1. Introduction
Ibuprofen, a nonsteroidal anti-inflammatory drug (NSAID), has become a ubiquitous presence in worldwide healthcare due to its widespread use for alleviating pain, reducing inflammation, and lowering fever. This widespread usage can be attributed to its over-the-counter availability, potent therapeutic benefits, and comparatively minimal side effects. Despite its pervasive presence, the synthesis of ibuprofen is still a subject of ongoing research, with continuous efforts to refine and improve its production process. The main focus of such research involves optimizing the synthetic procedure to maximize yield, reduce reaction time, and minimize the use of hazardous reagents or catalysts. As the production of ibuprofen continues to grow in response to worldwide demand, it becomes increasingly important to develop more efficient, sustainable, and safe manufacturing processes.
Dr. Stewart Adams, a pharmacologist from the United Kingdom, and his research team at the Boots Pure Drug Company in Nottingham created ibuprofen. Their research was initiated in the late 1950s, with the aim of developing a drug that could effectively relieve pain and inflammation without causing significant side effects. The Boots team applied for an ibuprofen patent in 1962, and it was approved in 1966. [1,2] Then clinical trials began to evaluate ibuprofen's safety and effectiveness in treating a variety of illnesses, including pain and inflammation. Ibuprofen was licensed for medicinal use in the United Kingdom in 1969 after successful laboratory testing and clinical trails. [3] In the 1970s, structural research on ibuprofen showed its particular binding interactions with COX enzymes, shedding light on its anti-inflammatory properties.[4] In the 1980s, research concentrated on ibuprofen absorption,distribution, metabolism, and excretion (pharmacokinetics). The research led to a better understanding of its bioavailability, half-life, and interactions with various tissues. Researchers investigated the stereochemistry of ibuprofen, which relates to the spatial arrangement of its atoms, in the 1990s. The pharmacological characteristics of ibuprofen stereoisomers were examined.[5]Further research was conducted in the 2000s to better understand the potential negative effects of ibuprofen, notably on the gastrointestinal system and cardiovascular health. Studies looked into the trade-off between its therapeutic effects and potential dangers.[6] In the 2010s, scientists researched nanotechnology-based ibuprofen formulations to improve solubility and bioavailability, potentially boosting efficacy and minimizing side effects.[7] In the 2020s, modern analytical techniques such as computer modeling and sophisticated spectroscopy were used to acquire a better knowledge of ibuprofen's molecular interactions. Ibuprofen has become one of the most widely used medications globally as its aboudant beneficial. For instance, pain relief, anti-inflammatory properties, over-the-Counter availablity, lower incidence of gastrointestinal irritation. Ibuprofen can be acquired over-the-counter, therefore it is thought that its medical benefits have greatly enhanced patients' lives. As a human anti-inflammatory medication. Ibuprofen has weak but distinct anti-inflammatory properties, comparable to aspirin milligram for milligram, with far less negative effects on the stomach. The medication's analgesic properties are most likely a result of its anti-inflammatory effects. Since it stops the production of prostaglandins and has no effect on the adrenopituitary axis, it is a nonsteroidal agent. Ibuprofen has been shown to be helpful in cases of rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, gout, and Bartter's syndrome.[8]
Over the years, various methods have been developed for the synthesis of ibuprofen, each has its own advantages and limitations. These pre-existing methods have contributed significantly to the availability and accessibility of ibuprofen, but they also face certain challenges. These limitations include low yield, lengthy reaction times, iterative use of mentallic reagents or catalysts and hazardous reagents or catalysts.
To overcome these limitations, researchers have focused on developing modifications to the synthesis techniques to improve yield and efficiency for many years. These modifications involve optimizing reaction conditions, exploring alternative catalysts, and utilizing greener and safer reagents. By solving and lessening these limitations, it is expected that the modified synthesis methods can enhance the overall production of ibuprofen and reduce its environmental impact.
In that case, this thesis aims to contribute to this ongoing research by exploring modifications in ibuprofen synthesis through mechanism learning. This thesis aim to dissect the history and evolution of ibuprofen synthesis, critique previous methods, and outline aims and objectives for future studies, with the ultimate goal of enhancing the production process of this essential therapeutic drug.Modifying and breaking the limitations of existing technologies through research on synthesis mechanism,the resulting synthetic effort is both a creative and inventive achievement(higher atom economy and greener) in synthetic strategy.
