1. Introduction
Military and veteran population are considered vulnerable to several mental disorders, the managements of which have long been a challenging task [1]. Despite the advances in conventional treatments including psychotherapies and pharmacotherapies, this unique population often exhibits distinctive symptoms presentation and comorbidities compared to the general population[2], resulting in suboptimal treatment response [3].Considering these challenges, researchers are exploring alternative treatment options, including psychoactive drugs. Psychoactive drugs have been historically used for recreational purposes and portrayed as extremely dangerous drugs for decades due to their ability to alter perception and mood[4]. However, in recent years, an increasing number of studies have investigated their medicinal value. Although still in early stages of research, these drugs show promise as novel and effective treatment options for mental disorders [5]. Considering these factors, evidence of medical use of these drugs for military and veteran populations is warranted. Cannabis and psychedelics are the two most used psychoactive substances.
1.1. Evidence of effectiveness of cannabis and psychedelics for mental disorders
Cannabis, targeting the ECB system [6], may have therapeutic potential for mental disorders, such as depression, anxiety, and PTSD, by reducing symptoms and improving cognitive performance [7]. Delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) are the two most well-known and studied components of cannabis [8]. THC is the primary psychoactive component of cannabis and exerts its effects by binding to CB-1 and CB-2 receptors in the ECB system[9]. Unlike THC, CBD does not have psychoactive properties[10], making it a popular choice for those seeking the potential benefits of cannabis without the “high risk” associated with THC. The properties of CBD seem to depend on several cannabinoid-dependent and independent mechanisms, including interacting with a number of different receptors, such as the CB1 and CB2 receptors, the GPR55 receptor, the TRPV1 receptor, the 5-HT1A receptor[11] and FAAH inhibition[12]. Besides THC and CBD, some synthetic cannabinoids have emerged in recent years, of which nabilone is the most explored one. Nabilone, a synthetic CB1 receptor agonist chemically similar to THC [13], has been shown to be safe and well-tolerated with little evidence of abuse or tolerance development[14]. It has also been used to relieve pain[15] and alleviate PTSD-related insomnia and nightmares [16].
Psychedelics, including substances such as 3,4-methylenedioxymethamphetamine (MDMA), psilocybin, lysergic acid diethylamide (LSD), 5-methoxytryptamine (5-MeO-DMT), and ibogaine, were first explored as therapeutic drugs during the 1960s. These psychedelics act primarily through activating serotonin receptors (e.g., 5-HT1A/2A/C)[17] and can produce substantial changes in sensory perception, mood, cognition, and behavior [18]. Recently, several studies have explored the use of psychedelics as adjunctive therapies in psychotherapy and have shown promising results in reducing symptoms of anxiety, depression, PTSD and substance abuse disorder [19-22].
1.2. Cannabis and psychedelics for military and veteran populations
The specific nature of the profession often exposes military service members to various traumatic events and high levels of stress, making them vulnerable to mental health disorders [23]. Moreover, military members often face unique challenges such as adapting to military culture, separation from loved ones during deployments, and reintegrating into civilian life after service. In the military context, prevalence of traumatic brain injury (TBI) is relatively high, which may result in a range of physical, cognitive, and emotional symptoms [24]. Additionally, somatization symptoms such as musculoskeletal conditions, chronic pain, and insomnia due to rigorous combat experiences and deployment history further affect their mental health [25]. Stigma is common among the military personnel [26]. Taken together, military and veteran populations exhibit considerable distinctions in illness development, symptom presentation and comorbidities, and is often less responsive to traditional mental health treatments compared to general population [27]. While studies have supported the potential therapeutic effects of cannabis and psychedelics for mental disorders among the general population, the extent to which they may be appropriate for military and veteran populations remain unclear. We haven’t found any review focusing on military population.
There has been growing interest in the potential therapeutic benefits of cannabis and psychedelics for mental disorders. Several systematic reviews have been conducted and the results are mainly positive [28-36]. However, relatively rare studies focus on military and veteran populations. Considering the high incidence and distinctive features of mental disorders in this unique population, the medical use profile of cannabis and classical psychedelic for them requires further exploration. Our goal is to collect evidence regarding the medical use of cannabis and psychedelics in treating mental health symptoms in military personnel and veterans.
2. Methods
The present systematic review was documented in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [37]. In 2 November 2023, Medline, PubMed and Web of science online databases were searched from their inception up to October 2023 . In addition, to be as comprehensive as possible, we manually searched through reference listings of included papers and high-related reviews for further studies. Only papers written in English were included. The full search strategies are shown in supplemental annex A; no protocol was registered. Table 1 shows the eligibility criteria. Due to the possible limited numbers of included studies, the assumed large heterogeneity in the type of designs and outcome measurements, a descriptive review was completed.
Two reviewers independently extracted the data on authors, date of publication, sample characteristics, interventions, psychedelics or cannabis details, study designs, control group, outcomes measures and main findings, and consensus were reached by discussion. Data were collected using a specifically designed table finally. For bias assessment, the Cochrane Risk of Bias (Rob) tool was used. Results and analysis are provided in supplemental annex C.
Table 1. PICOS table of eligibility criteria
Items | Details | |
Population | Military personnel and veterans (two studies including several policemen and firefighters were also included considering their similar working experience and the relatively small sample sizes) with symptoms of mental disorders, including PTSD, SUD, depression or anxiety disorders, to some extent | |
Intervention | Using cannabis (including THC, CBD, Nabilone) or classical psychedelics (including MDMA, psilocybin, LSD, 5-MeO-DMT, ibogaine) as monotherapies or adjunctive to conventional therapies | |
Comparator | Placebo or none | |
Outcome | Reported change of symptoms of mental disorders | |
Study design | Study design (S): randomized controlled trails (RCTs), self-control trails and case reports. Narrative and systematic reviews, meta-analyses editorial and book chapters were excluded. | |
PICOS, population, intervention, comparator, outcome, study design | ||
3. Results
Figure 1 shows the PRISMA flow diagram of included and excluded studies. In total, 8 studies were included in this systematic review. Of these, 5 were about cannabis (1 about nabilone, 4 about THC and/or CBD), and 3 were about psychedelics (2 about ibogaine and 5-MeO-DMT, 1 about MDMA). All the included studies in this systematic review were published between 2014 and 2020. Among these studies, three were RCTs [38-40], two were retrospective studies [41, 42], one was a case-matched controlled cross-sectional study [43], one was a self-control study [44], and one was a case study[45]. Four studies were conducted in the United States[17, 40, 43, 45], three studies in Canada[38, 39, 41], and the other in New Mexico [42]. Most of the studies focused on military personnel and veterans with PTSD, who often had comorbidities such as AUD and mood disorders. Of the eight studies, seven included only veterans, while one chose currently serving soldiers. Three RCTs had a small sample size of 10 to 80 participants, with only 6 to 20 participants in each arm. The other four open-label studies had larger sample sizes of 80 to 700 participants. Given the diversity in study designs, medications implicated, and outcome measures, we briefly introduced each study and reached conclusions at the end of the review. The main characteristics of the 8 studies are reported in table 2. Details of the 8 included studies are shown in supplemental annex B.
