1. Introduction
1.1. Background
The first mention of symptoms of scarlet fever was described by the Sicilian anatomist Giovas in 1553, offering a different perspective from the usual rash. [1]. In 1565, Johann, a scholar from the Netherlands, became the first to describe the sore throat during the onset of the disease[1]. Jean of Poitiers wrote the first definitive description of scarlet fever in France in 1578[1]. In 1675, the term scarlet fever was first mentioned in the relevant medical literature by sydenham, who identified scarlet fever as a different disease entity from the rash and measles[1]. In the 17th and 18th centuries, scarlet fever was very prevalent in European countries and North America, with a high fatality rate[1]. Beginning in the 1920s, George and Gladys discovered and demonstrated that hemolytic streptococcus produces scarlet fever toxin, which can cause sore throats[1]. The condition was controlled until antibiotic treatment became available in the 1940s[1]. The study of the pathogenesis and treatment of this disease plays an important role in preventing and controlling the outbreak of scarlet fever. It helps in developing effective treatment protocols, including appropriate antibiotics and supportive care. Ongoing research on scarlet fever may lead to discoveries in microbiology, immunology, and related fields. This disease will not only cause a threat to the patient’s life, but also have a great negative impact on the patient’s family and the social environment in which they live. While scarlet fever may not be as widespread as it once was, it remains a concern in certain regions. Understanding and studying the disease contributes to global health efforts, emphasizing the importance of disease surveillance, vaccination, and healthcare infrastructure. In summary, studying scarlet fever is meaningful for advancing medical knowledge, improving healthcare practices, and safeguarding public health, particularly in the context of infectious diseases and pediatric health.
1.1.1. Symptoms of scarlet fever
Scarlet fever arises from the presence of type A streptococcus, a member of the beta-hemolytic streptococcus family, and is more prevalent among school-age and adolescent children [1]. The condition manifests as sudden throat inflammation, typically within one to three days following infection, featuring symptoms like fever, aching throat, discomfort while swallowing, enlarged tonsils, swollen lymph nodes, vomiting, neck adenopathy, abdominal discomfort (frequently seen in young children), and a reddened tongue (commonly referred to as strawberry tongue, a characteristic sign of scarlet fever) [2]. This illness is frequently linked with streptococcus pyogenes throat inflammation in children and teenagers, presenting as a sandpapery, white eruption on the skin [1]. There are two concurrent categories of complications: suppurative, resulting from the deterioration or extension of the initial infection (e.g. bacterial pharyngitis progressing to otitis media, sinusitis, or bacterial meningitis), and non-suppurative, typically arising from an immune response after the primary infection has been resolved [1]. The query arises as to why the same bacterium is responsible for both scarlet fever and tonsillitis [1]. Streptococcus pyogenes infections can lead to typical phenomena like pharyngitis or a sore throat, as well as scarlet fever, often stemming from the identical bacterial strain that gives rise to tonsillitis [3]. Scarlet fever represents a healthcare-related pattern marked by the emergence of a skin eruption coupled with a streptococcus pyogenes infestation [3]. Variations in bacterial strains, virulence factors, and immune reactions collectively determine the organism’s clinical expressions [3].
1.1.2. The mortality rate of scarlet fever
During the span from the 1950s through the 1980s, scarlet fever was notably prevalent; however, from the 1980s to the 1990s, its occurrence progressively dwindled, reaching a relatively consistent and diminished level in China [4]. A notable resurgence has been observed since 2011. Over the course of eight years, spanning from 2011 to 2018, a cumulative count of 479,555 cases was documented, surpassing the combined tally of cases reported during the preceding 12-year period (1999-2000) [4].