2. Discussion
2.1. Boot's synthesis:
The Boots pure drug company developed for manufacture of ibuprofen in the 1961.[9] And this method was also named as Boot's synthesis of ibuprofen.In order to synthesize ibuprofen by this method,(see Figure 1) on the first step after the reaction of isobutylbenzene and acetic anhydride under the catalysis of AlCl3, The reaction of isobutylbenzene with acetic anhydride initiates the synthesis, producing 1-(4-isobutylphenyl)ethan-1-one.In the later phases of production, the para-acetyl group of this molecule is converted into the propionic acid substituent of ibuprofen. First, a Darzens reaction occurs between ethyl chloroacetate and the acetyl group to form an,α,β-epoxy ester ethyl 3-(4-isobutylphenyl)-3-methyloxirane-2-carboxylate. The aldehyde is then produced by hydrolysis and decarboxylation 2-(4-isobutylphenyl)propanal. Aldoxime (E)-2-(4-isobutylphenyl)propanal oxime is produced by condensation with hydroxylamine and is then dehydrated with acetic anhydride to yield nitrile 2-(4-isobutylphenyl)propanenitrile. Ibuprofen is created by hydrolyzing nitrile to a carboxylic acid while being affected by an acid.[10]
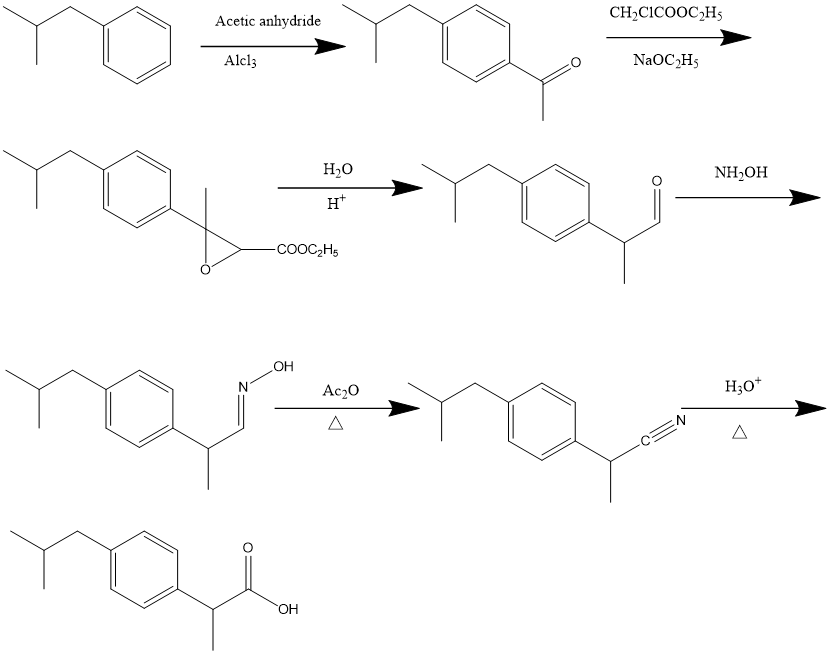
Figure 1. Synthesis route of Boot's synthesis of ibuprofen.
Mechanism:
①Figure 2 shows Ethyl chloroacetate is first deprotonated at its halogenated site, resulting in a carbanion that is stabilized by resonance with its enolate form. This nucleophile makes an attack on carbonyl carbon in compound 1-(4-isobutylphenyl)ethan-1-one. Following the expulsion of the chloride leaving group, the oxygen anion of the resultant tetrahedral intermediate takes part in an intramolecular SN2 reaction to create the,α,β-epoxy ester ethyl 3-(4-isobutylphenyl)-3-methyloxirane-2-carboxylate.

Figure 2. Mechanism in Boot's synthesis of ibuprofen.
②The,α,β-epoxy ester ethyl 3-(4-isobutylphenyl)-3-methyloxirane-2-carboxylate is changed into an aldehyde in the following process. The ester is first hydrolyzed by acid, producing the carboxylic acid. This molecule spontaneously decarboxylates in acidic circumstances to produce the aldehyde 2-(4-isobutylphenyl)propanal in balance with its enol tautomer.(see Figure 3)

Figure 3. Mechanism in Boot's synthesis of ibuprofen.
③As shown in Figufd 4 aldehyde 2-(4-isobutylphenyl)propanal and hydroxylamine are then condensed to produce aldoxime (E)-2-(4-isobutylphenyl)propanal oxime. The hydroxylamine's nucleophilic attack on the aldehyde carbonyl carbon is where the mechanism starts. Expulsion of a water molecule leaves the aldoxime after a few proton exchanges.
