1. Introduction
Vaginitis is a common gynecological disease and remains an important health issue among women worldwide. It includes a range of causes, including bacterial, fungal, and parasitic infections. Understanding the complex interactions between various pathogens, external factors, and hormonal fluctuations in the development of vaginitis is crucial for effective management and treatment. This comprehensive review aims to delve into the historical background and multifaceted characteristics of vaginitis, elucidate the intricate complexity of pathogenic factors and their impact on clinical practice. In addition, this study focuses on exploring the complex relationship between clindamycin and streptomycin, a class of antibiotics that are crucial in combating vaginal infections. By elucidating the unique characteristics and mechanism of action of clindamycin and its antagonist metronidazole, this article aims to provide a detailed understanding of their efficacy in the treatment of vaginitis. The exploration of their respective chemical structures and drug synthesis provides valuable insights that help optimize their therapeutic outcomes. Furthermore, this study aims to incorporate the results of relevant clinical trials and examine the safety profile and cost control strategies of using clindamycin and metronidazole. By evaluating the overall efficacy of these antibiotics, this study aims to promote improvement in treatment plans, patient care, and better disease management strategies. Ultimately, this comprehensive analysis aims to fill an important gap in our understanding of vaginitis treatment and pave the way for more targeted and effective treatment interventions.
2. Overview of disease
2.1. Introduction of vaginitis
Vaginitis is a common gynecological disease, with its onset potentially triggered by a variety of pathogens, external factors, and hormonal fluctuations. Among the diverse spectrum of vaginitis cases, it is noteworthy that approximately 90 percent can be attributed to the presence of bacterial vaginosis, candidiasis, or trichomoniasis. Bacterial vaginosis, often abbreviated as BV, holds a prominent position as one of the most common forms of vaginitis, being responsible for roughly 50 percent of all vaginal infections. BV can manifest as either symptomatic or asymptomatic, and it tends to affect women of reproductive age. A striking observation is that approximately half of women worldwide experience symptoms linked to BV infection, such as vaginal malodor and itching. Compounding the challenge of BV is its propensity for recurrence, as evidenced by numerous cases. Hence, early detection and timely treatment assume paramount importance in managing this condition effectively. The recurrent nature of BV underscores the significance of research, medical intervention, and public health awareness in addressing this prevalent gynecological concern [1].
2.2. History of vaginitis
Due to the belief that vaginal Haemophilus was the pathogen, BV was originally called 'Haemophilus vaginalis vaginitis' which was initially described by Gardner and Dukes in 1955. Subsequent research revealed H.vaginal vaginalis wasn’t one kind of the Haemophilus genus, leading to the bacterium being renamed Gardnerella vaginalis[1].
Nowadays, BV’s etiology still remains unclear. In a healthy vaginal ecosystem, Lactobacilli typically dominate, constituting about ninety to ninety-five percent of whole bacteria [1].
Abnormal vaginal discharge is the main reason of BV. BV has been researched for about sixty-five years. More than 60 years later, researchers studying BV still cannot elucidate the causes of BV and understand many aspects related to its consequences. This underscores that BV remains a clinical challenge [1].
2.3. The introduction of lincosamide
The lincosamide class of antibacterials originates [2]. Nowadays, lincomycin is widely used to treat infections [3]. It is primarily employed to combat gram-positive bacteria. The mechanism of action of these antibiotics involves binding to the 23S rRNA of the 50S subunit to disrupt microbial protein synthesis [4]. Lincomycin proves useful in treating connective tissue infections due to its excellent absorption in the skin and bones and its ability to counter strains that produce necrotizing toxins. Due to the apace development of resistance as well as gastrointestinal side effects the treatments of Clindamycin have been limited in the past [2].
3. Comparison of clindamycin and metronidazole
3.1. Clindamycin
3.1.1. The background of clindamycin. There is a great connection between clindamycin and lincosamide. The semi-synthetic lincosamide antibiotic——clindamycin is used to treat severe infections caused by microorganisms susceptible to it and can be used to treat common acne. Its spectrum of activity is relatively narrow. What’s more, clindamycin can also combat protozoa and has been used to treat other diseases such as Babesiosis.
Clindamycin is derived from lincomycin and has also significantly reduced the use of lincomycin [5].
