1. Introduction
Supramolecule is an ordered aggregates composed of many molecules. By using intermolecular force, also known as Van der Waals force, and different ways of combinations, several varieties of molecular collections can be formed that have different structures and properties to assume different roles. About eighty years ago, the term "Supramolecule" was proposed to explain the interaction between molecules. But in the following decades, they did not receive much attention. Until the 1960s, supramolecules were considered to be related to the environment [1]. Supramolecules are found to be related to the enthalpy and entropy values in the environment. Normally, the formation of supramolecules will lead to the increase of enthalpy and entropy [2]. In the natural environment, some supramolecules will be formed spontaneously without artificial synthesis. This is in accordance with the Thermodynamic Laws that entropy is increasing spontaneously. Supramolecules will assemble themselves to form aggregates. Due to different intermolecular forces, supramolecules will have different arrangements, which also leads to different characteristics [3]. In cells, aggregates formed by some supramolecules will even be further assembled into organelles. Different arrangements and characteristics lead to different and specific functions of these specific aggregates. The aggregates will react with particular reactants to produce specific biological reactions, and even can transform between their structures. Due to the progress of technology in recent years, the research on supramolecules has developed rapidly, and has been widely used in many fields. Many interdisciplinary research projects have been carried out, and a large number of outstanding research achievements have emerged on the application of supramolecules in biology. In view of the development speed of this field, this review attempts to integrate and introduce previous research reports, analyze how supramolecule present in biological cells, and discuss the practical application of supramolecules in the field. The review will first introduce and analyze the supermolecules naturally formed in cells, then talk about the artificial intervention by using existing nanotechnology on the characteristics of supermolecules and briefly introduce some technical achievements and specific developments in the area. Finally, the review will present personal subjective views with discussion.The wastes and plastic products discharged from the industrial production process are the main sources of marine pollutants. Marine pollution damages the environment, the health of all living things, and the structure of the global economy. National Geographic report that the Pacific Garbage Patch is one example, there are plastics and microplastics floating on and below the surface of swirling ocean currents between California and Hawaii in an area of about 1.6 million square kilometers (617,763 square miles) [1]. Research find that huge amount of human made plastic are follow to the ocean. Now there are polices made by government to reduce ocean pollution, they made some progress but still need large progress. According to a 2018 report from the United Nations, more than sixty countries have enacted regulations to limit or ban the use of disposable plastic items [1]. Also, people must make some actions, such as join ocean protections organizations. This paper gives people the opportunity to volunteer with ocean conservation groups and to find better alternatives to the harm caused by plastics.
2. Natural Supramolecules in The Cells
2.1. DNA
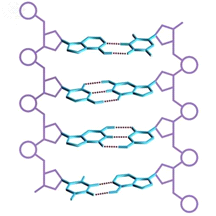
PDNA is one of the typical supramolecular aggregates. It is located at the central part of the cells, which is known as the nucleus of the eukaryotic cells. It carries the genetic information in cells. The sugar-phosphate strand on one side of DNA combines with the strand on the other side by matching the base pairs through hydrogen bond to form a double helix supramolecular aggregate.
|
Figure 1. DNA Schematic. |
The adenine and thymine of DNA will form two hydrogen bonds with each other, while guanine and cytosine will form three hydrogen bonds to hold two sugar-phosphate strand together.
2.2. Cell membrane
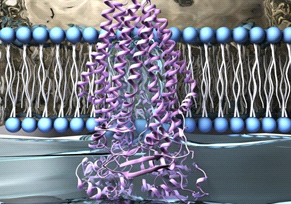
The cell membrane is one of the most important organelles of the cell, and it is the only medium for the inside of the cell to communicate with the external. Therefore, the cell membrane undertakes the functions of transportation, transduction and recognition. An important chemical molecule constituting cell membrane is phospholipid, which has a hydrophilic head capable of forming hydrogen bonds and a hydrophobic fatty acid tail. The formation of supramolecules is usually related to entropy, and cell membrane as a supramolecular aggregate is no exception. The hydrophobic fatty acid tail of phospholipid isolates water and forces water to surround the phospholipid molecule, which cannot freely form hydrogen bonds with other water molecules, which leads to the decrease of entropy value of the system. Since entropy tends to increase spontaneously in the system, water molecules tend to reduce the area of contact with the tail of hydrophobic fatty acids. In order to increase entropy, water molecules will drive a single phospholipid and other phospholipids together to form a bilayer structure, which only the hydrophilic head of phospholipid will contact with water molecules. In the phospholipid bilayer, the hydrophobic fatty acid tails gathered together will have Van der Waals force between them. The interreacted forces between hydrophilic heads and hydrophobic tails and the Van der Waals forces make the cell membrane, which is mainly composed of a phospholipid bilayer, become a supramolecular aggregate.