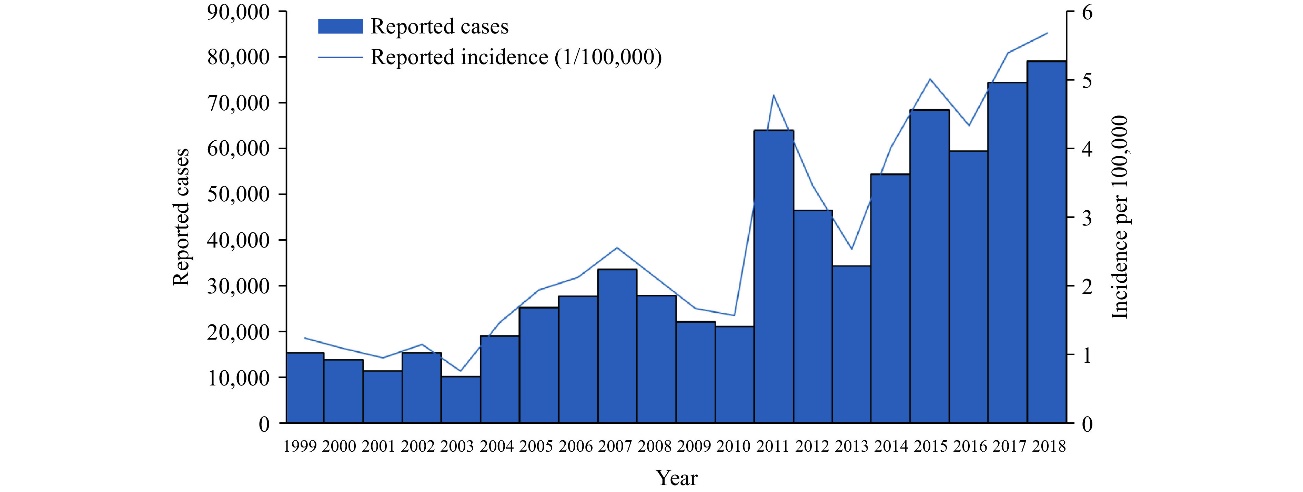
Figure 1. Number of reported cases of scarlet fever from 1999 to 2018 [3].
1.1.3. The impact on china and the world
Scarlet fever can infect individuals of all age groups, although its ease of transmission in settings like classrooms and daycare centers, coupled with the absence of immunity to Group A streptococcal protective antigens in these populations, has led to a notable vulnerability [5]. During the 18th and 19th eras, scarlet fever emerged as a significant contributor to childhood mortality, with fatalities reaching as high as 30 percent. Over time, the severity of scarlet fever has diminished, and instances of fatality have become exceedingly rare [6]. Predominantly affecting children aged 5 to 12, scarlet fever has an incidence rate of approximately 5 in 1,000 children, while occurrences among adults are less frequent [2]. In contrast to other childhood illnesses, scarlet fever can recur throughout an individual’s life, with its highest prevalence observed at around six years of age and a notable rise in cases among three to four-year-old children [2]. Across all age brackets, males face a higher risk compared to females, possibly attributed to suboptimal personal hygiene practices and increased physical interactions [6]. Consequently, future strategies and interventions aimed at addressing this concern must prioritize school-age children and boys [6].
In China, scarlet fever follows a dual seasonal pattern that varies across different regions [6]. The northern and southern areas experience biannual peaks in The months of “May-June” and “November-December.”, while southwestern regions exhibit a single peak in May-June [6]. Geographic disparities in these structures might be influenced by climatic and demographic factors [6]. Additionally, our research demonstrates a significant decline in scarlet fever incidence Amidst the school vacation period in China. This observed seasonal pattern aligns with that of the regions of Hong Kong and South Korea, yet various from Poland (with a peak in January and March) and the United Kingdom (with a peak from February to March) [6]. These distinctions in weather-related trends between East Asia and Europe could potentially stem from variations in the duration and timing of school breaks [6].
2. Description of chemical structures of drugs
2.1. One of the drugs used to treat scarlet fever—penicillin V
2.1.1. Origins
In the year 1929, Alexander Fleming effectively isolated penicillin from a type of Penicillium notatum [7]. Natural penicillins are generated through the brewing of mutated strains of Penicillium chrysogenum [8]. Penicillin V is synthesized by introducing phenoxyacetic acid into the nutrient medium [8]. By employing advanced deep fermentation techniques in the late 1940s, a multitude of synthetic penicillins were innovated, enabling the mass production of medications, albeit contributing to an elevation in the body’s resistance to these drugs [7]. Despite the emergence of alternative classes of anti-infective medications, penicillin retained its position as the foremost and crucial drug of choice.
2.1.2. Structures
All penicillins share a fundamental chemical composition comprising a beta-lactam loop, a thiazolidine loop, and a branch (6-aminopenicillanic acid) [7]. The beta-lactam loop contains the intrinsic antimicrobial effectiveness of penicillins. Modification of this loop structure leads to the formation of penicilloic acid, resulting in the loss of the compound’s antimicrobial potency [7].
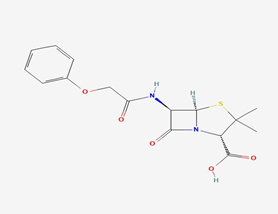
Figure 2. The structure of Phenoxymethylpenicillin [7].