3.1.2. Chemical structure. Understanding the chemical structure is of great help for further research on its role. Clindamycin’s chemical structure is shown below.
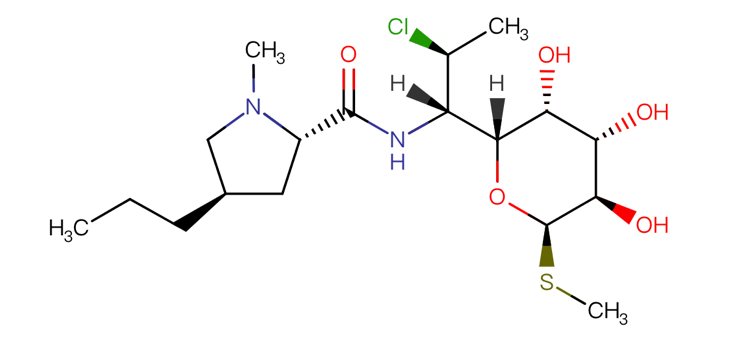
Figure 1. chemical structure of Clindamycin [5]
3.1.3. Drug pharmacology. In organisms, by affecting the process Clindamycin functions through specifically attaching to the 23S ribosomal RNA within the bacterial ribosome's 50S subunit, directly inhibiting peptide bond formation and disrupting the peptide chain initiation process [5].
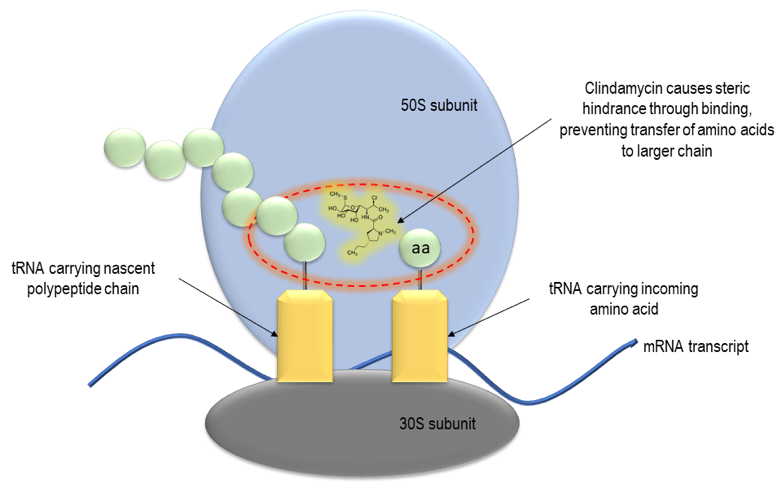
Figure 2. Mechanism of action of clindamycin [5]
3.1.4. Drug production. Clindamycin, derived from lincomycin as its 7(S)-chloro-7-deoxy variant, has proven its efficacy over time [5].
3.1.5. Pharmacological properties. Clindamycin hydrochloride is the hydrochloride form of Clindamycin, mainly to increase the water solubility, facilitate the absorption and distribution of drugs in the body, and thus improve the efficacy of drugs.
Regarding the absorption of clindamycin, approximately ninety pencent of clindamycin hydrochloride is absorbed in the gastrointestinal tract. The peak concentration is observed within 1 hour after administering a 150 mg dosage of clindamycin, and the average concentration decreases to less than 1 microgram per milliliter after 6 hours [7]. Residual food in the human body does not affect clindamycin hydrochloride inhalation into human blood significantly, although it may lead to a slight reduction in the extent of absorption.
3.1.6. Limitation. Clindamycin is likely to affect the gastrointestinal flora of breastfed infants. During breastfeeding, it is advisable not to discontinue breastfeeding solely due to the need for clindamycin. You can explore alternative medications that can replace clindamycin. Additionally, it is important to be vigilant about potential adverse effects on the gastrointestinal flora, such as diarrhea and fungal infections [6]. If blood appears in the stool, consideration should be given to the possibility of antibiotic-associated colitis.
Topical vaginal use of clindamycin is unlikely to cause side effects in infants, although approximately thirty percent of the vaginal dose is absorbed into the body. Local application for acne treatment is unlikely to have side effects in infants. However, if the infant is exposed to clindamycin applied topically to the breast, it may increase the risk of diarrhea.