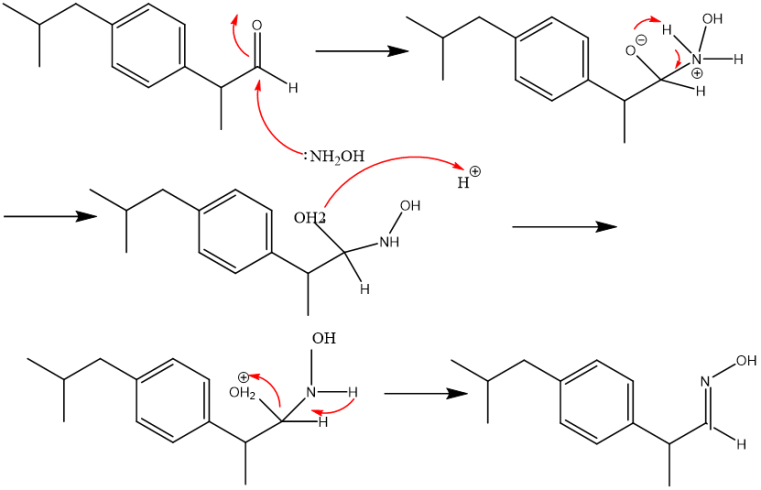
Figure 4. Mechanism in Boot's synthesis of ibuprofen.
④Following that, acetic anhydride is used to dehydrate the aldoxime (E)-2-(4-isobutylphenyl)propanal oxime. The nitrile product 2-(4-isobutylphenyl)propanenitrile is produced by acetylating compound (E)-2-(4-isobutylphenyl)propanal oxime, which results in an O-acetyl aldoxime that easily removes acetic acid. The nitrile 2-(4-isobutylphenyl)propanenitrile is then hydrolyzed under acid-catalyzed conditions. The carboxylic acid (ibuprofen) is produced by two nucleophilic additions of water and the removal of ammonium.(see Figure 5)
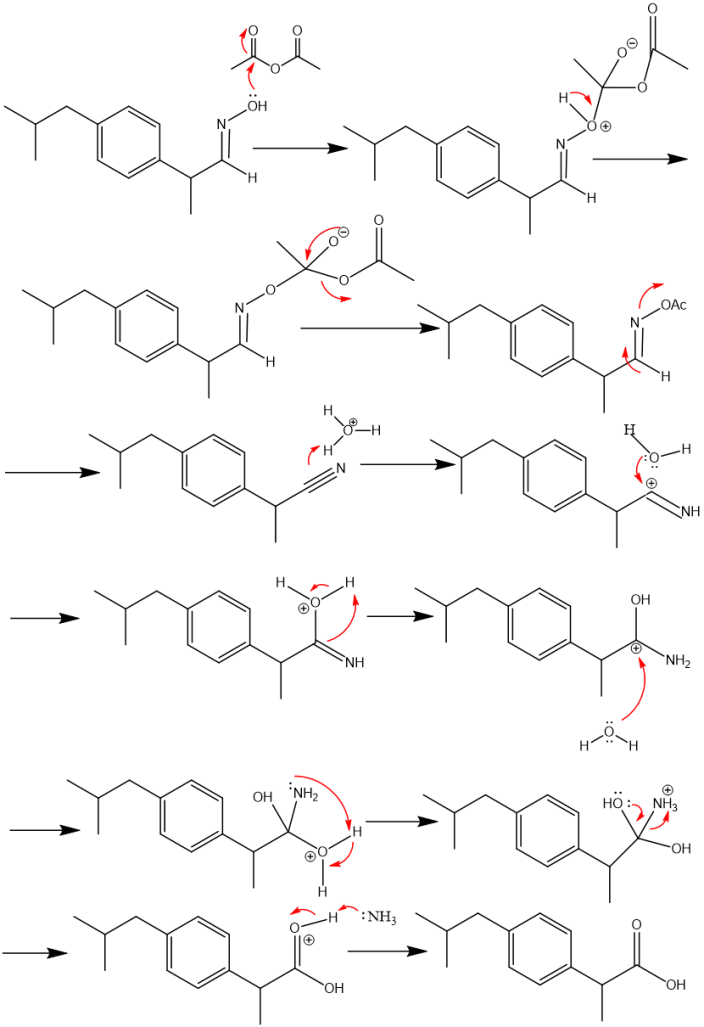
Figure 5. Mechanism in Boot's synthesis of ibuprofen.
Table 1. The Boots’ synthesis of ibuprofen atom economy [11].