2.1.3. Nomenclature and Chemical properties
MF: C16H18N2O5S [8] MW: 350.4g/mol [8]
Boil temperature: 289.00-291.00 °C [8]. @ 760.00 mm Hg (est) [8].
Melt temperature: 248-262 °F (Decomposes) <0.1 g/100mL [8].
Dissolvability: Below 1 mg/mL at 55 °F. At a pH of 1.8 (acidified using hydrochloric acid), the solubility in H2O is 24 mg per 100 ml; Dissolves in hydrophilic organic solvents; exhibits limited solubility within plant-based oils and liquid petroleum jelly [8]. Readily dissolves in ethanol and acetone; does not dissolve in fatty oils [8].
Durability and longevity: Maintains stability in air temperatures up to 37 °C; exhibits notable resistance to acidic conditions [8]. After mixing, penicillin V potassium oral solution should be cooled @ 2-8 °C, and any unutilized solution should be thrown away following a span of 14 days. Solutions containing penicillin start to degrade after several days, even when stored in chilly conditions [8].
Optical rotation: The bioactive conformation is the right-handed D-form, while the DL-form exhibits half the activity. [8]. L-Penicillin V exhibits minimal to negligible antimicrobial effectiveness, with peak absorption occurring at 268 and 274 nm (with molar absorptivity values of 1330 and 1100, respectively) [8].
PH: 2.5-4.0 (saturated solution); Acidic Pka: 2.735; Pka: 2.79 [8]
IUPAC Name: (2S, 5R, 6R)-3, 3-dimethyl-7-oxo-6- [(2-phenoxyacetyl) amino]-4-thia-1- azabicyclo [3.2.0]heptane-2-carboxylic acid [8].
Penicillin V is a derivative of penicillin characterized by a 6beta-(phenoxyacetyl) amino side-chain. This compound functions as a conjugated acid of phenoxymethyl penicillin (1-) and serves as a phenoxymethyl counterpart to penicillin G [8]. It appears as a crystal powder of white appearance under normal ambient temperature conditions and is alternatively known as phenoxy methylate penicillin or penicillin VK. This particular penicillin variant falls under the category of narrow-spectrum antibiotics and qualifies as an orally active natural penicillin [8]. Phenoxymethylpenicillin finds its application in treating several conditions, including mild to moderate upper breathing system infections, scarlet fever and minor cellulitis caused by Streptococcus, excluding cases of bacterial bloodstream infection [8].
Arrangement of penicillins into categories.
Table 1. The Classification of Penicillins [7].
Category and substances | Trade names1 | Availability |
Natural penicillins | ||
penicillin G potassium | Pfizerpen | Injectable: Vials of 5 and 20 microliters |
phenoxymethyl penicillin | Multifarious generics | Pills: Available in strengths of 250 and 500 milligrams |
Liquid Formulation: Concentrations of 125mg/5 mL and 250mg/5 mL | ||
penicillin G procaine | Wycillin | Injectable: Concentration of 600,000 units per millilite |
penicillin G benzathine | Bicillin L-A, Permapen | Injectable: Available in strengths of 300,000 units per milliliter and 600,000 units per milliliter |
penicillin G procaine/ penicillin G benzathine combination | Bicillin C-R | Injectable: Offered in concentrations of 150,000 units per 150,000 u/mL, 150,000 units per 450,000 u/mL, and 300,000 units per 30,000 u/mL |
Penicillinase-Resistant Penicillins | ||
methicillin | Staphcillin | Not currently accessible |
nafcillin | Multifarious generics | Injectable: Vials containing 20 mg/mL |
oxacillin | Bactocill | Encapsulated Form: Available in strengths of 250 and 500 milligrams |
Liquid Formulation: Concentration of 250 milligrams per 5 milliliters | ||
Injectable: Vials containing 500mg, 1g, 2g, and 4g | ||
Cloxacillin | Multifarious generics | Capsules available in strengths of 250 and 500 milligrams |
Liquid formulation with a concentration of 125mg per 5 mL | ||
dicloxacillin | Dynapen | Capsules come in options of 250 or 500 milligrams |
Liquid mixture with a concentration of 62.5 mg per 5 mL | ||
Aminopenicillins | ||
ampicillin | Principen | Capsules are provided in strengths of 250 and 500 mg. |
Liquid mixture available in concentrations of 125 mg per 5 mL and 250 mg per 5 mL | ||
Injectable: Vials containing 125, 250, 500 mg, 1, and 2 g | ||
amoxicillin | Amoxil, Trimox | Encapsulated doses: 250 mg and 500 mg |
Tablets designed to be chewed: Available in strengths of 125, 250, and 400 mg | ||
Tablets with a protective film coating: Available in strengths of 500 and 875 mg | ||