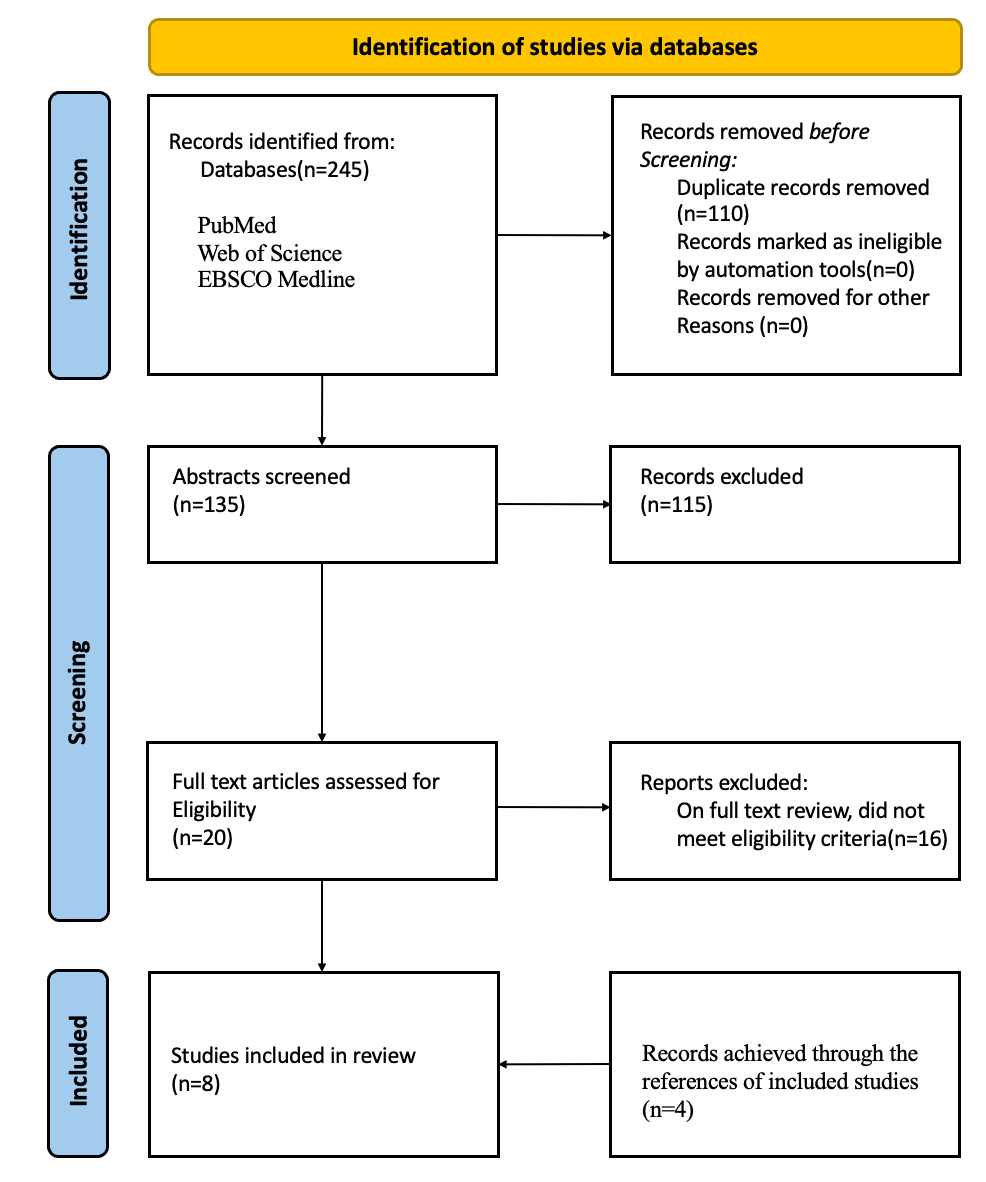
Figure 1. PRISMA flow diagram detailing the study selection process
Table 2. Summary of retrieved papers
Authors (country) | Study Design | Populat-ion | Intervention/ Comparator | Dose (s) and Duration of Medication | ROA | Out- comes | Adverse effects | Follow-up | Main Findings |
Jetly et al. 2014 (Canada) | RCT, crossover | 10 currently serving male soldiers with PTSD | NAB/ Placebo | The average dose achieved in each group was: NAB: 1.95 ± 0.9 mg. PBO: 2.78 ± 0.7mg.; 7 weeks | OS | CAPS CGI-C WBQ | Most common were mouth and headache and no patient dropped out because of adverse effects. | n.d. | NAB induced significant relief for military personnel with PTSD and reductions in symptoms of nightmares. |
Greer et al. 2014 (New Mexico) | A retrospective self-control study | 80 veterans with PTSD | Cannabis (THC+CBD)/ None | n.d.; n.d. | Smoked | CAPS | n.d. | n.d. | Reductions in PTSD symptoms in some patients. |
Smith et al. 2017 (Canada) | A retrospective self-control study | 100 veterans with PTSD | Cannabis (THC+CBD)/ None | 9.4 grams/day on average; n.d. | Smoked | A scale of 0 to 10 including various symptoms | n.d. | The time of follow-up ranged from less than 3 months to 18 months and improvement were significant between baseline and follow-up. | Significant improvements across all PTSD symptoms, as well as social and family impact outcomes and pain severity. |
Bonn-Miller et al. 2020 (Canada) | RCT, crossover | 80 veterans with PTSD | Cannabis (THC+CBD)/ None | Ad libitum use up to 1.8 grams/day; 3 weeks | Smoked | CAPS- 5 PCL-5 IDAS IPF ISI | Cardiac disorders; eye disorders; gastrointestinal disorders; general disorders; infections; injuries; nervous system disorders and psychiatric disorders; etc. | n.d. | All treatment groups, including placebo, showed good tolerability and significant improvements in PTSD symptoms during three weeks of treatment, but no active treatment statistically outperformed placebo in this brief, preliminary trial. |
Johnson et al. 2016 (United States) | A case-matched controlled cross-sectional study | 700 veterans with PTSD | Cannabis / Not using cannabis | n.d.; n.d. | Smoked | PCL-C PHQ-9 PQSIA BOMC TLFB ASSIST | n.d. | n.d. | No association between PTSD scores and frequency of cannabis use. |
Davis et al. 2020 (United States) | A self-control study | 51 Veterans with PTSD | Ibogaine and 5-MeO-DMT/ None | A single dose(10mg/kg) of ibogaine on day 1 and day 3 to 50mg 5-MeO-DMT on day 3;3days. | OS and inhaled | PCL-5 PHQ-2 GAD2-item DSISS MOS-CF subscale AAQ-II | n.d. | n.d. | Significant and large reductions in PTSD, depression and anxiety symptoms, suicidal ideations and cognitive impairment; increased psychological flexibility. |
Barsuglia 2018 (United States) | A case study | A veteran with PTSD and AUD and non-specified mood disorder | Ibogaine and 5-MeO-DMT/ None | 1550mg ibogaine HCl on day1 and 5–7mg 5-MeO-DMT on day3; 3days | Intravenous administrated and inhaled | SPECT examinations Self-report | Ataxia; vomiting; acute panic; hallucination. | The therapeutic effects for alcohol abuse disorder were sustained at 1month, with a partial return to mild alcohol use at 2 months. | Acute remission of alcohol use, reduced cravings for alcohol and improvements in cognition, mood, and interpersonal functioning; reduced orbitofrontal, temporal, occipital and cerebellar perfusion and increased perfusion in bilateral caudate nuclei, left putamen as well as in temporal, occipital and cerebellar regions. |
Mithoefer et al. 2018 (United States) | RCT, crossover | 26 veterans with PTSD | MDMA/ Active control | 30 mg, 75 mg, or 125 mg MDMA; n.d. | OS | CAPS-IV BDI-II PSQI PTGI NEO-PI-R DES-II GAF | 85 adverse events were reported and the most frequently included anxiety, headache, fatigue, and muscle tension. Most adverse reactions were mild to moderate in severity. | Scores on all secondary measures at 12-month follow-up showed improvement compared with baseline. | Active doses (75 mg and 125 mg) of MDMA with adjunctive psychotherapy in a controlled setting were effective and well tolerated in reducing PTSD, depression symptoms and improved sleep quality. |
Abbreviations: ROA: route of administration; n.d.: not documented; PTSD: post-traumatic stress disorder; Nabilone: NAB; CBD: cannabidiol; THC: tetrahydrocannabinol; 5-MeO-DMT:5-methoxy-N,N-dimethyltryptamine; MDMA:3,4-methylenedioxymethamphetamine; OS: oral administrated; PCL-C: PTSD Checklist-Civilian Version; PCL-5: patient-completed PTSD Checklist for the DSM-5; PHQ-2: Patient Health Questionnaire-2; GAD: Generalized Anxiety Disorder; DSISS: Depressive Symptom Index Suicidality Subscale; MOS-CF: Medical Outcomes Study—Cognitive Functioning; AAQ-II: Acceptance and Action Questionnaire II; AUD: alcohol use disorder; IPF: Inventory of Psychosocial Functioning; CMMQ: Comprehensive Marijuana Motives Questionnaire; IDAS: Inventory of Depression and Anxiety Scale; GAF: Global Assessment of Functioning; PCL-M: Post-Traumatic Stress Disorder Checklist-Military Version; CES: Combat Exposure Scale; PSQI: Pittsburgh Sleep Quality Index; NFQ: Nightmare Frequency Questionnaire- Revised; NES: Nightmare effects survey; CAPS: Clinical Administered PTSD Scale; CGI-C: Clinical Global Impression of Change; WBQ: General Well Being Questionnaire; n.d.: not documented; PHQ-9: Patient Health Questionnaire; PQSIA: Paykel questionnaire for suicidal ideations and attempts; BOMC: Blessed Orientation-Memory-Concentration Test; BDI-II: Beck Depression Inventory-II; PSQI: Pittsburgh Sleep Quality Index; PTGI: Post-Traumatic Growth Inventory; DES-II: Dissociative Experiences Scale II; NEO-PI-R: NEO Personality Inventory; TLFB: Timeline Followback; ASSIST: Alcohol, Smoking, and Substance Involvement Screening Test.
3.1. Cannabis