It is also important to rule out the possibility of clindamycin-induced pregnancy-associated dermatosis with pustules [8].
3.1.7. Pharmacoeconomics. Clindamycin is sold in various forms, including solutions for injection, tablet, and capsule. Different forms of Clindamycin from different manufacturers have different prices. The specific price of each Clindamycin is shown below [9].
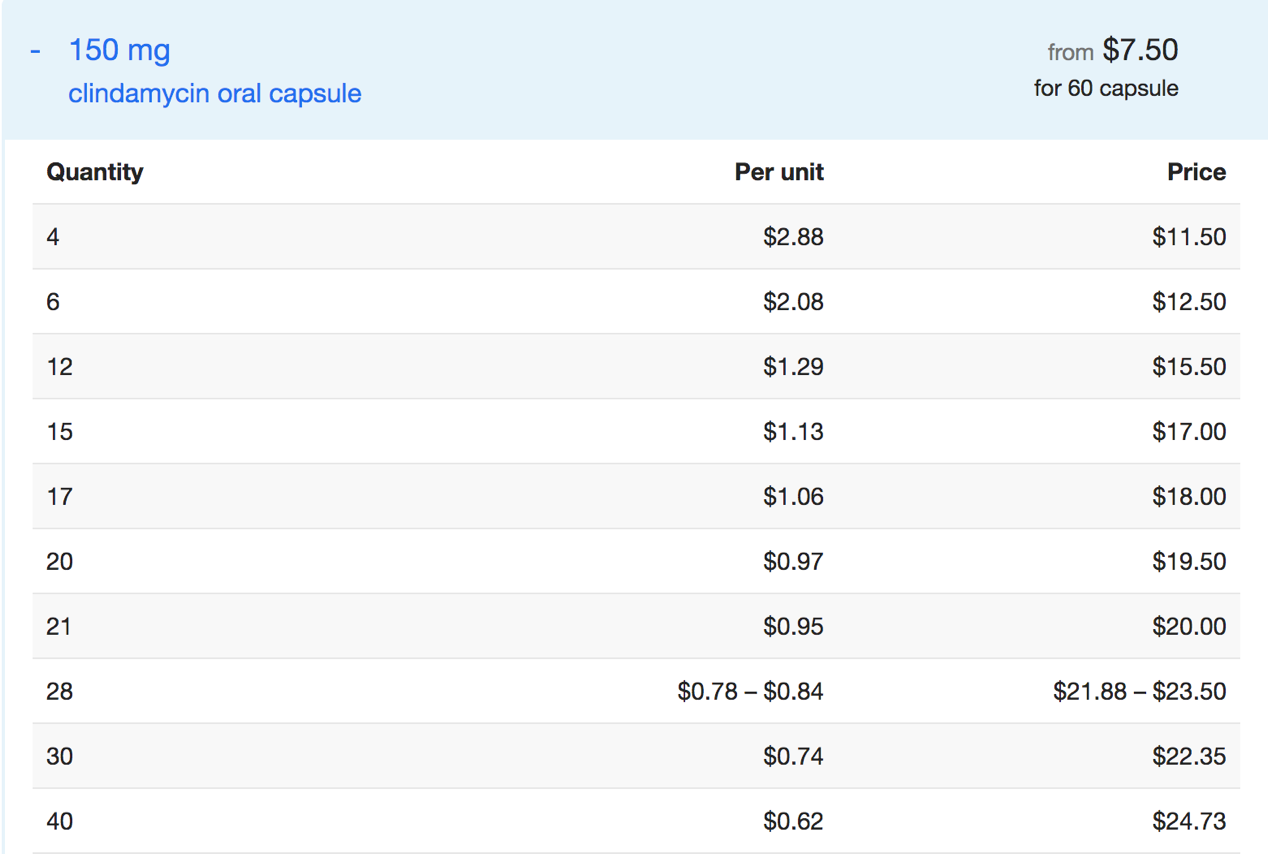
Figure 3. Price of Clindamycin oral capsule [9]
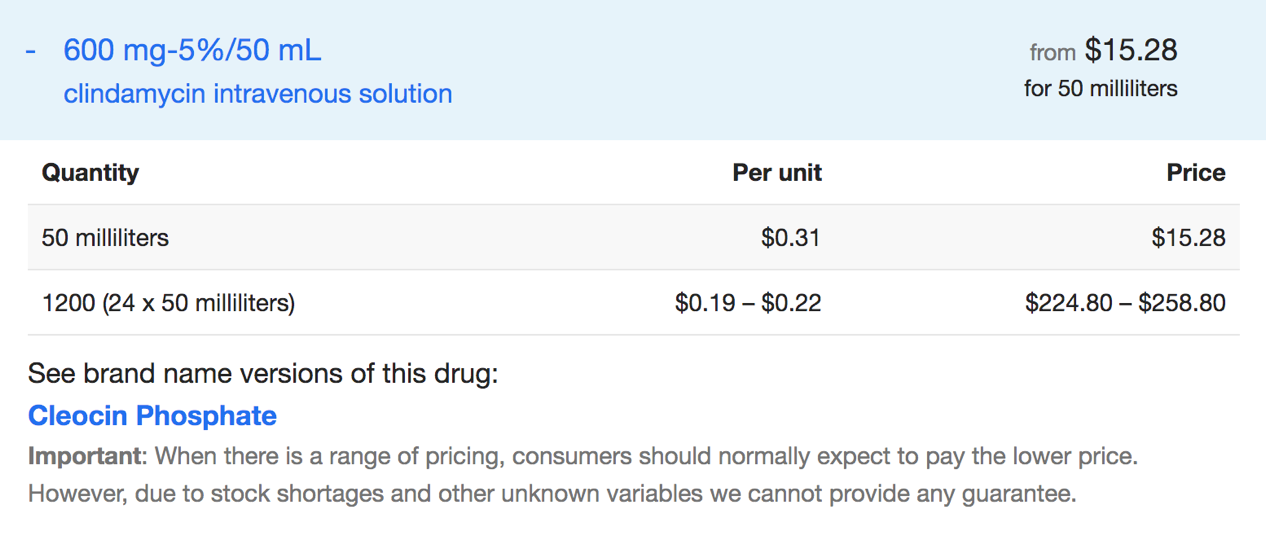
Figure 4. Price of Clindamycin intravenous solution [9]
3.2. Metronidazole
3.2.1. Background. Metronidazole, part of the nitroimidazole class of antibiotics, is used widely. It is employed to treat parasitic infections, such as gastrointestinal infections. Due to its effectiveness, Metronidazole has a long history of use [10]. Additionally, it serves as a preventive measure against postoperative infections.
