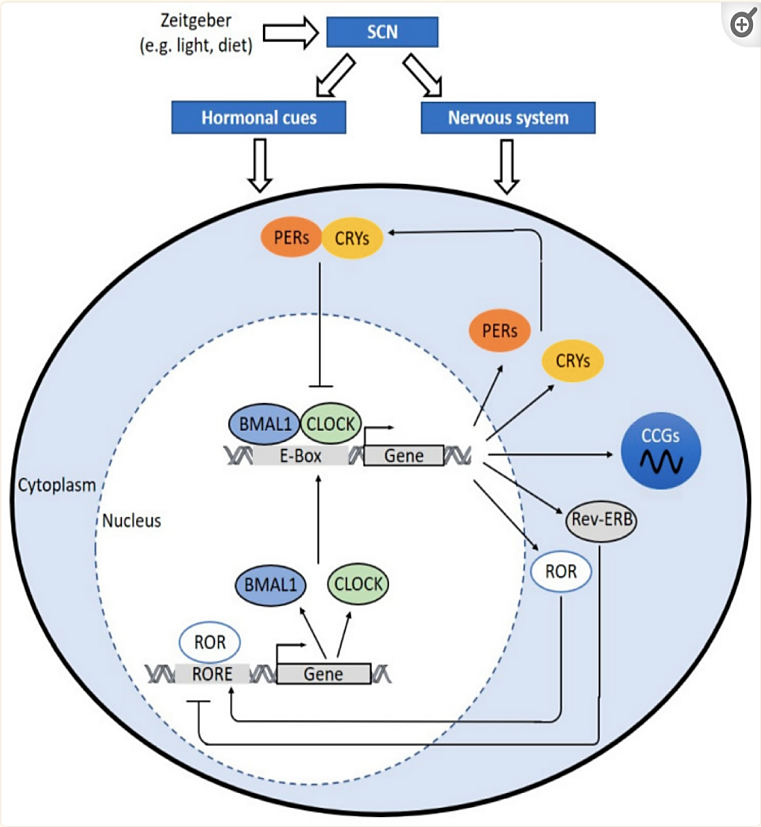
Figure 1. The molecular clock mechanism [1].
The intricate orchestration of our biological processes in response to daily environmental changes is governed by an intrinsic biological rhythm known as the circadian clock. This finely tuned process starts with the suprachiasmatic nucleus (SCN) in the hypothalamus, often referred to as the master clock, which receives external cues such as light and darkness. These cues are then translated into hormonal signals and nerve impulses that act as regulators for peripheral clocks situated throughout the body, including in immune cells, blood vessels, and perivascular adipose tissues. At the heart of this circadian system lie essential clock genes, with two key protagonists being brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein 1 (BMAL1) and circadian locomotor output cycles kaput (CLOCK). These genes, when their respective proteins interact, form a heterodimer that binds to specific DNA sequences known as E-boxes within gene promoters. This binding initiates the transcription of critical clock-related genes, including Period (PER) and Cryptochrome (CRY) genes. These genes, in turn, create proteins that constitute a repressor complex, which moves into the cell nucleus to inhibit the transcriptional activity of CLOCK:BMAL1, effectively serving as “brakes” on the circadian clock.
However, the circadian clock’s regulation extends beyond this central mechanism. It involves a network of interactions, including the involvement of reverse ERB (REV-ERB) and retinoic acid receptor-related orphan receptors (ROR). REV-ERB acts as a negative regulator, dampening the expression of BMAL1 and CLOCK, while ROR takes on the role of a positive regulator, enhancing the expression of these critical clock genes. This intricately woven molecular clockwork not only controls our daily rhythms but also extends its influence on the regulation of genes known as clock-controlled genes (CCGs). These CCGs encode proteins crucial for various physiological processes, including those involved in atherosclerosis development, hemostasis, inflammation, lipid metabolism, and macrophage trafficking. The rhythmic expression of these genes follows a tissue-specific or even cell-type-specific pattern, underscoring the remarkable complexity and precision of the circadian clock system.