Liquid mixture offered at concentrations of 50 mg/mL, 125 mg per 5 mL, 200 mg per 5 mL, 250 mg per 5 mL, and 400 mg per 5 mL | ||
bacampicillin | Spectrobid | Currently unavailable |
Carboxypenicillins | ||
carbenicillin | Geocillin | Tablets with a strength of 382 mg |
ticarcillin | Ticar | Injectable: Vials with contents of 1, 3, and 6 grams |
Ureidopenicillins and piperazine penicillin | ||
azlocillin | Azlin | No more accessible in the U.S. marketplace |
mezlocillin | Mezlin | No more accessible in the U.S. marketplace |
piperacillin | Pipracil | Injectable: Vials containing 2, 3, and 4 grams |
2.1.4. Mode of Delivery
Penicillin V is commonly administered via the oral route and demonstrates greater resistance to digestive acids compared to penicillin G [9]. After oral intake, phenoxymethylpenicillin is swiftly but not entirely absorbed, exhibiting a bioavailability ranging from 25 to 60 percent [8]. The calcium or potassium salt forms of phenoxymethylpenicillin exhibit improved absorption and utilization, with enhanced drug uptake also possible when taken in a fasting state [8]. UKHSA and PHS documented scarlet fever, tonsillitis, lower respiratory tract GAS infection, and pulmonary empyema as indicative presentations of invasive GAS infection [10]. Invasive GAS contagion is suggested by manifestations like lower breathing passage GAS contagion and lung abscess. Inside the EMIS medication ordering platform utilized by GP techniques, the recommended administration mode, and dosages for penicillin V in treating scarlet fever are outlined as follows [10]:
Preferred treatment options in order: Penicillin V, Amoxicillin, and Flucloxacillin (in succession, provided there is no documented penicillin sensitivity) [10].
Penicillin V - administered according to BNF-c includes penicillin [10].
For cases of scarlet fever, a treatment duration of 10 days is recommended [10]. In case of systemic illness, inability to ingest by-mouth antimicrobials, or lack of betterment within 48 hours, make a referral to secondary healthcare [10].
For children between 1 and 11 months of age, the recommended dosage is 62.5mg taken four times daily [10].
- Liquid formulation containing 125mg per 5ml [10].
- Administer 1.3ml* of the 250mg/5ml liquid (Round to the measurable dose; provide a medicine dropper for dispensing) [10].
For children between the ages of 1 and 5 years, the recommended dosage is 125mg taken four times daily [10].
- Liquid formulation containing 250mg per 5ml [10].
- Liquid formulation with a concentration of 125mg per 5ml [10].
For children aged 6 to 11 years, the recommended dosage is 250mg taken four times a day [10].
- Tablets containing 250mg [10].
- Liquid formulation with a concentration of 250mg per 5ml [10].
For children aged 12 years and older, the recommended dose is 500mg taken four times daily [10].
-Tablets with a strength of 250mg [10].
- Liquid formulations are saved for uncommon situations [10].
Kindly choose the most suitable form according to the given priority sequence [10].
Tablets can be broken down and mixed into a small quantity of liquid or a soft edible substance (like a teaspoon of jam) right before taking [10].
ENSURE THAT THE INDIVIDUAL CARRYING OUT THIS PROCEDURE IS NOT ALLERGIC TO PENICILLIN [10].
2.1.5. Mechanism of drug action and Drug targets
Phenoxymethylpenicillin demonstrates bactericidal effects against microorganisms sensitive to penicillin [8]. It operates by targeting these bacteria during their active multiplication phase, where it disrupts the formation of peptidoglycan in bacterial cellular barrier [8]. Microbial cell barriers are constructed from peptidoglycan or cytoplasmic envelopes, and the peptidoglycan formation process involves three stages: 1. Precursors form within the cytoplasm. 2. These precursor elements link together to form polymers. 3. Cross-linking occurs through transpeptidation [8]. Phenoxymethicillin obstructs the formation of cellular barriers mucopeptides through attachment to a distinct penicillin-binding protein (PBP) located within the structure of the microbial cell barriers, this action disrupts the third stage of microbial cell barrier formation, resulting in cell breakdown [8]. Penicillins are agents with bactericidal properties that carry out their mode of action by hindering the production of microbial cell barriers and inducing a self-destructive effect within bacteria [8].