3.2.2. Chemical structure. Metronidazole’s chemical formula is C6H9N3O3[10]. Metronidazole presents as a white to light-yellow crystalline powder with a faint aroma. It has a bitter and somewhat salty taste. The pH in a saturated aqueous solution is approximately 6.5[10].

Figure 5. chemical structure of Metronidazole [10]
3.2.3. Drug pharmacology. Metronidazole enters the organism and exerts its action by inhibiting protein synthesis through DNA interaction [11]. This results in a disruption of helical DNA structure and causes breaks in the DNA strands, ultimately leading to cell death in susceptible organisms [11].
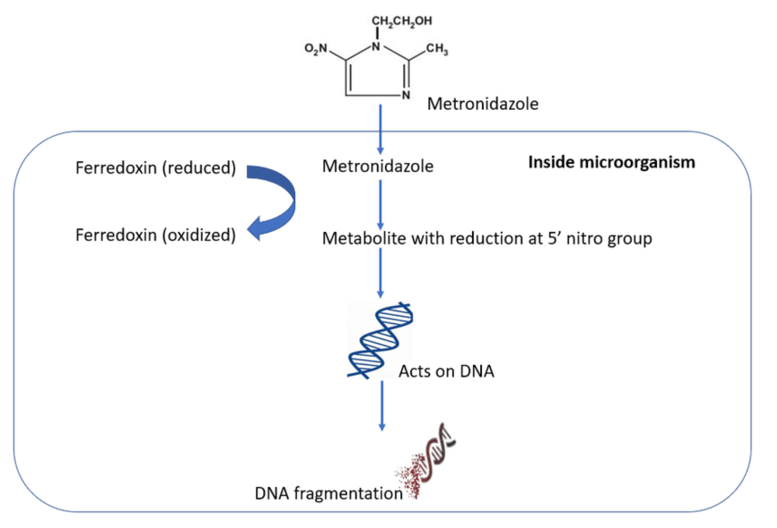
Figure 6. Mechanism of action metronidazole [12]
3.2.4. Drug production. Metronidazole is synthesized by initially nitration of 2-methylimidazole to produce 2-methyl-5-nitroimidazole (37.2.9). Subsequently, this compound undergoes a reaction with 2-chloroethanol or ethylene oxide, which can be readily converted into the final desired form of metronidazole [13].