So, what does this all have to do with blood pressure regulation? The answer lies in the circadian rhythms that naturally govern our bodies. Blood pressure, for instance, exhibits a circadian pattern characterized by higher values during the day and lower values at night. The coordination of these rhythms relies on the same clock genes, such as BMAL1 and CLOCK. During the light phase, BMAL1 and CLOCK proteins activate the transcription of specific genes, some of which are likely involved in blood pressure regulation. The details of these mechanisms are still subjects of ongoing research but likely encompass the regulation of genes related to vasodilation, vasoconstriction, and other cardiovascular processes.
In summary, our circadian clock, orchestrated by genes like BMAL1 and CLOCK, is a fundamental regulator of our biological processes. While its role in blood pressure regulation is complex and multifaceted, it is evident that disruptions to these circadian rhythms can lead to blood pressure dysregulation, potentially contributing to conditions such as hypertension. Understanding these intricate molecular mechanisms holds promise for uncovering novel therapeutic targets for cardiovascular diseases and may pave the way for innovative approaches to improving overall health and well-being.
2.2. Dysregulation of blood pressure
Given the important role of the adrenal gland in orchestrating circadian oscillations, Tanaka et. al. investigates the adrenal gland circadian clock in spontaneously hypertensive rats (SHR) and control Wistar-Kyoto rats maintained under a 12-hour light-dark cycle, It investigates the circadian clock in the adrenal glands of these rats and analyzes the expression of clock genes, including Bmal1 and Per2, as well as the secretion of corticosterone and aldosterone. Eventually, the authors found out that SHR exhibits aberrant circadian rhythms that can impact the adrenal gland and steroid hormone secretion, potentially leading to cardiovascular consequences, including dysregulated blood pressure [2].
To further support that irregular BP circadian variation can increase the probability of cardiovascular diseases, corroborative, clinical features reflect marked circadian cardiovascular arrhythmicity. discusses the association between diurnal systolic BP (SBP) profiles, particularly the nocturnal SBP dipping patterns, and the risk of chronic kidney disease (CKD) progression. It notes that individuals with non-dipping and reverse-dipping patterns are at a higher risk for CKD progression, highlighting the significance of BP circadian variation in kidney health [3]. Further exemplifying the intimate nexus between circadian arrhythmias and cardiovascular maladies.
3. Disturbances in Circadian Rhythms and Pathophysiological Sequelae
3.1. Shift workers
Concomitantly, the entrainment of circadian rhythms is profoundly enmeshed in an individual’s occupational or lifestyle patterns. The proliferation of shift work, notably among healthcare professionals, to meet the continuous demands of healthcare services has raised concerns regarding its impact on well-being, health promotion, disease prevention, and overall quality of life. This comprehensive review delves into the intricate relationship between shift work and the prevalence of cardiovascular diseases and cancers among healthcare workers. Prolonged exposure to irregular shift work patterns has been consistently associated with an elevated risk of cardiovascular diseases. The disruption of circadian rhythms and cardiopulmonary processes resulting from shift work can have detrimental effects on health [4]. Yet, research in this field faces challenges, chiefly stemming from inconsistent definitions of shift work and varying measurement criteria. This lack of uniformity has led to the misclassification of study populations and underscores the pressing need for standardizing shift work definitions and developing effective intervention strategies.
In specific industries such as the automotive sector, identifying modifiable risk factors among shift workers becomes pivotal for implementing successful preventive measures. A study conducted in a South African car manufacturing company exposed a significant portion of automotive shift workers to risk factors for cardiovascular diseases. A substantial percentage fell within the pre-obese or obese category, while a noteworthy proportion exhibited hypertension. Key parameters like body mass index, blood pressure, cholesterol levels, and age displayed interconnections, hinting at potential links between these factors and cardiovascular risk [5]. In addition, experimental rat models have provided valuable insights into the effects of shift work on metabolic disorders and cardiac function. These models reveal that shift work significantly alters gene expression patterns in the heart, with rhythmic gene expression phases becoming clustered at specific times during the day. Interestingly, restricting food consumption during shift work can impact hundreds of genes in the heart, including those associated with cardiac hypertrophy and fibrosis. These findings caution that interventions aimed at mitigating metabolic disorders in shift workers may inadvertently influence cardiovascular health [6].
In sum, the complex interplay between shift work and cardiovascular well-being underscores the need for a deeper understanding of its underlying mechanisms and the development of targeted strategies to minimize its adverse effects, particularly in industries where shift work is prevalent.