Reagent Utilized in ibuprofen Unutilized in ibuprofen
Formula Mr Formula Mr Formula Mr
C10H14 134 C10H13 133 H 1
C4H6O3 102 C2H3 27 C2H3O3 75
C4H7ClO2 122.5 CH 13 C3H6ClO2 109.5
C2H5ONa 68 0 C2H5ONa 68
H3O 19 0 H3O 19
NH3O 33 0 NH3O 33
H4O2 36 HO2 33 H3 3
Total Ibuprofen Waste products
C20H42NO10ClNa 514.5 C13H18O2 206 C7H24NO8ClNa 308.5
Atom economy=206/514.5=40%
Analyse:
By using this method ibuprofen can be made of materials that are readily accessible.
Additionally,it can also lower the energy cost and equipment corrosion. Table 1 shows the atom economy of Boot's synthesis is only 40%, in other words, the ingredients utilized in the synthesis were wasted to a greater than 50% extent.For example, the UK market for ibuprofen is about 3 ×10 6 Kg per year,and the waste will be 4.5 ×10 6 Kg.[11] Besides, the reactions are complex(6 steps)and the use of Alcl3 can cause environmental pollution.
2.2. BHC synthesis:
The Boots-Hoechst-Celanese business created the BHC synthesis of ibuprofen in 1992[9], which was a considerable advancement over the original Boots technique. Figure 6 shows in this reaction, isobutylbenzene undergoes a Friedel-Crafts acylation to produce 1-(4-isobutylphenyl)ethan-1-one, which is the first step in the BHC synthesis of ibuprofen. However, this pathway differs significantly in how this intermediate is converted to ibuprofen. First, a catalytic hydrogenation reduces the aromatic ketone of 1-(4-isobutylphenyl)ethan-1-one to an alcohol 1-(4-isobutylphenyl)ethan-1-ol. In the presence of a palladium catalyst, carbon monoxide is carbonylated with 1-(4-isobutylphenyl)ethan-1-ol to generate ibuprofen.[12]
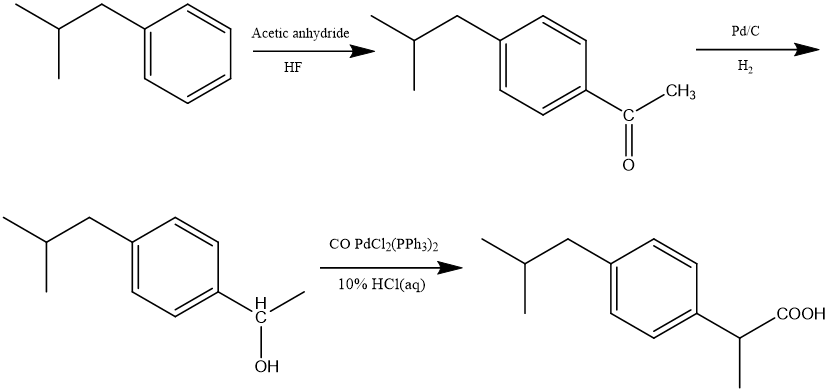
Figure 6. BHC synthesis of ibuprofen.
Mechanism: The Friedel-Crafts acylation of isobutylbenzene to 1-(4-isobutylphenyl)ethan-1-one proceeds in the same way mechanically as the Boots procedure, as was previously demonstrated. However, HF takes the place of the Alcl3. The aromatic ketone 1-(4-isobutylphenyl)ethan-1-one is hydrogenated to the alcohol 1-(4-isobutylphenyl)ethan-1-ol in the following synthesis process. By activating H2 by surface adsorption, the palladium catalyst speeds up the process. Additionally, the ketone adheres to the palladium surface [13]. By bringing it close to the activated H2, this encourages hydrogenation.