Five studies evaluated the effectiveness of cannabis for psychological issues in military personnel and veterans. A double-blind, placebo-controlled crossover study was conducted by Jetly et al.[82] to investigate the efficacy of NAB in reducing the frequency and intensity of nightmares in male Canadian military personnel with PTSD who experience trauma-related nightmares despite standard treatment. The study suggests that synthetic endocannabinoids, like nabilone, may have potential as a medication for PTSD-related nightmares in the military population, but further studies with larger cohorts are needed to validate these findings. In a study by Greer et al.[42], psychometric data on PTSD symptoms were analyzed in 80 military veterans applying to the New Mexico Medical Cannabis Program from 2009 to 2011. Patients in this sample reported over 75% reduction in all three clusters of PTSD symptoms while using cannabis[42], adding support to the conclusion that reasonable cannabis use may be conducive to PTSD symptom relief in some military veterans. However, details of medication, including dose and duration, were not mentioned, and adverse effects during the medication were not reported. To evaluate the outcomes of medical cannabis for military and police veterans with PTSD, Smith et al.[41] conducted a retrospective study among 100 patients from January 2014 to January 2016. The study concluded that cannabis use resulted in improvements in all PTSD symptoms, social and family outcomes, and pain severity, making it an effective therapy for military veterans with PTSD. However, no data about the side effects of cannabis were collected. Aimed to collect preliminary data on the safety and potential efficacy of three different active concentrations of smoked cannabis (i.e., High THC = approximately 12% THC and < 0.05% CBD; High CBD = 11% CBD and 0.50% THC; THC+CBD = approximately 7.9% THC and 8.1% CBD) compared to placebo (< 0.03% THC and < 0.01% CBD) in the treatment of PTSD among military veterans, Bonn-Miller et al. [38]conducted a randomized, double-blind, placebo-controlled, cross-over design study, assigning 80 participants to 4 groups. This study provided preliminary evidence supporting the potential therapeutic effect and safety of medical cannabis among military personnel, with generally mild and transient side effects reported. Although the above studies reported positive findings on the therapeutic effects of cannabis, including THC, CBD, and nabilone, for symptoms of mental disorders among military and veteran samples, one study reported inconsistent conclusions. Johnson et al.[43]conducted a case-controlled cohort study among a large clinical population of veterans with probable PTSD and concluded that PTSD symptomatology was not associated with the frequency of cannabis use.
3.2. Psychedelics
The efficacy of psychedelic medicine in the treatment of psychological problems among military personnel was examined in three studies. Davis et al. [44] conducted a retrospective cohort study to examine the efficacy of psychedelic treatment with ibogaine and 5-MeO-DMT for trauma-related psychological and cognitive impairment among 65 U.S. Special Operations Forces Veterans. Given the repeated trauma exposure and nature of such exposure experienced by Special Operations Forces, this population may have unique treatment needs. The study suggested that psychedelic-assisted therapy may hold unique promise for this population but did not report follow-up and adverse effects data. Barsuglia [45] presented a case report on the potential therapeutic effects of ibogaine and 5-MeO-DMT in a 31-year-old male military veteran with comorbidity of AUD and PTSD and suggested a short-term therapeutic effect of ibogaine and 5-MeO-DMT.To investigate the efficacy and safety of MDMA-assisted psychotherapy for chronic PTSD, A randomized, double-blind, dose-response, phase 2 trial with 26 participants diagnosed with PTSD investigated the efficacy and safety of MDMA-assisted psychotherapy[40]. The study supported the safety and potential efficacy of MDMA for PTSD and indicated that the efficacy was closely related to the doses. An active dose (75 mg and 125 mg) of MDMA combined with psychotherapy was effective and well-tolerated in alleviating symptoms of PTSD.
4. Discussion
The current article aimed to review the medical applications of cannabis and psychedelics in the context of military and veteran personnel. To our knowledge, this is the first systematic review investigating the available evidence for these psychoactive drugs in treating symptoms of mental disorders among this unique population. Summarizing the existing studies, we found that the conclusion both positive and negative. According to our systematic review, cannabis and psychedelics may offer an innovative, rapid-acting, well-tolerated, and potentially cost-effective intervention for the military population suffering from mental problems. Nevertheless, there are still many disputes and disagreements regarding the application of these drugs.
4.1. Positive
1. Rapid-acting: Cannabis and psychedelics have a unique advantage in their ability to produce quick effects. Patients may experience noticeable improvements in symptoms after taking them for a short period of time or just a few times. Of the five studies included in our review that used cannabis, two reported the duration of medication [38, 39]. One study found that seven weeks of nabilone use provided significant relief for symptoms of nightmares[39], and another found that three weeks of cannabis use showed significant improvements in PTSD symptoms[38]. Psychedelic medicine, on the other hand, does not require daily use for months or years, but rather can be used on one or a few occasions during psychotherapy sessions. Conventional pharmacological treatment and psychotherapy are often time-consuming and result in low patient compliance. Additionally, studies have found that psychedelics can increase patients’ psychological flexibility more rapidly than traditional psychotherapy[46]. Therefore, many patients are willing to adhere to medication and view the treatment process as a positive life experience.