3.2. Myocardial Pathophysiology
In addition, circadian rhythm disturbance amplifies pathophysiology. By comparing mice with normal 24-hour rhythmic patterns to those with 20-hour irregular rhythmic mice. The 20hr mice demonstrate a reduction in vascular wall thickness, decrease in myocyte cross-sectional area, elevation in blood pressure, and increase in left ventricular end-systolic and end-diastolic dimensions [7].
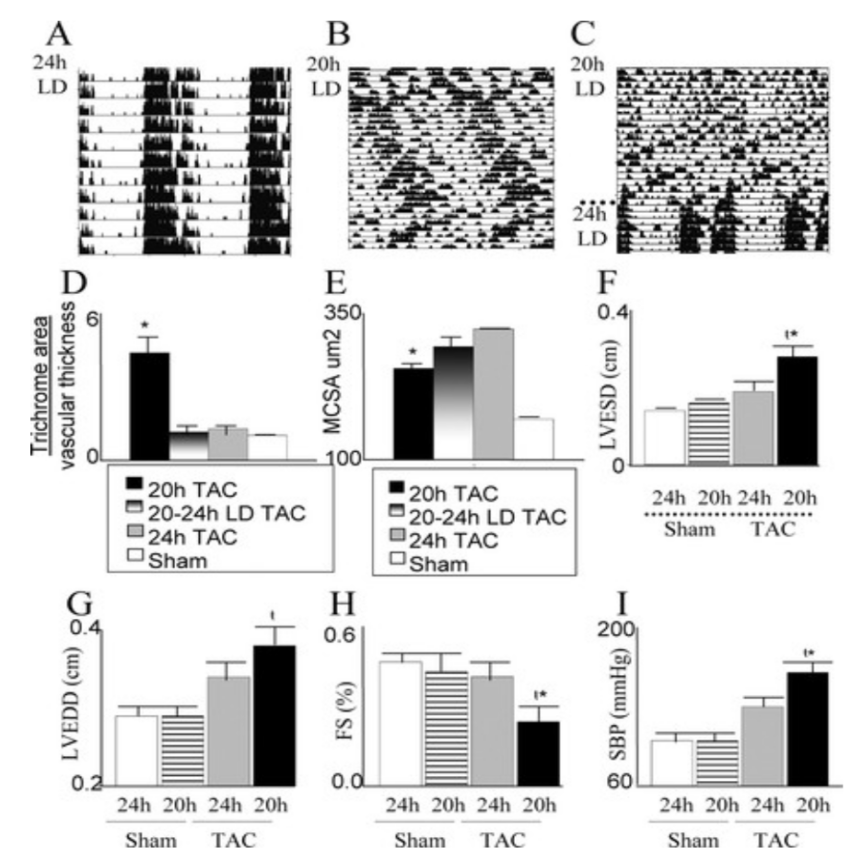
Figure 2. “Cardiac dysfunction is associated with disturbing central and peripheral clocks: remodeling and pathophysiology”[7].
Since the vascular wall thickness is a key factor in maintaining vascular integrity, the thin vascular wall accentuates rupture vulnerabilities. Myocytes are the heart muscle cells responsible for the contraction of the heart, thus the smaller myocardial muscles can reflect insufficient cardiac contractility, meaning less pump of blood, leading to the potential failure of circulation and increased risk of abnormal blood clotting. In addition, the increase of left ventricle end-systolic dimension is associated with cardiovascular death [8], as Increased end-systolic size may indicate impaired ventricular contraction or volume overload, which may indicate impaired cardiac function. Lastly, the left ventricular end-diastolic diameter is a measure of the size of the ventricle at maximum diastole and filling. Elevations may indicate impaired ventricular filling or impaired ventricular dilation, which may be associated with heart failure or other cardiovascular diseases.
4. Circadian Arrhythmicity and Vascular Dynamics
Beyond the myocardium, circadian rhythms wield influence over vascular homeostasis encompassing vasoconstriction, dilation, and clot formation. A study investigated platelet responsiveness among hypertensive individuals to aggregating agents (adrenaline and ADP) during different times of the day. The researchers also discuss whether these changes were associated with changes in platelet membrane α2-adrenoceptors profiles, levels of certain hormones (plasma catecholamines and cortisol), and blood pressure values, which are also influenced by circadian rhythms. The authors found out that the platelets from hypertensive patients show an increased sensitivity to adrenaline in the morning, possibly due to morning blood pressure fluctuations [9]. This anomalous responsiveness, ensnared within circadian arrhythmicity, resonates with augmented proclivities toward myocardial infarction and sudden cardiac demise [10].