2.1.6. Drug resistance
Enzymatic degradation represents a prevalent how beta-lactam antimicrobials encounter defiance, primarily orchestrated by beta-lactam biological catalysts that decompose the beta-lactam bond within antibiotics. This process leads to the breakdown of the antibiotics’ antibacterial efficacy [7]. Resistance to beta-lactam drugs is also linked to a reduction in the quantity of porins. Among these, OmpF and OmpC are the key porins, and the absence of OmpF porins in mutants can prompt resistance due to the slower penetration rate of the remaining OmpC porins, coupled with an escalation in beta-lactam enzyme activity [7]. The capacity of penicillin to bind with PBP (penicillin-binding protein) is pivotal for its antimicrobial efficacy, with distinct PBPs exhibiting varying affinities for penicillin [7]. Bacterial mutations can alter their sensitivity to penicillin, potentially leading to a decreased affinity of PBPs for penicillin and consequently influencing bacterial responsiveness to the drug’s concentration [7]. To address bacterial resistance to penicillin, a strategy involving combination antimicrobial therapy may prove beneficial. This approach entails the use of synergistic drugs alongside chemotherapy to potentially counteract the emergence of resistance [7].
2.2. The other drugs used to treat scarlet fever--azithromycin
2.2.1. Original
In 1950, the initial group of macrolide antibiotics was obtained from Streptomyces strains, and these antibiotics are categorized based on the dimensions of the macrolide loop, encompassing variations with 12-, 14-, 15-, or 16-ringed [11]. The majority of these macrolides feature amino or uncharged carbohydrate components attached to the cyclic ester linkage through glycosidic connections [11]. Following this discovery, numerous derivatives have been synthesized to enhance acid stability and bioavailability, resulting in the emergence of the second-generation macrolide, azithromycin [11]. Azithromycin represents a broad-spectrum macrolide antibiotic characterized by its prolonged half-life and remarkable tissue penetration capacity [11]. Its primary application lies in addressing lung and airway infections, digestive system, and urogenital system [11].
2.2.2. Structures
Azithromycin, classified as an azalide as part of the macrolide category of antimicrobials, bears the chemical name [9-deoxo-9a-aza-9a-methyl-9a-homoerythromycin]. It features a 15-membered ring structure, it exhibits a 15-ringed configuration, where a methylated nitrogen takes the place of a carbonylic functional cluster at the 9a site on the non-sugar loop. This modification serves to inhibit its metabolism, setting azithromycin apart from other macrolide variants [11, 12].
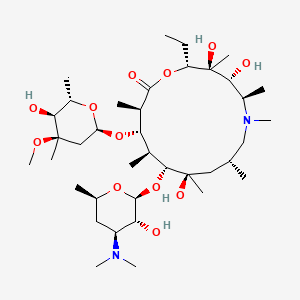
Figure 3. The structure of azithromycin [10].
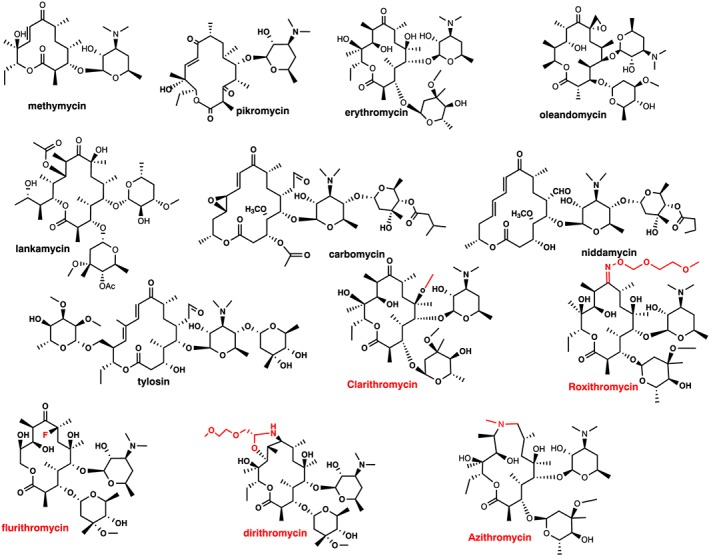
Figure 4. The structure of macrolides [10].