2.Cost-effective. Smith et al. found that during the use of medicinal cannabis, most patients were able to reduce the dosage of other PTSD-related drugs, with some even stopping all drugs during follow-up [41]. A pharmacoeconomic evaluation found that this resulted in an average savings of $2,300 to $3,800 per patient per year. Another longitudinal study found reductions in the use of prescribed psychiatric medication three months after initiation of cannabis[47]. Therefore, if cannabis and psychedelics could replace other expensive conventional pharmacotherapies to some extent among military personnel, the treatment-related costs and financial burden could be considerably reduced.
3. Well-tolerated. Although some side effects were reported in a few studies using cannabis and psychedelics, most were mild or moderate in severity and did not lead to discontinuation[40]. In contrast, traditional therapies, especially pharmacotherapy, are often discontinued due to intolerable side effects such as decreased libido, extreme changes in weight and metabolic function, sleep disruption, anorgasmia, and orgasmic delay[48, 49].
4.2. Negative
1. Limited high-quality evidence. Although interest in using cannabis and psychedelics as adjunctive or alternative treatments for mental health problems has grown dramatically in recent years, there is a notable lack of high-quality evidence regarding their efficacy and safety, especially their long-term effects and delayed or residual side effects. Furthermore, given the documented problems associated with these drugs, such as addiction[38], and the strict regulation of these drugs in many countries, research on their application to treat psychological problems progresses slowly, especially among the military population. Although only a few studies have briefly reported the side effects in our systematic review, indicating they were relatively mild or moderate in severity[38-40, 43], few studies have investigated the long-term effects of these drugs. In terms of available literature, it is not easy to draw a definitive conclusion on the safety of these drugs based on current limited evidence.
2. Lack of standardized protocols. One of the major challenges in using cannabis and psychedelics lies in the diversity of psychoactive ingredients and protocols of administration, none of which has been standardized. For example, cannabis can be found in many different natural plants and synthetic products, and the exact content of psychoactive ingredients can vary significantly. Even though heterogeneity can be minimized under extremely strict conditions, it is rather difficult to set standards of potency and purity[50]. Additionally, different preparations also have different routes of administration, such as smoking, swallowing, absorption sublingually, or topical application[51]. Previous research has shown that the route of administration can directly affect the therapeutic potential and risk of problematic use [52]. There’s no doubt that dose is a direct factor affecting the efficacy and safety of drugs. Although some studies have explored the relationship between dose and efficacy, no standard prescribed dose has been specified.
The systematic review provides valuable insights into the medical use of cannabis and psychedelics among military and veteran populations, but the landscape is complex with mixed results. Currently, there is a severe lack of definitive evidence-based medical guidance on the use of these drugs. Therefore, larger randomized controlled trials with high methodological quality are urgently needed to provide more conclusive evidence on the efficacy and safety of these drugs compared to placebos and conventional therapies. Longitudinal studies are also necessary to evaluate the long-term effects and potential side effects. Additionally, standardized protocols for administration, including specific doses, routes of administration, and treatment duration, need to be developed to allow for uniformity and comparability across studies. Evidence-based medicine is critical for developing clinical guidelines on the medical use of these drugs for mental health issues among military personnel.
5. Conclusions
The relatively high prevalence and unique features of mental disorders among the military and veteran population highlight the need for novel and effective strategies, and some researchers are exploring the potential of psychoactive drugs. The current systematic review focused on collecting evidence on the medical use of two of these drugs--cannabis and psychedelics. The existing evidence is promising but limited, and further research is necessary to provide more robust evidence on the efficacy, safety, and clinical utility of these interventions. Addressing methodological and practical challenges associated with their implementation is also critical. If proven effective and safe, cannabis and classic psychedelics have the potential to improve the lives of military personnel, veterans, and others struggling with mental health disorders who often face unique challenges and barriers to accessing conventional mental health care. The systematic review also has several limitations, including small sample sizes, a limited number of available studies, heterogeneity in experimental design and medication details. The small sample sizes may lead to exaggerated effects of psychoactive drugs and limit the generalizability of conclusions. Additionally, only three out of the eight included studies were randomized controlled trials, while the rest were cohort or self-control studies, including one case report, which may limit the persuasiveness of the results. Most studies did not investigate participants’ past use of psychoactive drugs, which could have affected their response. Finally, most studies relied on self-reported experimental results, which may be subject to bias and reduce the reliability of the studies.
References
[1]. Bogaers, R., E. Geuze, J. van Weeghel, F. Leijten, D. van de Mheen, N. Greenberg, A. D. Rozema and E. Brouwers. “Mental health issues and illness and substance use disorder (non-)disclosure to a supervisor: A cross-sectional study on beliefs, attitudes and needs of military personnel.” BMJ Open 13 (2023): e063125. 10.1136/bmjopen-2022-063125.
[2]. Richardson, J. D., A. Thompson, L. King, F. Ketcheson, P. Shnaider, C. Armour, K. St Cyr, J. Sareen, J. D. Elhai and M. A. Zamorski. “Comorbidity patterns of psychiatric conditions in canadian armed forces personnel.” Can J Psychiatry 64 (2019): 501-10. 10.1177/0706743718816057.
[3]. Straud, C. L., J. Siev, S. Messer and A. K. Zalta. “Examining military population and trauma type as moderators of treatment outcome for first-line psychotherapies for ptsd: A meta-analysis.” J Anxiety Disord 67 (2019): 102133. 10.1016/j.janxdis.2019.102133.
[4]. Mayer, F. P., D. Luethi, L. B. Areal and H. H. Sitte. “Editorial: Old and new psychoactive substances: Pharmacology and potential applications.” Front Psychiatry 13 (2022): 1087005. 10.3389/fpsyt.2022.1087005.
[5]. Varker, T., L. Watson, K. Gibson, D. Forbes and M. L. O’Donnell. “Efficacy of psychoactive drugs for the treatment of posttraumatic stress disorder: A systematic review of mdma, ketamine, lsd and psilocybin.” J Psychoactive Drugs 53 (2021): 85-95. 10.1080/02791072.2020.1817639.
[6]. Le Boisselier, R., J. Alexandre, V. Lelong-Boulouard and D. Debruyne. “Focus on cannabinoids and synthetic cannabinoids.” Clin Pharmacol Ther 101 (2017): 220-29. 10.1002/cpt.563.
[7]. Sharafi, A., S. Pakkhesal, A. Fakhari, N. Khajehnasiri and A. Ahmadalipour. “Rapid treatments for depression: Endocannabinoid system as a therapeutic target.” Neurosci Biobehav Rev 137 (2022): 104635. 10.1016/j.neubiorev.2022.104635.
[8]. Adams, I. B. and B. R. Martin. “Cannabis: Pharmacology and toxicology in animals and humans.” Addiction 91 (1996): 1585-614.
[9]. Castillo-Arellano, J., A. Canseco-Alba, S. J. Cutler and F. León. “The polypharmacological effects of cannabidiol.” Molecules 28 (2023): 10.3390/molecules28073271.
[10]. Crippa, J. A., F. S. Guimarães, A. C. Campos and A. W. Zuardi. “Translational investigation of the therapeutic potential of cannabidiol (cbd): Toward a new age.” Front Immunol 9 (2018): 2009. 10.3389/fimmu.2018.02009.
[11]. Pacher, P., N. M. Kogan and R. Mechoulam. “Beyond thc and endocannabinoids.” Annu Rev Pharmacol Toxicol 60 (2020): 637-59. 10.1146/annurev-pharmtox-010818-021441.