Another palladium-catalyzed reaction, carbonylation of alcohol 1-(4-isobutylphenyl)ethan-1-ol with carbon monoxide, is the last step. Following is a potential mechanism [14]. Alcohol 1-(4-isobutylphenyl)ethan-1-ol is in equilibrium with its benzyl chloride analog in the presence of copious hydrochloric acid. The benzyl chloride is added oxidatively to the palladium core to start the catalytic cycle. The Pd-C sigma bond is subsequently completed by the addition of carbon monoxide. Finally, the catalyst is revived and the ibuprofen is released by water's nucleophilic attack on the acyl intermediate.(see Figure 7)
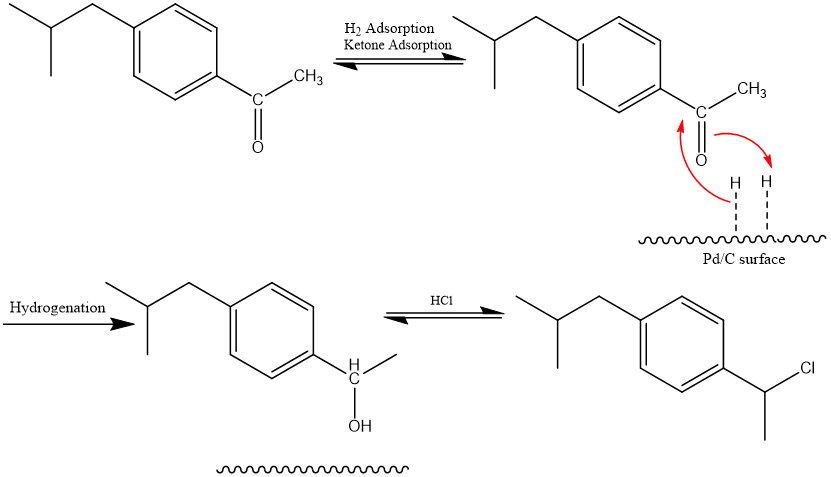
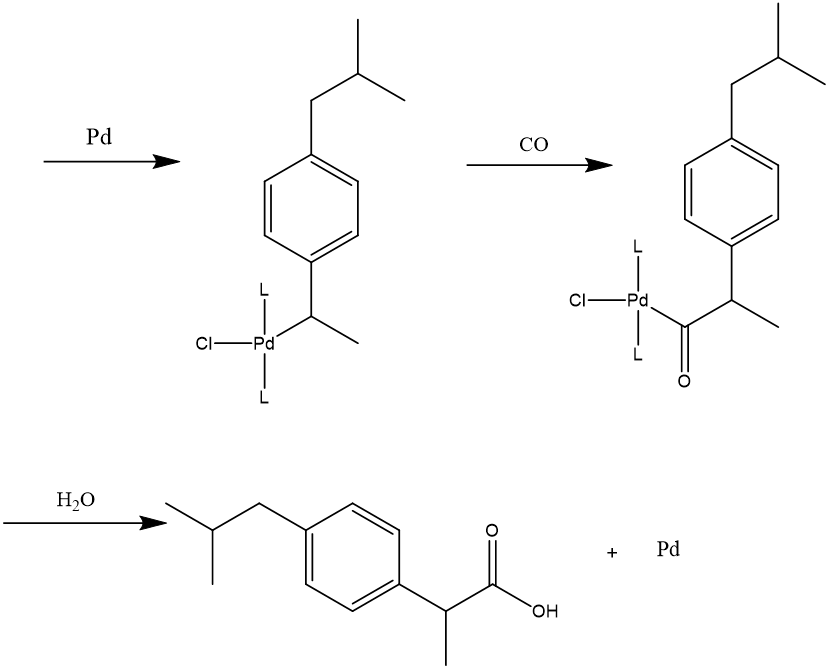
Figure 7. Mechanism in BHC synthesis of ibuprofen.
Table 2. The BHC synthesis of ibuprofen [11].
Reagent Mr Utilized in ibuprofen Mr Unutilized in ibuprofen Mr
C10H14 134 C10H13 133 H 1
C4H6O3 102 C2H3O 43 C2H3O2 59
H2 2 H2 2 0
CO 28 CO 28 0
Total Ibuprofen Waste products
C15H22O4 266 C13H18O2 206 C2H4O2 60
Atom economy=206/266=77%
Analyse:
The BHC synthesis is ultimately a much "greener" method since it offers a number of advantages over the Boots synthesis. The synthesis, obviously, requires fewer steps to complete than the latter.The conversion of isobutylbenzene to ibuprofen takes place in three steps as opposed to six in the Boots method.The technique also achieves twice the atom utilization (77%) of the original Boots process (40%), demonstrating improved atom economy(As can be seen from the Table 2 that the BHC synthesis has a higher atomic economy than Boot's synthesis).And BHC synthesis does not use aluminum chloride that causes pollution to the environment.Last but not least, the procedure featured effective separation and recovery of catalysts and reaction byproducts, which reduced the amount of waste generated.
The BHC synthesis stil use hazardous material(HF) .Besides,the atom economy is only 77%, and using this method to produce ibuprofen in large quantities would result in significant waste.In the future,a simpler, more effective, and stereo-specific method is still needed for ibuprofen's synthesis..