Macrolide configuration exhibit different generations of compounds. Initial generation, there are three categories based on the number of members in the ring: 12‐ringed (methymycin), 14‐ringed (pikromycin, erythromycin, oleandomycin, and lankamycin), and 16‐ringed (carbomycin, niddamycin, and tylosin). All of these fall under the category of organic compounds [10]. Moving on to the successor generation, it comprises 14‐ringed (clarithromycin, roxithromycin, flurithromycin, and dirithromycin) and 15‐ringed (azithromycin) compounds [10]. The modifications introduced in the erythromycin compound to create the successor generation of 14‐ and 15‐ringed macrolides are highlighted in red [10].
2.2.3. Nomenclature and Chemical properties
Molecular Formula: C38H72N2O12 [11].
Dissolvability: It dissolves well in alcohol and DSMO, showing limited solubility in H2O. In aqueous environments, it registers around 2.37 mg/L at 25 °C (estimated) or 5.14e-01 g/L. [11].
Melt temperature: Between 113 and 115 degrees Celsius [11].
pKa = 8.74 [11].
IUPAC Name:
R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-11- [(2S,3R,4S,6R)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-2-ethyl-3,4,10-trihydroxy-13- [(2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-3,5,6,8,10,12,14-heptamethyl-1-oxa-6-azacyclopentadecan-15-one [11]. Azithromycin falls within the category of azalides, which are a subset of large cyclic lipid antimicrobials [13]. It originates from erythromycin, yet it has a distinct chemical composition compared to erythromycin due to the inclusion of a nitrogen particle with a methyl group within the internal ring [13]. The chemical formula this compound is C38H72N2012, with a chemical weight of 749.0 [13].
2.2.4. Mode of delivery
Azithromycin comes in tablet format and is also offered as an extended-release suspension (liquid) and a standard suspension (liquid) intended for oral consumption. The tablets and suspension (marketed as Zithromax) are commonly ingested once a day, with or without food, over a span of 1 to 5 days [14]. It is crucial for patients to strictly adhere to the prescribed instructions when taking azithromycin [14].
The drug dosage and recommended oral administration approach for treating scarlet fever are as follows.
Table 2. Drug dosage and oral method used to treat scarlet fever [14].
Possible approaches to manage scarlet fever | |||
Medication and administration method | Age of the individual receiving care | Suggested dosage | Length of time |
Phenoxymethylpenicillin (pencillin V), Ingested | Ages spanning from 1 to 6 years | 125mg every six hours | 10 days |
Ages spanning from 6 to 12 years | 250mg every six hours | ||
Ages spanning from 12 to 18 years | Administering 250 to 500 milligrams every six-hours period | ||
Adults | Administering 500 milligrams every six-hours period | ||
Cefalexin, Ingested | Ages spanning from 1 to 5 years | Administering 125mg twice daily | 10 days |
Ages spanning from 5 to 12 years | Administering 250mg twice daily | ||
Ages 12 and older | Administering 500mg twice daily | ||
Clarithromycin, Ingested | Ages spanning from 1 to 12 years | Administering 7.5mg per kilogram per dose, two times a day (maximum 250mg) | 10 days |
Ages 12 and older | Administering 250mg twice daily | ||
Azithromycin, Ingested | A span of 6 months to 12 years | Giving 12mg per kilogram (up to a maximum of 500mg) once daily | 5 days |
2.2.5. Mechanism of drug action and Drug target
Azithromycin, classified within the macrolide drug category, features a dual-ring structure and primarily operates by impeding bacterial protein synthesis. This is achieved by specifically targeting the 50s subunit of susceptible bacterial ribosomes, resulting in reduced protein production in correlation with heightened macrolide concentration [15]. Azithromycin attaches to specific sites on bacterial ribosomes referred to as “neopeptide exit channels,” which serve as pathways for newly formed protein chains to exit the ribosome. This binding partially obstructs these pathways, hindering the movement of recently synthesized protein chains [15]. Azithromycin demonstrates a high degree of active absorption across various cell types and exhibits efficacy against beta-lactam-resistant bacteria and various suppurative bacteria in laboratory settings [15]. Its attributes encompass antibacterial modulation, immunomodulatory capacity, and anti-inflammatory effects, rendering it therapeutically beneficial for an array of respiratory inflammatory conditions [15]. Azithromycin’s mechanism of action encompasses the suppression of proinflammatory cytokine production, inhibition of bacterial protein synthesis, curbing of neutrophil infiltration, and modulation of macrophage polarization, equipping it to combat a diverse range of microorganisms [15].