[12]. Sbarski, B. and I. Akirav. “Cannabinoids as therapeutics for ptsd.” Pharmacol Ther 211 (2020): 107551. 10.1016/j.pharmthera.2020.107551.
[13]. Fernández-Ruiz, J., I. Galve-Roperh, O. Sagredo and M. Guzmán. “Possible therapeutic applications of cannabis in the neuropsychopharmacology field.” European Neuropsychopharmacology 36 (2020):
[14]. Bajtel, Á., T. Kiss, B. Tóth, S. Kiss, P. Hegyi, N. Vörhendi, B. Csupor-Löffler, N. Gede, J. Hohmann and D. Csupor. “The safety of dronabinol and nabilone: A systematic review and meta-analysis of clinical trials.” Pharmaceuticals (Basel) 15 (2022): 10.3390/ph15010100.
[15]. Bilbao, A. and R. Spanagel. “Medical cannabinoids: A pharmacology-based systematic review and meta-analysis for all relevant medical indications.” BMC Med 20 (2022): 259. 10.1186/s12916-022-02459-1.
[16]. Rehman, Y., A. Saini, S. Huang, E. Sood, R. Gill and S. Yanikomeroglu. “Cannabis in the management of ptsd: A systematic review.” AIMS Neurosci 8 (2021): 414-34. 10.3934/Neuroscience.2021022.
[17]. McClure-Begley, T. D. and B. L. Roth. “The promises and perils of psychedelic pharmacology for psychiatry.” Nat Rev Drug Discov 21 (2022): 463-73. 10.1038/s41573-022-00421-7.
[18]. Ziff, S., B. Stern, G. Lewis, M. Majeed and V. R. Gorantla. “Analysis of psilocybin-assisted therapy in medicine: A narrative review.” Cureus 14 (2022): e21944. 10.7759/cureus.21944.
[19]. Carhart-Harris, R. L., M. Bolstridge, J. Rucker, C. M. Day, D. Erritzoe, M. Kaelen, M. Bloomfield, J. A. Rickard, B. Forbes, A. Feilding, et al. “Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study.” Lancet Psychiatry 3 (2016): 619-27. 10.1016/s2215-0366(16)30065-7.
[20]. Carhart-Harris, R., B. Giribaldi, R. Watts, M. Baker-Jones, A. Murphy-Beiner, R. Murphy, J. Martell, A. Blemings, D. Erritzoe and D. J. Nutt. “Trial of psilocybin versus escitalopram for depression.” N Engl J Med 384 (2021): 1402-11. 10.1056/NEJMoa2032994.
[21]. Kelly, D. F., K. Heinzerling, A. Sharma, S. Gowrinathan, K. Sergi and R. J. Mallari. “Psychedelic-assisted therapy and psychedelic science: A review and perspective on opportunities in neurosurgery and neuro-oncology.” Neurosurgery 92 (2023): 680-94. 10.1227/neu.0000000000002275.
[22]. da Costa, S. C., T. Oesterle, T. A. Rummans, E. Richelson and M. Gold. “Psychedelic drugs for psychiatric disorders.” J Neurol Sci 440 (2022): 120332. 10.1016/j.jns.2022.120332.
[23]. Parastouei, K., H. Rostami and M. Chambari. “The association between a priori dietary patterns and psychological disorders in military personnel.” BMC Psychiatry 23 (2023): 203. 10.1186/s12888-023-04650-x.
[24]. Graham, N. S. N., G. Blissitt, K. Zimmerman, D. Friedland, M. E. Dumas, E. Coady, A. Heslegrave, H. Zetterberg, V. Escott-Price, S. Schofield, et al. “Advance-tbi study protocol: Traumatic brain injury outcomes in uk military personnel serving in afghanistan between 2003 and 2014 - a longitudinal cohort study.” BMJ Open 13 (2023): e069243. 10.1136/bmjopen-2022-069243.
[25]. Easterbrook, B., R. A. Plouffe, S. A. Houle, A. Liu, M. C. McKinnon, A. R. Ashbaugh, N. Mota, T. O. Afifi, M. W. Enns, J. D. Richardson, et al. “Moral injury associated with increased odds of past-year mental health disorders: A canadian armed forces examination.” Eur J Psychotraumatol 14 (2023): 2192622. 10.1080/20008066.2023.2192622.
[26]. Fikretoglu, D., M. L. Sharp, A. B. Adler, S. Bélanger, H. Benassi, C. Bennett, R. Bryant, W. Busuttil, H. Cramm, N. Fear, et al. “Pathways to mental health care in active military populations across the five-eyes nations: An integrated perspective.” Clin Psychol Rev 91 (2022): 102100. 10.1016/j.cpr.2021.102100.
[27]. Liu, J. J., N. Ein, C. Forchuk, S. G. Wanklyn, S. Ragu, S. Saroya, A. Nazarov and J. D. Richardson. “A meta-analysis of internet-based cognitive behavioral therapy for military and veteran populations.” BMC Psychiatry 23 (2023): 223. 10.1186/s12888-023-04668-1.
[28]. Rice, L. J., L. Cannon, N. Dadlani, M. M. Y. Cheung, S. L. Einfeld, D. Efron, D. R. Dossetor and E. J. Elliott. “Efficacy of cannabinoids in neurodevelopmental and neuropsychiatric disorders among children and adolescents: A systematic review.” Eur Child Adolesc Psychiatry (2023): 10.1007/s00787-023-02169-w.
[29]. Narayan, A. J., L. A. Downey, B. Manning and A. C. Hayley. “Cannabinoid treatments for anxiety: A systematic review and consideration of the impact of sleep disturbance.” Neurosci Biobehav Rev 143 (2022): 104941. 10.1016/j.neubiorev.2022.104941.
[30]. Black, N., E. Stockings, G. Campbell, L. T. Tran, D. Zagic, W. D. Hall, M. Farrell and L. Degenhardt. “Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: A systematic review and meta-analysis.” Lancet Psychiatry 6 (2019): 995-1010. 10.1016/s2215-0366(19)30401-8.
[31]. Orsolini, L., S. Chiappini, U. Volpe, D. Berardis, R. Latini, G. D. Papanti and A. J. M. Corkery. “Use of medicinal cannabis and synthetic cannabinoids in post-traumatic stress disorder (ptsd): A systematic review.” Medicina (Kaunas) 55 (2019): 10.3390/medicina55090525.
[32]. van der Meer, P. B., J. J. Fuentes, A. A. Kaptein, J. W. Schoones, M. M. de Waal, A. E. Goudriaan, K. Kramers, A. Schellekens, M. Somers, M. G. Bossong, et al. “Therapeutic effect of psilocybin in addiction: A systematic review.” Front Psychiatry 14 (2023): 1134454. 10.3389/fpsyt.2023.1134454.
[33]. De Aquino, J. P., A. Bahji, O. Gómez and M. Sofuoglu. “Alleviation of opioid withdrawal by cannabis and delta-9-tetrahydrocannabinol: A systematic review of observational and experimental human studies.” Drug Alcohol Depend 241 (2022): 109702. 10.1016/j.drugalcdep.2022.109702.
[34]. Dos Santos, R. G., J. C. Bouso, M. Alcázar-Córcoles and J. E. C. Hallak. “Efficacy, tolerability, and safety of serotonergic psychedelics for the management of mood, anxiety, and substance-use disorders: A systematic review of systematic reviews.” Expert Rev Clin Pharmacol 11 (2018): 889-902. 10.1080/17512433.2018.1511424.