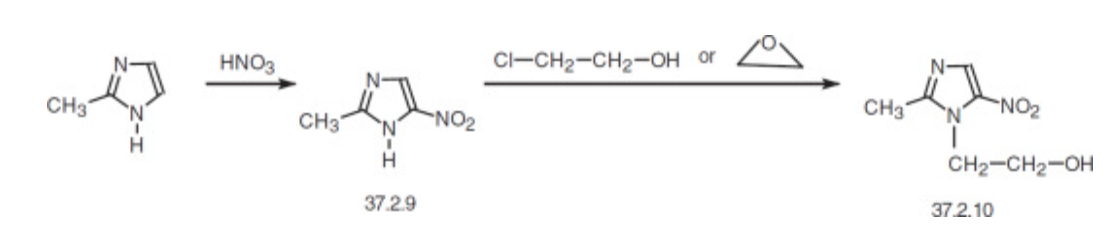
Figure 7. synthesis of Metronidazole [13]
3.2.5. Pharmacological properties. Metronidazole has a low protein binding rate (<20%). In adults, the Volume of distribution under steady state is among 0.51 to 1.1 L/kg [10].
Oral administration of Metronidazole results in nearly complete absorption, the bioavailability of the tablet exceeds 90%, and its absorption is not affected by infection. Rectal absorption accounts for 67% to 82% of the dose, while vaginal absorption is about 20% to 56%. Metronidazole can penetrate various tissues, including the central nervous system, reaching 60% to 100% of the plasma concentration. However, its concentration in placental tissue is not high. Extensive liver metabolism produces five metabolites. Among them, the hydroxy metabolites exhibit thirty percent to sixty-five of the biological activity [14].
3.2.6. Limitation. Nausea is a prevalent side effect associated with Metronidazole, while vomiting, headaches, and a metallic taste are among the additional side effects [15]. What’s more, Metronidazole also has some neurological effects such as ataxia, dizziness and seizures. What’ more, metronidazole-induced encephalopathy (MIE) was reported as a case which typically occurs when the total dose exceeds the standard dosage [15].
Metronidazole is generally well-tolerated, which the most common being mild side effects; however, moderate side effects are not uncommon, such as nausea, abdominal pain, and diarrhea. However, there have been rare reports of more severe issues such as neurotoxicity, optic neuropathy, peripheral neuropathy and encephalopathy [16].
3.2.7. Pharmacoeconomics. The absorption mode of different forms in the body also varies, which may lead to different action times. The specific administration method should be analyzed based on the individual patient's situation and cannot be generalized. Metronidazole is sold in various forms, including solutions for injection, film coated tablet and coated capsule [10]. Different forms of Metronidazole with different routes have different prices. For example, the price of sustained-release metronidazole may be higher than that of general metronidazole due to the more complex preparation process. The specific price of Metronidazole each is shown below.