2.2.6. Drug resistance
Azithromycin resistance predominantly stems from two factors: Inadequate utilization of azithromycin serves as a primary catalyst in the development of antibiotic-resistant bacteria, potentially giving rise to the propagation of bacteria that possess resistance to the medication [15]. The first factor involves resistance triggered by alterations in drug targets and the involvement of efflux pumps. Specifically, mutations within the mrR coding region led to heightened expression of the MtrCDE efflux system [15]. In addition, genetic mutations influencing a specific segment of the bacterial ribosome (23s rRNA subunits) can result in diminished binding capability or interaction with azithromycin in Neisseria gonorrhoeae. The modification of the drug’s target involves the methylation of the 23s ribosomal subunit or mutations in the rrl allele of the 23s rRNA gene, thereby impeding the attachment of the macrolide to this subunit [15].
3. Conclusion
Although phenoxymethicillin (penicillin V) and azithromycin have different treatment mechanisms for scarlet fever, they can eventually kill the bacteria that cause this disease and play a therapeutic role. Penicillins are agents with bactericidal properties that carry out their mode of action by hindering the production of microbial cell barriers and inducing a self-destructive effect within bacteria. Azithromycin, features a dual-ring structure and primarily operates by impeding bacterial protein synthesis. These two antibiotics not only have different mechanisms of action, but also have different modes of administration. Although both have therapeutic effects, penicillin still occupies the main proportion in the treatment of scarlet fever from the perspective of clinical application and experiments. In terms of drug resistance, A more effective solution to drug resistance is the most worthy direction of further research at this stage. The bacteria that cause scarlet fever continue to evolve and develop antibodies with the use of antibiotics, which means that the efficacy of penicillin and azithromycin will continue to diminish. Exploring new drugs and altering the chemical structure of these two classes of antibiotics to have new therapeutic effects to deal with bacterial resistance can help in the development of resistance. What is more, a variety of drug combination therapy with synergistic functions has become an exploratory program, and relevant personnel’s research in scarlet fever is still continuing. As a class of epidemic, scarlet fever is highly contagious, and relevant medical departments in various countries should also do a good job of corresponding control to avoid large-scale outbreaks again.
References
[1]. Ferretti, J., & Köhler, W. (2016). History of streptococcal research. University of Oklahoma Health Sciences Center.
[2]. InformedHealth.org [Internet]. Cologne, Germany: Institute for Quality and Efficiency in Health Care (IQWiG); 2006-. Scarlet fever: Overview. [Updated 2020 Jul 16]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279620/
[3]. Azithromycin. (n.d.). Medlineplus.gov. Retrieved August 14, 2023, from https://medlineplus.gov/druginfo/meds/a697037.html
[4]. You, Y., Qin, Y., Walker, M. J., Feng, L., & Zhang, J. (2019). Increased Incidence of Scarlet Fever - China, 1999-2018. China CDC weekly, 1(5), 63–66. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8393181/
[5]. Scarlet fever: A deadly history and how it prevails. (2023, January 24). Asm.org. https://asm.org/Articles/2023/January/Scarlet-Fever-A-Deadly-History-and-How-it-Prevails
[6]. Liu, Y., Chan, T. C., Yap, L. W., Luo, Y., Xu, W., Qin, S., Zhao, N., Yu, Z., Geng, X., & Liu, S. L. (2018). Resurgence of scarlet fever in China: a 13-year population-based surveillance study. The Lancet. Infectious diseases, 18(8), 903–912. https://doi.org/10.1016/S1473-3099(18)30231-7
[7]. Penicillins. (n.d.). Antimicrobe.org. Retrieved August 14, 2023, from http://www.antimicrobe.org/d24.asp
[8]. National Center for Biotechnology Information (2023). PubChem Compound Summary for CID 6869, Penicillin V. Retrieved July 31, 2023 from https://pubchem.ncbi.nlm.nih.gov/compound/Penicillin-V.
[9]. Britannica, T. Editors of Encyclopaedia (2023, June 5). Penicillium. Encyclopedia Britannica. https://www.britannica.com/science/Penicillium
[10]. (N.d.). Nhs.uk. Retrieved August 14, 2023, from https://www.clinicalguidelines.scot.nhs.uk/media/3651/ggc-interim-guidance-antibiotics-in-gas-v9-16-dec-2022.pdf
[11]. Dinos G. P. (2017). The macrolide antibiotic renaissance. British journal of pharmacology, 174(18), 2967–2983. Drug dosage and oral method used to treat scarlet fever National Center for Biotechnology Information (2023). PubChem Compound Summary for CID 447043, Azithromycin. Retrieved August 9, 2023 from https://pubchem.ncbi.nlm.nih.gov/compound/Azithromycin.