[35]. Ko, K., E. I. Kopra, A. J. Cleare and J. J. Rucker. “Psychedelic therapy for depressive symptoms: A systematic review and meta-analysis.” J Affect Disord 322 (2023): 194-204. 10.1016/j.jad.2022.09.168.
[36]. Berkovitch, L., B. Roméo, L. Karila, R. Gaillard and A. Benyamina. “[efficacy of psychedelics in psychiatry, a systematic review of the literature].” Encephale 47 (2021): 376-87. 10.1016/j.encep.2020.12.002. Efficacité des psychédéliques en psychiatrie, une revue systématique.
[37]. Page, M. J., D. Moher, P. M. Bossuyt, I. Boutron, T. C. Hoffmann, C. D. Mulrow, L. Shamseer, J. M. Tetzlaff, E. A. Akl, S. E. Brennan, et al. “Prisma 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews.” Bmj 372 (2021): n160. 10.1136/bmj.n160.
[38]. Bonn-Miller, M. O., S. Sisley, P. Riggs, B. Yazar-Klosinski, J. B. Wang, M. J. E. Loflin, B. Shechet, C. Hennigan, R. Matthews, A. Emerson, et al. “The short-term impact of 3 smoked cannabis preparations versus placebo on ptsd symptoms: A randomized cross-over clinical trial.” PLoS One 16 (2021): e0246990. 10.1371/journal.pone.0246990.
[39]. Jetly, R., A. Heber, G. Fraser and D. Boisvert. “The efficacy of nabilone, a synthetic cannabinoid, in the treatment of ptsd-associated nightmares: A preliminary randomized, double-blind, placebo-controlled cross-over design study.” Psychoneuroendocrinology 51 (2015): 585-8. 10.1016/j.psyneuen.2014.11.002.
[40]. Mithoefer, M. C., A. T. Mithoefer, A. A. Feduccia, L. Jerome, M. Wagner, J. Wymer, J. Holland, S. Hamilton, B. Yazar-Klosinski, A. Emerson, et al. “3,4-methylenedioxymethamphetamine (mdma)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: A randomised, double-blind, dose-response, phase 2 clinical trial.” Lancet Psychiatry 5 (2018): 486-97. 10.1016/s2215-0366(18)30135-4.
[41]. Smith, P. A., S. Chan, A. Blake, A. Wolt and S. O’Hearn. “Medical cannabis use in military and police veterans diagnosed with post-traumatic stress disorder (ptsd).” Journal of Pain Management 10 (2017): 397-405.
[42]. Greer, G. R., C. S. Grob and A. L. Halberstadt. “Ptsd symptom reports of patients evaluated for the new mexico medical cannabis program.” J Psychoactive Drugs 46 (2014): 73-7. 10.1080/02791072.2013.873843.
[43]. Johnson, M. J., J. D. Pierce, S. Mavandadi, J. Klaus, D. Defelice, E. Ingram and D. W. Oslin. “Mental health symptom severity in cannabis using and non-using veterans with probable ptsd.” J Affect Disord 190 (2016): 439-42. 10.1016/j.jad.2015.10.048.
[44]. Davis, A. K., L. A. Averill, N. D. Sepeda, J. P. Barsuglia and T. Amoroso. “Psychedelic treatment for trauma-related psychological and cognitive impairment among us special operations forces veterans.” Chronic Stress (Thousand Oaks) 4 (2020): 2470547020939564. 10.1177/2470547020939564.
[45]. Barsuglia, J. P., M. Polanco, R. Palmer, B. J. Malcolm, B. Kelmendi and T. Calvey. “A case report spect study and theoretical rationale for the sequential administration of ibogaine and 5-meo-dmt in the treatment of alcohol use disorder.” Prog Brain Res 242 (2018): 121-58. 10.1016/bs.pbr.2018.08.002.
[46]. Watts, R. and J. B. Luoma. “The use of the psychological flexibility model to support psychedelic assisted therapy.” Journal of Contextual Behavioral Science 15 (2020): 92-102.
[47]. Gruber, S. A., K. A. Sagar, M. K. Dahlgren, M. T. Racine, R. T. Smith and S. E. Lukas. “Splendor in the grass? A pilot study assessing the impact of medical marijuana on executive function.” Front Pharmacol 7 (2016): 355. 10.3389/fphar.2016.00355.
[48]. Cohen, J., Z. Wei, J. Phang, R. B. Laprairie and Y. Zhang. “Cannabinoids as an emerging therapy for posttraumatic stress disorder and substance use disorders.” J Clin Neurophysiol 37 (2020): 28-34. 10.1097/wnp.0000000000000612.
[49]. Alexander, W. “Pharmacotherapy for post-traumatic stress disorder in combat veterans: Focus on antidepressants and atypical antipsychotic agents.” P t 37 (2012): 32-8.
[50]. Haney, M., E. W. Gunderson, J. Rabkin, C. L. Hart, S. K. Vosburg, S. D. Comer and R. W. Foltin. “Dronabinol and marijuana in hiv-positive marijuana smokers. Caloric intake, mood, and sleep.” J Acquir Immune Defic Syndr 45 (2007): 545-54. 10.1097/QAI.0b013e31811ed205.
[51]. Strasser, F., D. Luftner, K. Possinger, G. Ernst, T. Ruhstaller, W. Meissner, Y. D. Ko, M. Schnelle, M. Reif and T. Cerny. “Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: A multicenter, phase iii, randomized, double-blind, placebo-controlled clinical trial from the cannabis-in-cachexia-study-group.” J Clin Oncol 24 (2006): 3394-400. 10.1200/jco.2005.05.1847.
[52]. Van Dam, N. T. and M. Earleywine. “Pulmonary function in cannabis users: Support for a clinical trial of the vaporizer.” Int J Drug Policy 21 (2010): 511-3. 10.1016/j.drugpo.2010.04.001.
Cite this article
Zhao,H.;Liang,K.;Fang,Y. (2024). Medical use of cannabis and psychedelics in treating symptoms of mental disorders among military and veteran populations: A systematic review. Theoretical and Natural Science,32,298-312.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Bogaers, R., E. Geuze, J. van Weeghel, F. Leijten, D. van de Mheen, N. Greenberg, A. D. Rozema and E. Brouwers. “Mental health issues and illness and substance use disorder (non-)disclosure to a supervisor: A cross-sectional study on beliefs, attitudes and needs of military personnel.” BMJ Open 13 (2023): e063125. 10.1136/bmjopen-2022-063125.
[2]. Richardson, J. D., A. Thompson, L. King, F. Ketcheson, P. Shnaider, C. Armour, K. St Cyr, J. Sareen, J. D. Elhai and M. A. Zamorski. “Comorbidity patterns of psychiatric conditions in canadian armed forces personnel.” Can J Psychiatry 64 (2019): 501-10. 10.1177/0706743718816057.
[3]. Straud, C. L., J. Siev, S. Messer and A. K. Zalta. “Examining military population and trauma type as moderators of treatment outcome for first-line psychotherapies for ptsd: A meta-analysis.” J Anxiety Disord 67 (2019): 102133. 10.1016/j.janxdis.2019.102133.
[4]. Mayer, F. P., D. Luethi, L. B. Areal and H. H. Sitte. “Editorial: Old and new psychoactive substances: Pharmacology and potential applications.” Front Psychiatry 13 (2022): 1087005. 10.3389/fpsyt.2022.1087005.