In addition to phospholipid, there are also various proteins, which also are supramolecules formed by hydrogen bonding, that make up the cell membrane. Compared with phospholipid, the functional groups of cell membrane are more proteins. A lot of active transport through the cell membrane uses these proteins. For example, a class of proteins on the cell membrane is specially used to transport ions, which is usually called ion pump. This kind of protein usually carries crown ethers. Crown ethers with different amounts of carbon can selectively pair different ions, allowing the cell membrane to selectively let ions pass through as needed. In this way, cell can change the voltage of inside and outside to take cellular action, such as generate cellular response, release bioelectricity, and so on.
Phospholipid molecules have hydrophilic heads (blue) and hydrophobic tails (white). Hydrophobic fatty acid tails cross with each other to produce strong Van der Waals force, which makes the whole phospholipid bilayer form a large supermolecule. Channel protein (purple) is an ion pump, and the secondary structure of protein formation is a supramolecule assembled of hydrogen bonds. The hydrophilic fatty acid heads of phospholipid molecules can form hydrogen bonds. These two supramolecules are composed together to form a large, complete cell membrane.
|
Figure 2. DNA Schematic. |
3. Currently Nanotechnologies Apply to Cell By Using Properties Of Supramolecules
3.1. DNA
Now nanotechnology has been able to artificially control and construct DNA, which is also at the nanoscale, by using supramolecular properties. This technology is mainly used in the field of genetic engineering and has become mature. The recombinant DNA can be introduced into the biological receptor cells to carry out normal biological activities, such as replication and transcription. At the linear DNA ends, a segment of extra unpaired nucleotides is called "overhangs", which are also known as "sticky ends". The sequence of sticky ends is changeable and can be paired with DNA fragments [4]. The special property of double stranded supramolecular DNA can used for DNA recombination. By pairing specific sequences, DNA can be designed, so some transgenic products have special capabilities that their original genes do not have; Through gene repair and editing, some human genetic diseases can even be treated.
3.2. Cell membranes
Cell membrane is a multifunctional organelle, which selectively prevents external substances from entering cells. This helps cells maintain a healthy state, but it also brings trouble to medical means such as drug delivery. However, through nanotechnology, specific substances can be allowed to enter cells without damaging cells, so this technology has been widely used in the medical industry.
Materials can be transported through proteins on the cell membrane into the cell. It is worth noting that the current technology has allowed the artificial construction of crown ethers to break the independent ionic balance of cells, and some special crown ethers can be used with other drugs, such as penicillin, to improve the antibacterial effect of drugs[5].
After passing through the cell membrane, the membrane can transport materials through endocytosis and exocytosis. The vesicles are involved in this transport. Both vesicles and cell membranes are composed of Phospholipid. Unlike proteins fixed on the cell membrane, vesicles can easily leave the cell membrane to transport substances to other organelles, which as known as Endomembrane system, or even other cells. Also different from the short-range transport vesicles in the Endomembrane system, extracellular Vesicles, as a medium with high capacity, which can travel freely between cells and transport for a long distance, are considered to be a good means for artificial intervention in cell response. There is also a special type of extracellular vesicles called exosomes. These vesicles will carry small RNA, release substances from the outside into the cell, and be able to re-establish intermolecular forces with the cell membrane and reintegrate into the supramolecular assembly of the cell membrane. By artificially regulating the substances in the exosomes, even the exosomes' membranes, the exosomes can fuse with the receptor cells, so that the substances can enter the cells and even integrate into the cell membranes, allowing the receptor cells to produce controllable changes [6]. In addition to regulating exosomes secreted by cells themselves, it is also proposed to construct supramolecular vesicles via vitro synthesis for drug delivery. The artificial nano molecule is more accurate in drug delivery, and has other functions, such as improving drug efficiency and reducing drug toxicity [7].
4. Discussion
Supramolecules can be naturally formed, but because the bonds that combine them are usually intermolecular forces, the possibility of supramolecules being manually manipulated is greatly increased, and even further artificial synthesis can be carried out. Because of the characteristics of intermolecular forces, supramolecules will be affected by the surrounding environment, including pH, temperature, entropy and other factors, and these will cause different changes in supramolecules, such as structural morphology. Because of the development of nanotechnology, supramolecules can now be artificial operated at the nano level, which gives supramolecules more room for development. Biotechnology can be said to be one of the most promising fields in this century, and a large number of supramolecules naturally used in biological cells are bound to become one of the most important breakthroughs. It is bound to promote the development of biotechnology by artificially changing the shape of supramolecules or artificially synthesizing specific supramolecules and then applying them to biological cells or organisms. If a science will become a technology when it reaches the bottleneck, then the research on supramolecules in the biological field is also walking on this road and has seen the dawn. Perhaps some technical achievements are not mature yet, and more exploration and experiments are needed to continuously verify and improve them, but the main direction of this field has been clarified, and only time is needed to constantly experience and improve.
5. Conclusion
In biological cells, supramolecules are usually formed by intermolecular forces, but are also related to the enthalpy of the system. The two main supramolecule aggregates in cells are DNA and cell membrane. With current nanotechnology, supramolecules can be artificially broken down and synthesized, and supramolecules can be allowed to have specific functions by designing supramolecular structures. In this way, DNA and cell membrane can also be artificially interfered by nanotechnology.