2.3. Electrochemical carboxylation for ibuprofen synthesis:
Any process induced or accompained by the passage of an electric current, including. The transfer of electrons between two substances is referred to as an electrochemical reaction. An electrochemical cell is represented by the two electrodes and the ionic conductor in between. The process that occurs in the cell as a whole is a redox process in which one species is reduced while another is oxidized.[15]
Traditionally, 2-hydroxy-2-arylpropanoic acids can be synthesized using hydrogen cyanide from the appropriate cyanohydrins. However, the requirement to use quite poisonous cyanides makes this approach less appealing and somewhat challenging from the standpoint of industry. It is preferable to construct a high yield system with an undivided cell, performing electrochemical carboxylations, in order to establish a convenient and economically useful synthesis process of ibuprofen. Electrochemical carboxylatiopn would refer to a method of introducing a carboxyl group into the ibuprofen molecule using an electrochemical reaction. Ibuprofen's chemical structure comprises a propionic acid moiety, which might be selectively changed to add a carboxyl group via electrochemical carboxylation. [16]Early studies of electrocarboxylation of alkyl aryl ketones revealed a low yield of carbonxylated products from acetophenones; nevertheless, it was later demonstrated that acetophenone may be electrochemically carboxylated with significant yields in an undivided celi employing an oxalate electrode. electronchemical reactions
Analyse:
Electochemical reactions can often be carried out under milder conditions compared to traditional chemical reactions, which might reduce the formation of unwanted byproducts or the degradation of senstive moleculesd. This method can be considered as an environmentally friendly approach since they can eliminate the need for certain hazardous reagents or generate less waste. Electrochemical reactions often exhibit high elctron transfer efficiency, making them efficient for the conversion of molecules. Moreover, elctrochemical reactions can sometimes offer high selectivity, allowing people to target specific functional groups for modification. This could potentially enable the introduction of a carbpxyl group at a desired position in the molecule.
However, Electrochemical reactions can be complex, necessitationg meticulous tuning of reaction variables such as electode materials, electrolytes, current density, and reaction duration. Because of this complexity, developing and replicating the process can be difficult. Modifying the chemical structure of ibuprofen can significantly impact its biological activity, pharmacokinetics, and safety profile. Any modifications should be carefully evaluated to ensure that the resulting compund retains its intend therapeutic effects. While small-scale electrochemical reactions in a laboratory setting may be feasible, scaling up the process for industrial production may provide hurdles in terms of maintaing consistent and efficient reactions.[17] Electrochemical processes may not always exhibit the necessary selectivity, and the possibility of side reactions or the generation of undesirable byproducts should be concerned.
2.4. Continuous-flow synthesis of ibuprofen
In recent years,the manufacture of ibuprofen using continuous flow is gaining popularity.Firstly, propionic acid is added to isobutylbenzene and heated to 150 ° C under the condition of triflic acid catalysis for 5 minutes The mixture is then continued to react in a solution dominated by PhI(OAc)2 HC(OMe)3, with MeOH as the catalyst, at a temperature of 50 ° C for 2 minutes.At this point, a manganese-containing compound is formed. The compound was transferred to KOH solution and continued to react with MeOH as catalyst at 65 ° C for 3 minutes. (see Figure 8)

Figure 8. Cntinuous-flow synthesis of ibuprofen.
Analyse
Continuous-flow synthesis allows for greater control over reaction conditions, potentially resulting in improved yields and purities. The technique is easily scaled up without requiring significant changes, making it suited for large-scale production. When compared to batch procedures, flow chemistry frequently entails handling smaller volumes of hazardous compounds at any given time, lowering the risk of accidents and exposure. Continuous-flow systems provide for exact control of reaction parameters like temperature, pressure, and reaction time. This can increase selectivity and limit the generation of undesirable byproducts. When compared to traditional batch synthesis, the effective use of reagents, reduced waste generation, and enhanced energy efficiency in flow processes can result in a smaller environmental imprint. Complex multistep syntheses can be incorporated easily into a single continuous-flow system, eliminating the need for intermediate isolations and purifications.
Nonetheless, setting up a continuous-flow system necessitates the use of specialized equipment such as pumps, reactors, and control systems. The initial investment might be substantial, especially for small-scale companies. Continuous-flow systems can become clogged or blocked, particularly when working with solid reactants or precipitates. This can cause the process to be disrupted and require cleaning and maintenance. While flow chemistry allows for faster optimization, it can also make identifying optimal conditions more challenging due to the dynamic nature of the reactions and the intricate interplay of variables.