[5]. Varker, T., L. Watson, K. Gibson, D. Forbes and M. L. O’Donnell. “Efficacy of psychoactive drugs for the treatment of posttraumatic stress disorder: A systematic review of mdma, ketamine, lsd and psilocybin.” J Psychoactive Drugs 53 (2021): 85-95. 10.1080/02791072.2020.1817639.
[6]. Le Boisselier, R., J. Alexandre, V. Lelong-Boulouard and D. Debruyne. “Focus on cannabinoids and synthetic cannabinoids.” Clin Pharmacol Ther 101 (2017): 220-29. 10.1002/cpt.563.
[7]. Sharafi, A., S. Pakkhesal, A. Fakhari, N. Khajehnasiri and A. Ahmadalipour. “Rapid treatments for depression: Endocannabinoid system as a therapeutic target.” Neurosci Biobehav Rev 137 (2022): 104635. 10.1016/j.neubiorev.2022.104635.
[8]. Adams, I. B. and B. R. Martin. “Cannabis: Pharmacology and toxicology in animals and humans.” Addiction 91 (1996): 1585-614.
[9]. Castillo-Arellano, J., A. Canseco-Alba, S. J. Cutler and F. León. “The polypharmacological effects of cannabidiol.” Molecules 28 (2023): 10.3390/molecules28073271.
[10]. Crippa, J. A., F. S. Guimarães, A. C. Campos and A. W. Zuardi. “Translational investigation of the therapeutic potential of cannabidiol (cbd): Toward a new age.” Front Immunol 9 (2018): 2009. 10.3389/fimmu.2018.02009.
[11]. Pacher, P., N. M. Kogan and R. Mechoulam. “Beyond thc and endocannabinoids.” Annu Rev Pharmacol Toxicol 60 (2020): 637-59. 10.1146/annurev-pharmtox-010818-021441.
[12]. Sbarski, B. and I. Akirav. “Cannabinoids as therapeutics for ptsd.” Pharmacol Ther 211 (2020): 107551. 10.1016/j.pharmthera.2020.107551.
[13]. Fernández-Ruiz, J., I. Galve-Roperh, O. Sagredo and M. Guzmán. “Possible therapeutic applications of cannabis in the neuropsychopharmacology field.” European Neuropsychopharmacology 36 (2020):
[14]. Bajtel, Á., T. Kiss, B. Tóth, S. Kiss, P. Hegyi, N. Vörhendi, B. Csupor-Löffler, N. Gede, J. Hohmann and D. Csupor. “The safety of dronabinol and nabilone: A systematic review and meta-analysis of clinical trials.” Pharmaceuticals (Basel) 15 (2022): 10.3390/ph15010100.
[15]. Bilbao, A. and R. Spanagel. “Medical cannabinoids: A pharmacology-based systematic review and meta-analysis for all relevant medical indications.” BMC Med 20 (2022): 259. 10.1186/s12916-022-02459-1.
[16]. Rehman, Y., A. Saini, S. Huang, E. Sood, R. Gill and S. Yanikomeroglu. “Cannabis in the management of ptsd: A systematic review.” AIMS Neurosci 8 (2021): 414-34. 10.3934/Neuroscience.2021022.
[17]. McClure-Begley, T. D. and B. L. Roth. “The promises and perils of psychedelic pharmacology for psychiatry.” Nat Rev Drug Discov 21 (2022): 463-73. 10.1038/s41573-022-00421-7.
[18]. Ziff, S., B. Stern, G. Lewis, M. Majeed and V. R. Gorantla. “Analysis of psilocybin-assisted therapy in medicine: A narrative review.” Cureus 14 (2022): e21944. 10.7759/cureus.21944.
[19]. Carhart-Harris, R. L., M. Bolstridge, J. Rucker, C. M. Day, D. Erritzoe, M. Kaelen, M. Bloomfield, J. A. Rickard, B. Forbes, A. Feilding, et al. “Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study.” Lancet Psychiatry 3 (2016): 619-27. 10.1016/s2215-0366(16)30065-7.
[20]. Carhart-Harris, R., B. Giribaldi, R. Watts, M. Baker-Jones, A. Murphy-Beiner, R. Murphy, J. Martell, A. Blemings, D. Erritzoe and D. J. Nutt. “Trial of psilocybin versus escitalopram for depression.” N Engl J Med 384 (2021): 1402-11. 10.1056/NEJMoa2032994.
[21]. Kelly, D. F., K. Heinzerling, A. Sharma, S. Gowrinathan, K. Sergi and R. J. Mallari. “Psychedelic-assisted therapy and psychedelic science: A review and perspective on opportunities in neurosurgery and neuro-oncology.” Neurosurgery 92 (2023): 680-94. 10.1227/neu.0000000000002275.
[22]. da Costa, S. C., T. Oesterle, T. A. Rummans, E. Richelson and M. Gold. “Psychedelic drugs for psychiatric disorders.” J Neurol Sci 440 (2022): 120332. 10.1016/j.jns.2022.120332.
[23]. Parastouei, K., H. Rostami and M. Chambari. “The association between a priori dietary patterns and psychological disorders in military personnel.” BMC Psychiatry 23 (2023): 203. 10.1186/s12888-023-04650-x.
[24]. Graham, N. S. N., G. Blissitt, K. Zimmerman, D. Friedland, M. E. Dumas, E. Coady, A. Heslegrave, H. Zetterberg, V. Escott-Price, S. Schofield, et al. “Advance-tbi study protocol: Traumatic brain injury outcomes in uk military personnel serving in afghanistan between 2003 and 2014 - a longitudinal cohort study.” BMJ Open 13 (2023): e069243. 10.1136/bmjopen-2022-069243.
[25]. Easterbrook, B., R. A. Plouffe, S. A. Houle, A. Liu, M. C. McKinnon, A. R. Ashbaugh, N. Mota, T. O. Afifi, M. W. Enns, J. D. Richardson, et al. “Moral injury associated with increased odds of past-year mental health disorders: A canadian armed forces examination.” Eur J Psychotraumatol 14 (2023): 2192622. 10.1080/20008066.2023.2192622.
[26]. Fikretoglu, D., M. L. Sharp, A. B. Adler, S. Bélanger, H. Benassi, C. Bennett, R. Bryant, W. Busuttil, H. Cramm, N. Fear, et al. “Pathways to mental health care in active military populations across the five-eyes nations: An integrated perspective.” Clin Psychol Rev 91 (2022): 102100. 10.1016/j.cpr.2021.102100.
[27]. Liu, J. J., N. Ein, C. Forchuk, S. G. Wanklyn, S. Ragu, S. Saroya, A. Nazarov and J. D. Richardson. “A meta-analysis of internet-based cognitive behavioral therapy for military and veteran populations.” BMC Psychiatry 23 (2023): 223. 10.1186/s12888-023-04668-1.
[28]. Rice, L. J., L. Cannon, N. Dadlani, M. M. Y. Cheung, S. L. Einfeld, D. Efron, D. R. Dossetor and E. J. Elliott. “Efficacy of cannabinoids in neurodevelopmental and neuropsychiatric disorders among children and adolescents: A systematic review.” Eur Child Adolesc Psychiatry (2023): 10.1007/s00787-023-02169-w.
[29]. Narayan, A. J., L. A. Downey, B. Manning and A. C. Hayley. “Cannabinoid treatments for anxiety: A systematic review and consideration of the impact of sleep disturbance.” Neurosci Biobehav Rev 143 (2022): 104941. 10.1016/j.neubiorev.2022.104941.