5. Autonomic nervous system
As mentioned above, generally the heart rate and blood pressure tend to be high during the day and low at night. Synchronization of SCN with the autonomic nervous system, including the sympathetic and parasympathetic branches governing heart rate and vasoconstriction, bears testament to circadian rhythm’s sway over cardiovascular dynamics. Irregular circadian rhythms may affect the function of the sympathetic nervous system as leading to an imbalance between the body’s internal clock and external signals, potentially affecting the sympathetic response and overall cardiovascular health.
On the other hand, circadian arrhythmias elicit an ebbing of regulatory markers controlled by the vagus nerve. As the vagus nerve is an important part of the parasympathetic nervous system and plays an important role in regulating heart rate and other autonomic nervous functions, impairments in cardiac vagal regulation cause implications for heart health and overall physiological balance. The activation of the vagus nerve leads to a reduction of heart rate, as it releases acetylcholine that binds to receptors on heart cells and slows the electrical impulses that control the heart’s contraction. In addition to controlling the heart rate, the parasympathetic nervous system also affects the conduction of electrical signals within the heart. It slows down the conduction of the AV node, giving the ventricles enough time to fill with blood before contracting. This ensures effective blood pumping and prevents arrhythmias. Eventually, the vagal dysfunction caused by the circadian arrhythmia led to the failure to properly regulate heart rate resulting in a high resting heart rate. Also, with vagus nerve dysfunction, this regulatory mechanism of BP could be impaired, resulting in the accumulation of scar tissue and increased blood pressure.
Furthermore, the SCN governs hormone release timing, spanning stress hormones like cortisol, epinephrine, and norepinephrine, with ramifications for heart rate, blood pressure, and blood vessel function.
6. Peripheral clocks
In parallel to the master clock located in the SCN, peripheral cells in various tissues of the body also have their own biological clocks. These peripheral clocks allow the activities of different tissues to be synchronized with the central circadian rhythm regulated by the SCN. Although the SCN itself does not express corticosteroid receptors (MR and GR), the receptors that bind to corticosteroid hormones, such as cortisol, peripheral cells do express these receptors at varying levels. This means that the peripheral biological clock may be influenced by cortisol, which is the adrenal gland’s response to stress, and follows a circadian rhythm (fluctuating throughout the day). Throughout the cardiovascular system, there are many MR-responsive cardiovascular tissues such as heart, blood vessels, vascular endothelium, and etc. Excessive MR Activation leads to increased activity of the sympathetic nervous system, often referred to as sympathetic arousal, resulting in increased heart rate and blood pressure, and changes in the contractility of the heart muscle, all of which contribute to cardiovascular stress. In addition, this excessive activation can also lead to renal retention, which contributes to fluid retention, hypertension, and other cardiovascular issues. Lastly, prolonged MR activation can lead to cardiac remodeling including fibrosis, and the accumulation of scar tissue, which can impair heart function and contribute to heart failure [11].
7. Cardiovascular Events
Research has shown that the timing of certain cardiovascular events, such as heart attacks and strokes, may also be influenced by the body’s circadian rhythms. The higher frequency of these events at certain times of day is thought to be related to the body’s natural rhythms and the influence of the SCN. A meta-analysis examining 11816 stroke patient symptom onset times highlights morning predominance [12].