3. Conclusion
In conclusion, this essay has examined how ibuprofen synthesis techniques have evolved over the past many decades. Even though the original Boots synthesis had limitations including low atom economy and the use of potentially dangerous chemicals, it developed the first feasible approach for the manufacturing of ibuprofen. With fewer stages, less waste, and greater atom economy than the Boots technique, the BHC synthesis greatly outperformed it. To further improve sustainability, efficiency, and selectivity, more contemporary methods including electrochemical carboxylation and continuous flow systems have been developed.
The specific methods continue to advance even as the fundamental goals—maximizing productivity and purity while reducing cost, waste, and environmental impact—remain consistent. Every new technique expands on what has come before and fixes flaws in older techniques. This incremental breakthrough has greatly improved the way this vital analgesic medication is produced. There is still a lot of room for the ibuprofen synthesis process to be made even more efficient, secure, and eco-friendly with the use of contemporary green chemistry concepts and cutting-edge technology.
To help with the construction of synthetic routes, research should concentrate on completely clarifying reaction processes going forward. Spectroscopic investigation as well as computer modeling can offer insightful molecular-level information. Processes should be evaluated using thorough lifecycle and sustainability criteria that cover everything from the procurement of raw materials to the disposal of trash. Emerging ibuprofen manufacturing techniques can strive for optimal synthesis—efficient, selective, scalable, safe, and producing minimal waste—by adopting a holistic approach. The objective of developing completely green, next-generation procedures for this essential medication may be achieved with persistent research and innovation.
References
[1]. Anon., 2015. BBC. [Online] Available at: https://www.bbc.com/news/health-34798438 [Accessed 2023].
[2]. Stewart S. Adams PhD, 1992. The Propionic Acids: A Personal Perspective. Chemical Pharmacology, 32(4), pp. 317-323.
[3]. Gayle M. Halford, 2012. 50th anniversary of the discovery of ibuprofen: an interview with Dr Stewart Adams. Platelets, 23(6), pp. 415-422.
[4]. K. D. Rainsford, 2011. Fifty years since the discovery of ibuprofen. Inflammopharmacology, Volume 19, pp. 293-297.
[5]. S C Tan, B. K. P. S. H. J. C. G. S. A. J. H., 1999. Ibuprofen stereochemistry: double-the-trouble?. Enantiomer, Volume 4, pp. 195-203.
[6]. Al-Saeed, A., 2011. Gastrointestinal and Cardiovascular Risk of Nonsteroidal Anti-inflammatory Drugs. Oman medical journal, 26(6), pp. 385-391.
[7]. Anon., 2020. Nanocarriers for the Delivery of Medical, Veterinary, and Agricultural Active Ingredients. ACS Nano, 14(3), pp. 2678-2701.
[8]. KANTOR, T. G. (1979). Ibuprofen. Annals of Internal Medicine, 91(6), 877-882.
[9]. Ha, M.W., Paek, S.M., 2021. Recent advances in the synthesis of ibuprofen and naproxen. Molecules. doi:10.3390/molecules26164792
[10]. Ahmadi, A., Daniali, M., Kazemi, S., Azami, S., & Alizade, N. (2014). Synthesis of ibuprofen with modified and economical process as an NSAID drug. Journal of Applied Chemical Research, 8(3), 91-95.
[11]. Imperial college london [Online] Available at: https://www.ch.ic.ac.uk/marshall/4I6/Ibuprofen2.pdf [Accessed 2023].
[12]. Cann, M. C., & Connelly, M. E. (2000). The BHC Company Synthesis of Ibuprofen, a Greener Synthesis of Ibuprofen Which Creates Less Waste and Fewer Byproducts, chapter from “Real World Cases in Green Chemistry”. Publisher American Chemical Society, Washington DC, 19-24.
[13]. Sungbom, C., & Tanaka, K. (1982). Concentration Dependence of Ketone Hydrogenation Catalyzed by Ru, Pd, and Pt. Evidence for Weak Ketone Adsorption on Pd Surface. Bulletin of the Chemical Society of Japan, 55(7), 2275-2276.
[14]. Cavinato,G.,&Toniolo,L.(1993). Palladium-catalyzed carbonylation of benzyl alcohol derivatives to phenylacetic acid derivatives.Journal of molecular catalysis,78(2),131-142.
[15]. Xiao-Fei Liu, K. Z. L. T. X.-B. L. W.-Z. Z., 2022. Recent advances in electrochemical carboxylation reactions using carbon dioxide. Green Chemical Engineering, 3(2), pp. 125-137.