DNA is the carrier of cell gene information. Nanotechnology that mainly researches and designs recombinant supramolecular DNA is called genetic engineering. By programming and editing the DNA sequence, the gene information carried by DNA will be changed to cure human genetic diseases, or change the original characteristics of cells and develop new varieties.
The cell membrane is mainly composed of phospholipid and protein. Therefore, at present, the nanotechnology of cell membrane, a supramolecular aggregate, is mainly focused on the two items of artificial interference with phospholipid bilayer and protein channel. By artificially constructing the crown ether structure of the protein, the ionic balance of the cell itself can be broken, and special crown ethers can even play the role of auxiliary drugs. Phospholipid bilayer is mainly involved in cell transport by forming vesicles. Special extracellular vesicles, such as exosomes, have attracted the attention of the industry. Through manual intervention and synthesis of supramolecular vesicles, it is easy to transport drugs to cells, and even targeted transport can be carried out.
This review only focuses on the DNA and cell membrane in cells as supramolecular aggregates, but other supramolecular aggregates in cells are not mentioned and discussed. Similarly, the interactions between these supramolecular organelles have not been discussed and analyzed. It is possible to conduct similar discussions and analyses on these topics in the future.
Acknowledgment
Thank Professor Erik Luijten of Northwestern University and Professor Tang Gang of Tongji University for their guidance on supramolecular knowledge for this review. Professor Luijten taught about supramolecules and enthalpy, and provided ideas for connecting supramolecules with biological organelles. Professor Tang taught about supramolecular synthesis, and application in the industry. Thank for their help for this review.
Thanks to the BASIS International School Park Lane Harbour for the data support provided for this review. A large and detailed literature library is the basis of the review paper.
References
[1]. Schalley, C. A. (2007). Analytical Methods in Supramolecular Chemistry. Wiley.
[2]. Zhou, G. D., & Duan, L. Y. (Eds.). (2022, September 14). Fundamentals of Structural Chemistry (5th ed.). Peking University Press.
[3]. Mak, Lee, & Zhou. (2006, May). Advanced Inorganic Structural Chemistry (2nd ed.). Peking University Press.
[4]. Seeman N. C. (2005). DNA Enables Nanoscale Control of the Structure of Matter. Quarterly Reviews of Biophysics, 38(4), 363–371. https://doi.org/10.1017/S0033583505004087
[5]. Gokel, M. R., McKeever, M., Meisel, J. W., Negin, S., Patel, M. B., Yin, S., & Gokel, G. W. (2021). Crown ethers having side arms: a diverse and versatile supramolecular chemistry. Journal of Coordination Chemistry, 74(1–3), 14–39. https://doi.org/10.1080/00958972.2021.1878352
[6]. Vlassov, A. V., Magdaleno, S., Setterquist, R., & Conrad, R. (2012). Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochimica et biophysica acta, 1820(7), 940–948. https://doi.org/10.1016/j.bbagen.2012.03.017
[7]. Hua, Y., Chen, L., Hou, C., Liu, S., Pei, Z., & Lu, Y. (2020). Supramolecular Vesicles Based on Amphiphilic Pillar[n]arenes for Smart Nano-Drug Delivery. International journal of nanomedicine, 15, 5873–5899. https://doi.org/10.2147/IJN.S255637
Cite this article
Peng,K. (2023). The applications of supramolecules in biological cells. Theoretical and Natural Science,4,206-210.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Biological Engineering and Medical Science (ICBioMed 2022), Part II
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Schalley, C. A. (2007). Analytical Methods in Supramolecular Chemistry. Wiley.
[2]. Zhou, G. D., & Duan, L. Y. (Eds.). (2022, September 14). Fundamentals of Structural Chemistry (5th ed.). Peking University Press.
[3]. Mak, Lee, & Zhou. (2006, May). Advanced Inorganic Structural Chemistry (2nd ed.). Peking University Press.
[4]. Seeman N. C. (2005). DNA Enables Nanoscale Control of the Structure of Matter. Quarterly Reviews of Biophysics, 38(4), 363–371. https://doi.org/10.1017/S0033583505004087
[5]. Gokel, M. R., McKeever, M., Meisel, J. W., Negin, S., Patel, M. B., Yin, S., & Gokel, G. W. (2021). Crown ethers having side arms: a diverse and versatile supramolecular chemistry. Journal of Coordination Chemistry, 74(1–3), 14–39. https://doi.org/10.1080/00958972.2021.1878352
[6]. Vlassov, A. V., Magdaleno, S., Setterquist, R., & Conrad, R. (2012). Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochimica et biophysica acta, 1820(7), 940–948. https://doi.org/10.1016/j.bbagen.2012.03.017
[7]. Hua, Y., Chen, L., Hou, C., Liu, S., Pei, Z., & Lu, Y. (2020). Supramolecular Vesicles Based on Amphiphilic Pillar[n]arenes for Smart Nano-Drug Delivery. International journal of nanomedicine, 15, 5873–5899. https://doi.org/10.2147/IJN.S255637