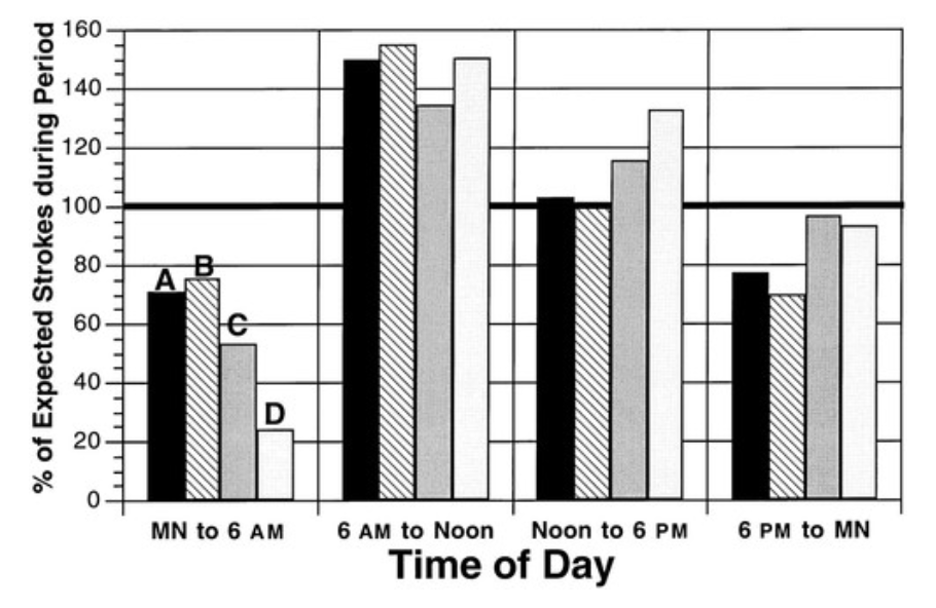
Figure 3. “Circadian patterns of onset of symptoms of stroke, subdivided by subtype of stroke, according to four 6-hour time periods” [12].
8. Conclusion
The interplay between circadian rhythms and cardiovascular dynamics illuminates a nuanced interdependence that extends beyond conventional boundaries. This intricate relationship, explored in this review, reveals the profound influence of circadian rhythms on cardiovascular health. Research findings highlight the multifaceted nature of this connection, underscoring its significance and laying the foundation for future investigations. The disruption of circadian synchronization is a central component in cardiovascular vulnerability. Studies exploring how genes code for circadian oscillations reveal an intimate relationship between these two elements. The genetic makeup, such as BMAL1 and CLOCK, offers insights about the impact on blood pressure regulation. The complications arising from this relationship could extend to inflammation and the peripheral organs further highlighting the comprehensive interaction between the two elements of cardiovascular health.
However, the current body of research leaves room for improvement. While studies have illuminated key aspects of the circadian-cardiovascular nexus, many questions remain unanswered. Future research should delve into the pathophysiological intricacies of this relationship, exploring the precise mechanisms by which circadian disruptions manifest as cardiovascular vulnerabilities. Additionally, interventions targeting circadian rhythms to fortify cardiovascular health require further investigation, offering potential avenues for preventive strategies and clinical applications. Looking ahead, the field of circadian-cardiovascular research holds promise for significant advancements. As our understanding deepens, we can anticipate the development of targeted interventions to mitigate the adverse effects of circadian arrhythmias on cardiovascular health. These interventions may include chronotherapeutic approaches tailored to individual circadian profiles. Furthermore, the integration of circadian considerations into public health policy and clinical practice could yield substantial benefits, reducing the burden of cardiovascular diseases on a global scale.
In conclusion, the intricate interplay between circadian rhythms and cardiovascular dynamics reveals a nuanced relationship with profound implications. Disruptions to circadian synchrony, coupled with complex molecular orchestrations, result in vulnerabilities within the cardiovascular system. This review calls for a deeper exploration of pathophysiological intricacies and underscores the imperative of safeguarding circadian rhythmicity to fortify cardiovascular health. Beyond cardiovascular domains, circadian arrhythmias can perturb various physiological and pathophysiological facets, including inflammation regulation. As we navigate this fascinating intersection of circadian biology and cardiovascular health, the journey ahead promises a brighter future for preventive cardiovascular care and public health policies.
References
[1]. Man AWC, Li H, Xia N. Circadian Rhythm: Potential Therapeutic Target for Atherosclerosis and Thrombosis. Int J Mol Sci. 2021 Jan 12;22(2):676. doi: 10.3390/ijms22020676. PMID: 33445491; PMCID: PMC7827891.
[2]. Tanaka, S., Ueno, T., Tsunemi, A. et al. The adrenal gland circadian clock exhibits a distinct phase advance in spontaneously hypertensive rats. Hypertens Res 42, 165–173 (2019). https://doi.org/10.1038/s41440-018-0148-8.