[16]. Dr. Rong Zhao, Z. L. I. M. J. S. P. L. A., 2022. Electrochemical Cross-Electrophile-Coupling for Transition-Metal-Free Allylic Carboxylation with Ambient CO2. chemistry Europe, 9(21).
[17]. Caio Lenon Chaves Carvalho, G. d. A. R. J. L. M. R. A. d. S. L. E. T. S. G. d. S. W. C., 2021. Effect of Ibuprofen on the electrochemical properties of Prussian blue/single-walled carbon nanotubes nanocomposite modified electrode. Surfaces and Interfaces, Volume 25.
Cite this article
Hu,X.;Song,Y.;Li,J.;Huang,Y. (2024). Modification of ibuprofen synthesis through the mechanism analysis. Theoretical and Natural Science,45,168-178.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Anon., 2015. BBC. [Online] Available at: https://www.bbc.com/news/health-34798438 [Accessed 2023].
[2]. Stewart S. Adams PhD, 1992. The Propionic Acids: A Personal Perspective. Chemical Pharmacology, 32(4), pp. 317-323.
[3]. Gayle M. Halford, 2012. 50th anniversary of the discovery of ibuprofen: an interview with Dr Stewart Adams. Platelets, 23(6), pp. 415-422.
[4]. K. D. Rainsford, 2011. Fifty years since the discovery of ibuprofen. Inflammopharmacology, Volume 19, pp. 293-297.
[5]. S C Tan, B. K. P. S. H. J. C. G. S. A. J. H., 1999. Ibuprofen stereochemistry: double-the-trouble?. Enantiomer, Volume 4, pp. 195-203.
[6]. Al-Saeed, A., 2011. Gastrointestinal and Cardiovascular Risk of Nonsteroidal Anti-inflammatory Drugs. Oman medical journal, 26(6), pp. 385-391.
[7]. Anon., 2020. Nanocarriers for the Delivery of Medical, Veterinary, and Agricultural Active Ingredients. ACS Nano, 14(3), pp. 2678-2701.
[8]. KANTOR, T. G. (1979). Ibuprofen. Annals of Internal Medicine, 91(6), 877-882.
[9]. Ha, M.W., Paek, S.M., 2021. Recent advances in the synthesis of ibuprofen and naproxen. Molecules. doi:10.3390/molecules26164792
[10]. Ahmadi, A., Daniali, M., Kazemi, S., Azami, S., & Alizade, N. (2014). Synthesis of ibuprofen with modified and economical process as an NSAID drug. Journal of Applied Chemical Research, 8(3), 91-95.
[11]. Imperial college london [Online] Available at: https://www.ch.ic.ac.uk/marshall/4I6/Ibuprofen2.pdf [Accessed 2023].
[12]. Cann, M. C., & Connelly, M. E. (2000). The BHC Company Synthesis of Ibuprofen, a Greener Synthesis of Ibuprofen Which Creates Less Waste and Fewer Byproducts, chapter from “Real World Cases in Green Chemistry”. Publisher American Chemical Society, Washington DC, 19-24.
[13]. Sungbom, C., & Tanaka, K. (1982). Concentration Dependence of Ketone Hydrogenation Catalyzed by Ru, Pd, and Pt. Evidence for Weak Ketone Adsorption on Pd Surface. Bulletin of the Chemical Society of Japan, 55(7), 2275-2276.
[14]. Cavinato,G.,&Toniolo,L.(1993). Palladium-catalyzed carbonylation of benzyl alcohol derivatives to phenylacetic acid derivatives.Journal of molecular catalysis,78(2),131-142.
[15]. Xiao-Fei Liu, K. Z. L. T. X.-B. L. W.-Z. Z., 2022. Recent advances in electrochemical carboxylation reactions using carbon dioxide. Green Chemical Engineering, 3(2), pp. 125-137.
[16]. Dr. Rong Zhao, Z. L. I. M. J. S. P. L. A., 2022. Electrochemical Cross-Electrophile-Coupling for Transition-Metal-Free Allylic Carboxylation with Ambient CO2. chemistry Europe, 9(21).
[17]. Caio Lenon Chaves Carvalho, G. d. A. R. J. L. M. R. A. d. S. L. E. T. S. G. d. S. W. C., 2021. Effect of Ibuprofen on the electrochemical properties of Prussian blue/single-walled carbon nanotubes nanocomposite modified electrode. Surfaces and Interfaces, Volume 25.