[12]. “ZITHROMAX, ZMAX (Azithromycin)” (2017) in Antibiotics Manual. Chichester, UK: John Wiley & Sons, Ltd, pp. 439–441.
[13]. Wessels MR. Pharyngitis and Scarlet Fever. 2016 Feb 10 [Updated 2016 Mar 25]. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: Basic Biology to Clinical Manifestations [Internet]. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2016-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK333418/
[14]. Heidary, M., Ebrahimi Samangani, A., Kargari, A., Kiani Nejad, A., Yashmi, I., Motahar, M., Taki, E., & Khoshnood, S. (2022). Mechanism of action, resistance, synergism, and clinical implications of azithromycin. Journal of Clinical Laboratory Analysis, 36(6). https://doi.org/10.1002/jcla.24427
[15]. Azithromycin. (n.d.). Medlineplus.gov. Retrieved August 14, 2023, from https://medlineplus.gov/druginfo/meds/a697037.html
Cite this article
Mei,X. (2024). Comparing penicillin and azithromycin in treating scarlet fever. Theoretical and Natural Science,45,179-188.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Ferretti, J., & Köhler, W. (2016). History of streptococcal research. University of Oklahoma Health Sciences Center.
[2]. InformedHealth.org [Internet]. Cologne, Germany: Institute for Quality and Efficiency in Health Care (IQWiG); 2006-. Scarlet fever: Overview. [Updated 2020 Jul 16]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279620/
[3]. Azithromycin. (n.d.). Medlineplus.gov. Retrieved August 14, 2023, from https://medlineplus.gov/druginfo/meds/a697037.html
[4]. You, Y., Qin, Y., Walker, M. J., Feng, L., & Zhang, J. (2019). Increased Incidence of Scarlet Fever - China, 1999-2018. China CDC weekly, 1(5), 63–66. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8393181/
[5]. Scarlet fever: A deadly history and how it prevails. (2023, January 24). Asm.org. https://asm.org/Articles/2023/January/Scarlet-Fever-A-Deadly-History-and-How-it-Prevails
[6]. Liu, Y., Chan, T. C., Yap, L. W., Luo, Y., Xu, W., Qin, S., Zhao, N., Yu, Z., Geng, X., & Liu, S. L. (2018). Resurgence of scarlet fever in China: a 13-year population-based surveillance study. The Lancet. Infectious diseases, 18(8), 903–912. https://doi.org/10.1016/S1473-3099(18)30231-7
[7]. Penicillins. (n.d.). Antimicrobe.org. Retrieved August 14, 2023, from http://www.antimicrobe.org/d24.asp
[8]. National Center for Biotechnology Information (2023). PubChem Compound Summary for CID 6869, Penicillin V. Retrieved July 31, 2023 from https://pubchem.ncbi.nlm.nih.gov/compound/Penicillin-V.
[9]. Britannica, T. Editors of Encyclopaedia (2023, June 5). Penicillium. Encyclopedia Britannica. https://www.britannica.com/science/Penicillium
[10]. (N.d.). Nhs.uk. Retrieved August 14, 2023, from https://www.clinicalguidelines.scot.nhs.uk/media/3651/ggc-interim-guidance-antibiotics-in-gas-v9-16-dec-2022.pdf
[11]. Dinos G. P. (2017). The macrolide antibiotic renaissance. British journal of pharmacology, 174(18), 2967–2983. Drug dosage and oral method used to treat scarlet fever National Center for Biotechnology Information (2023). PubChem Compound Summary for CID 447043, Azithromycin. Retrieved August 9, 2023 from https://pubchem.ncbi.nlm.nih.gov/compound/Azithromycin.
[12]. “ZITHROMAX, ZMAX (Azithromycin)” (2017) in Antibiotics Manual. Chichester, UK: John Wiley & Sons, Ltd, pp. 439–441.
[13]. Wessels MR. Pharyngitis and Scarlet Fever. 2016 Feb 10 [Updated 2016 Mar 25]. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: Basic Biology to Clinical Manifestations [Internet]. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2016-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK333418/
[14]. Heidary, M., Ebrahimi Samangani, A., Kargari, A., Kiani Nejad, A., Yashmi, I., Motahar, M., Taki, E., & Khoshnood, S. (2022). Mechanism of action, resistance, synergism, and clinical implications of azithromycin. Journal of Clinical Laboratory Analysis, 36(6). https://doi.org/10.1002/jcla.24427
[15]. Azithromycin. (n.d.). Medlineplus.gov. Retrieved August 14, 2023, from https://medlineplus.gov/druginfo/meds/a697037.html