[3]. Park CH, Jhee JH, Chun KH, Seo J, Lee CJ, Park SH, Hwang JT, Han SH, Kang SW, Park S, Yoo TH. Nocturnal systolic blood pressure dipping and progression of chronic kidney disease. Hypertens Res. 2023 Jul 14. doi: 10.1038/s41440-023-01368-x. Epub ahead of print. PMID: 37452154.
[4]. Yau A, Haque M. Shiftwork Association with Cardiovascular Diseases and Cancers Among Healthcare Workers: A Literature Review. Medeni Med J. 2019;34(4):387-395. doi: 10.5222/MMJ.2019.54775. Epub 2019 Dec 26. PMID: 32821466; PMCID: PMC7433719.
[5]. Travill AL, Soeker F, Overmeyer D, Rickers F. Cardiovascular and metabolic risk factors of shift workers within the automotive industry. Health SA. 2019 Oct 14;24:1227. doi: 10.4102/hsag.v24i0.1227. PMID: 31934435; PMCID: PMC6917447.
[6]. Trott AJ, Greenwell BJ, Karhadkar TR, Guerrero-Vargas NN, Escobar C, Buijs RM, Menet JS. Lack of food intake during shift work alters the heart transcriptome and leads to cardiac tissue fibrosis and inflammation in rats. BMC Biol. 2022 Mar 3;20(1):58. doi: 10.1186/s12915-022-01256-9. PMID: 35236346; PMCID: PMC8892784.
[7]. Tami A. Martino, Nazneen Tata, Denise D. Belsham, Jennifer Chalmers, Marty Straume, Paul Lee, Horia Pribiag, Neelam Khaper, Peter P. Liu, Fayez Dawood, Peter H. Backx, Martin R. Ralph and Michael J. Sole. Disturbed Diurnal Rhythm Alters Gene Expression and Exacerbates Cardiovascular Disease With Rescue by Resynchronization. Originally published5 Mar 2007https://doi.org/10.1161/HYPERTENSIONAHA.106.083568Hypertension. 2007;49:1104–1113.
[8]. Narayanan K, Reinier K, Teodorescu C, Uy-Evanado A, Aleong R, Chugh H, Nichols GA, Gunson K, London B, Jui J, Chugh SS. Left ventricular diameter and risk stratification for sudden cardiac death. J Am Heart Assoc. 2014 Sep 16;3(5):e001193. doi: 10.1161/JAHA.114.001193. PMID: 25227407; PMCID: PMC4323796.
[9]. Mores, Nadia; Martire, Maria; Pistritto, Guiseppa; Volpe, Anna-Rita*; Menini, Edoardo†; Folli, Giuseppe‡; Cardillo, Carmine. Platelet alpha2-adrenoceptors and diurnal changes of platelet aggregability in hypertensive patients. Journal of Hypertension 12(8):p 939-946, August 1994.
[10]. Geoffrey H. Tofler, M.B., Damian Brezinski, Andrew I. Schafer, M.D., Charles A. Czeisler, Ph.D., M.D., John D. Rutherford, M.B., Stefan N. Willich, M.D., Ray E. Gleason, Ph.D., Gordon H. Williams, M.D., and James E. Muller, M.D. Concurrent Morning Increase in Platelet Aggregability and the Risk of Myocardial Infarction and Sudden Cardiac Death
[11]. Sztechman D, Czarzasta K, Cudnoch-Jedrzejewska A, Szczepanska-Sadowska E, Zera T. Aldosterone and mineralocorticoid receptors in regulation of the cardiovascular system and pathological remodelling of the heart and arteries. J Physiol Pharmacol. 2018 Dec;69(6). doi: 10.26402/jpp.2018.6.01. Epub 2019 Mar 18. PMID: 30898981.
[12]. William J. Elliott. Circadian Variation in the Timing of Stroke OnsetA Meta-analysis. 1 May 1998https://doi.org/10.1161/01.STR.29.5.992Stroke. 1998;29:992–996.
Cite this article
Lin,A. (2024). Impact of the circadian clock on cardiovascular physiology and pathophysiology. Theoretical and Natural Science,29,170-177.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Man AWC, Li H, Xia N. Circadian Rhythm: Potential Therapeutic Target for Atherosclerosis and Thrombosis. Int J Mol Sci. 2021 Jan 12;22(2):676. doi: 10.3390/ijms22020676. PMID: 33445491; PMCID: PMC7827891.