[30]. Black, N., E. Stockings, G. Campbell, L. T. Tran, D. Zagic, W. D. Hall, M. Farrell and L. Degenhardt. “Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: A systematic review and meta-analysis.” Lancet Psychiatry 6 (2019): 995-1010. 10.1016/s2215-0366(19)30401-8.
[31]. Orsolini, L., S. Chiappini, U. Volpe, D. Berardis, R. Latini, G. D. Papanti and A. J. M. Corkery. “Use of medicinal cannabis and synthetic cannabinoids in post-traumatic stress disorder (ptsd): A systematic review.” Medicina (Kaunas) 55 (2019): 10.3390/medicina55090525.
[32]. van der Meer, P. B., J. J. Fuentes, A. A. Kaptein, J. W. Schoones, M. M. de Waal, A. E. Goudriaan, K. Kramers, A. Schellekens, M. Somers, M. G. Bossong, et al. “Therapeutic effect of psilocybin in addiction: A systematic review.” Front Psychiatry 14 (2023): 1134454. 10.3389/fpsyt.2023.1134454.
[33]. De Aquino, J. P., A. Bahji, O. Gómez and M. Sofuoglu. “Alleviation of opioid withdrawal by cannabis and delta-9-tetrahydrocannabinol: A systematic review of observational and experimental human studies.” Drug Alcohol Depend 241 (2022): 109702. 10.1016/j.drugalcdep.2022.109702.
[34]. Dos Santos, R. G., J. C. Bouso, M. Alcázar-Córcoles and J. E. C. Hallak. “Efficacy, tolerability, and safety of serotonergic psychedelics for the management of mood, anxiety, and substance-use disorders: A systematic review of systematic reviews.” Expert Rev Clin Pharmacol 11 (2018): 889-902. 10.1080/17512433.2018.1511424.
[35]. Ko, K., E. I. Kopra, A. J. Cleare and J. J. Rucker. “Psychedelic therapy for depressive symptoms: A systematic review and meta-analysis.” J Affect Disord 322 (2023): 194-204. 10.1016/j.jad.2022.09.168.
[36]. Berkovitch, L., B. Roméo, L. Karila, R. Gaillard and A. Benyamina. “[efficacy of psychedelics in psychiatry, a systematic review of the literature].” Encephale 47 (2021): 376-87. 10.1016/j.encep.2020.12.002. Efficacité des psychédéliques en psychiatrie, une revue systématique.
[37]. Page, M. J., D. Moher, P. M. Bossuyt, I. Boutron, T. C. Hoffmann, C. D. Mulrow, L. Shamseer, J. M. Tetzlaff, E. A. Akl, S. E. Brennan, et al. “Prisma 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews.” Bmj 372 (2021): n160. 10.1136/bmj.n160.
[38]. Bonn-Miller, M. O., S. Sisley, P. Riggs, B. Yazar-Klosinski, J. B. Wang, M. J. E. Loflin, B. Shechet, C. Hennigan, R. Matthews, A. Emerson, et al. “The short-term impact of 3 smoked cannabis preparations versus placebo on ptsd symptoms: A randomized cross-over clinical trial.” PLoS One 16 (2021): e0246990. 10.1371/journal.pone.0246990.
[39]. Jetly, R., A. Heber, G. Fraser and D. Boisvert. “The efficacy of nabilone, a synthetic cannabinoid, in the treatment of ptsd-associated nightmares: A preliminary randomized, double-blind, placebo-controlled cross-over design study.” Psychoneuroendocrinology 51 (2015): 585-8. 10.1016/j.psyneuen.2014.11.002.
[40]. Mithoefer, M. C., A. T. Mithoefer, A. A. Feduccia, L. Jerome, M. Wagner, J. Wymer, J. Holland, S. Hamilton, B. Yazar-Klosinski, A. Emerson, et al. “3,4-methylenedioxymethamphetamine (mdma)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: A randomised, double-blind, dose-response, phase 2 clinical trial.” Lancet Psychiatry 5 (2018): 486-97. 10.1016/s2215-0366(18)30135-4.
[41]. Smith, P. A., S. Chan, A. Blake, A. Wolt and S. O’Hearn. “Medical cannabis use in military and police veterans diagnosed with post-traumatic stress disorder (ptsd).” Journal of Pain Management 10 (2017): 397-405.
[42]. Greer, G. R., C. S. Grob and A. L. Halberstadt. “Ptsd symptom reports of patients evaluated for the new mexico medical cannabis program.” J Psychoactive Drugs 46 (2014): 73-7. 10.1080/02791072.2013.873843.
[43]. Johnson, M. J., J. D. Pierce, S. Mavandadi, J. Klaus, D. Defelice, E. Ingram and D. W. Oslin. “Mental health symptom severity in cannabis using and non-using veterans with probable ptsd.” J Affect Disord 190 (2016): 439-42. 10.1016/j.jad.2015.10.048.
[44]. Davis, A. K., L. A. Averill, N. D. Sepeda, J. P. Barsuglia and T. Amoroso. “Psychedelic treatment for trauma-related psychological and cognitive impairment among us special operations forces veterans.” Chronic Stress (Thousand Oaks) 4 (2020): 2470547020939564. 10.1177/2470547020939564.
[45]. Barsuglia, J. P., M. Polanco, R. Palmer, B. J. Malcolm, B. Kelmendi and T. Calvey. “A case report spect study and theoretical rationale for the sequential administration of ibogaine and 5-meo-dmt in the treatment of alcohol use disorder.” Prog Brain Res 242 (2018): 121-58. 10.1016/bs.pbr.2018.08.002.
[46]. Watts, R. and J. B. Luoma. “The use of the psychological flexibility model to support psychedelic assisted therapy.” Journal of Contextual Behavioral Science 15 (2020): 92-102.
[47]. Gruber, S. A., K. A. Sagar, M. K. Dahlgren, M. T. Racine, R. T. Smith and S. E. Lukas. “Splendor in the grass? A pilot study assessing the impact of medical marijuana on executive function.” Front Pharmacol 7 (2016): 355. 10.3389/fphar.2016.00355.
[48]. Cohen, J., Z. Wei, J. Phang, R. B. Laprairie and Y. Zhang. “Cannabinoids as an emerging therapy for posttraumatic stress disorder and substance use disorders.” J Clin Neurophysiol 37 (2020): 28-34. 10.1097/wnp.0000000000000612.
[49]. Alexander, W. “Pharmacotherapy for post-traumatic stress disorder in combat veterans: Focus on antidepressants and atypical antipsychotic agents.” P t 37 (2012): 32-8.
[50]. Haney, M., E. W. Gunderson, J. Rabkin, C. L. Hart, S. K. Vosburg, S. D. Comer and R. W. Foltin. “Dronabinol and marijuana in hiv-positive marijuana smokers. Caloric intake, mood, and sleep.” J Acquir Immune Defic Syndr 45 (2007): 545-54. 10.1097/QAI.0b013e31811ed205.
[51]. Strasser, F., D. Luftner, K. Possinger, G. Ernst, T. Ruhstaller, W. Meissner, Y. D. Ko, M. Schnelle, M. Reif and T. Cerny. “Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: A multicenter, phase iii, randomized, double-blind, placebo-controlled clinical trial from the cannabis-in-cachexia-study-group.” J Clin Oncol 24 (2006): 3394-400. 10.1200/jco.2005.05.1847.
[52]. Van Dam, N. T. and M. Earleywine. “Pulmonary function in cannabis users: Support for a clinical trial of the vaporizer.” Int J Drug Policy 21 (2010): 511-3. 10.1016/j.drugpo.2010.04.001.