[2]. Tanaka, S., Ueno, T., Tsunemi, A. et al. The adrenal gland circadian clock exhibits a distinct phase advance in spontaneously hypertensive rats. Hypertens Res 42, 165–173 (2019). https://doi.org/10.1038/s41440-018-0148-8.
[3]. Park CH, Jhee JH, Chun KH, Seo J, Lee CJ, Park SH, Hwang JT, Han SH, Kang SW, Park S, Yoo TH. Nocturnal systolic blood pressure dipping and progression of chronic kidney disease. Hypertens Res. 2023 Jul 14. doi: 10.1038/s41440-023-01368-x. Epub ahead of print. PMID: 37452154.
[4]. Yau A, Haque M. Shiftwork Association with Cardiovascular Diseases and Cancers Among Healthcare Workers: A Literature Review. Medeni Med J. 2019;34(4):387-395. doi: 10.5222/MMJ.2019.54775. Epub 2019 Dec 26. PMID: 32821466; PMCID: PMC7433719.
[5]. Travill AL, Soeker F, Overmeyer D, Rickers F. Cardiovascular and metabolic risk factors of shift workers within the automotive industry. Health SA. 2019 Oct 14;24:1227. doi: 10.4102/hsag.v24i0.1227. PMID: 31934435; PMCID: PMC6917447.
[6]. Trott AJ, Greenwell BJ, Karhadkar TR, Guerrero-Vargas NN, Escobar C, Buijs RM, Menet JS. Lack of food intake during shift work alters the heart transcriptome and leads to cardiac tissue fibrosis and inflammation in rats. BMC Biol. 2022 Mar 3;20(1):58. doi: 10.1186/s12915-022-01256-9. PMID: 35236346; PMCID: PMC8892784.
[7]. Tami A. Martino, Nazneen Tata, Denise D. Belsham, Jennifer Chalmers, Marty Straume, Paul Lee, Horia Pribiag, Neelam Khaper, Peter P. Liu, Fayez Dawood, Peter H. Backx, Martin R. Ralph and Michael J. Sole. Disturbed Diurnal Rhythm Alters Gene Expression and Exacerbates Cardiovascular Disease With Rescue by Resynchronization. Originally published5 Mar 2007https://doi.org/10.1161/HYPERTENSIONAHA.106.083568Hypertension. 2007;49:1104–1113.
[8]. Narayanan K, Reinier K, Teodorescu C, Uy-Evanado A, Aleong R, Chugh H, Nichols GA, Gunson K, London B, Jui J, Chugh SS. Left ventricular diameter and risk stratification for sudden cardiac death. J Am Heart Assoc. 2014 Sep 16;3(5):e001193. doi: 10.1161/JAHA.114.001193. PMID: 25227407; PMCID: PMC4323796.
[9]. Mores, Nadia; Martire, Maria; Pistritto, Guiseppa; Volpe, Anna-Rita*; Menini, Edoardo†; Folli, Giuseppe‡; Cardillo, Carmine. Platelet alpha2-adrenoceptors and diurnal changes of platelet aggregability in hypertensive patients. Journal of Hypertension 12(8):p 939-946, August 1994.
[10]. Geoffrey H. Tofler, M.B., Damian Brezinski, Andrew I. Schafer, M.D., Charles A. Czeisler, Ph.D., M.D., John D. Rutherford, M.B., Stefan N. Willich, M.D., Ray E. Gleason, Ph.D., Gordon H. Williams, M.D., and James E. Muller, M.D. Concurrent Morning Increase in Platelet Aggregability and the Risk of Myocardial Infarction and Sudden Cardiac Death
[11]. Sztechman D, Czarzasta K, Cudnoch-Jedrzejewska A, Szczepanska-Sadowska E, Zera T. Aldosterone and mineralocorticoid receptors in regulation of the cardiovascular system and pathological remodelling of the heart and arteries. J Physiol Pharmacol. 2018 Dec;69(6). doi: 10.26402/jpp.2018.6.01. Epub 2019 Mar 18. PMID: 30898981.
[12]. William J. Elliott. Circadian Variation in the Timing of Stroke OnsetA Meta-analysis. 1 May 1998https://doi.org/10.1161/01.STR.29.5.992Stroke. 1998;29:992–996.