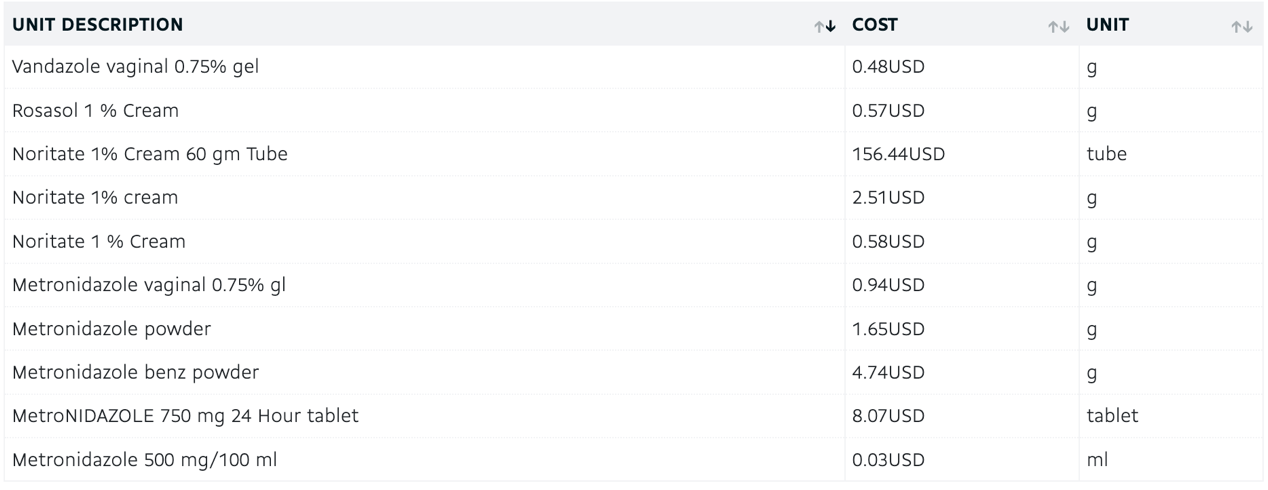
Figure 8. price of Metronidazole [10]
4. Clinical trials
4.1. Safety
Bacterial vaginosis is a common condition among gynecology patients, prompting several types of therapy. This trial aimed to assess the effectiveness of clindamycin vaginal cream in treating bacterial vaginosis. The study included sixty patients with symptoms of bacterial vaginosis, of which 46 completed the trial. Among them, 23 patients applied 5g of 2% clindamycin vaginal cream intravaginally before bedtime for one week, along with oral placebo tablets twice a day for one week. The remaining 23 patients took 500mg of oral metronidazole tablets twice daily for one week and used 5g of placebo vaginal cream intravaginally for one week. Comparing the cure rates of such two treatment regimens. In the clindamycin group, 22 patients (97%) exhibited improvement or cure during the initial follow-up, while the metronidazole group saw 19 patients (83%) showing improvement or cure. Adverse effects associated with such two approaches were similar. In conclusion, 2% clindamycin vaginal cream demonstrates a level of efficacy and safety akin to the established oral metronidazole therapy for treating BV [17].
4.2. Cost containment
On August 2, 1988, the University of British Columbia-affiliated hospital implemented a series of measures for cost control in an attempt to curb the rising expenditure on antibiotics. One of these measures involved reducing the frequency of patient doses of clindamycin and metronidazole by automating the dosing interval through the pharmacy. Clindamycin prescriptions were changed to once every 8 hours, while metronidazole was changed to once every 12 hours. During the post-implementation period, the average dose per course of metronidazole was reduced. Over the seven-month post-implementation period, this measure saved approximately $7,300. If this measure continued to be implemented, it was estimated to save over ten thousand dollars within a year. This cost control strategy successfully saved cost for the UBC-affiliated university hospital site [18].
4.3. Efficacy
Women diagnosed with bacterial vaginosis were instructed to use 100mg of clindamycin vaginal suppositories continuously for three days, along with taking placebo capsules orally twice a day for one week. Another group was given two 250mg capsules of metronidazole, totaling 500mg, to be taken orally twice a day for a week, along with placebo vaginal suppositories for three consecutive days. A predetermined sample size was used to achieve a likelihood of 0.84 for drawing accurate conclusions, indicating a 15% higher success rate with clindamycin compared to the expected 75% success rate for metronidazole.
This clinical trial involved a total of 399 participants, with 233 qualifying for efficacy assessment. In this subgroup, 77 out of 113 individuals, or 68.1%, experienced some improvement with clindamycin treatment, while 80 out of 120 individuals, accounting for 66.7% of the total, were completely cured with metronidazole treatment. Adverse events related to treatment occurred more frequently in the group receiving metronidazole. It is worth noting that systemic symptoms such as nausea and taste disturbances were the main reasons for the differences between the two groups. A three-day course of clindamycin administered in the form of vaginal suppositories showed comparable efficacy and superior tolerability when compared to the regimen of oral metronidazole 500mg taken twice daily for a continuous week in treating bacterial vaginosis [19].
5. Conclusion
To summarize, BV is a serious disease that troubles many women around the world. Infection with BV requires severe symptoms, even during pregnancy, which may affect the fetus through the connection between the mother and the child. Despite the high recurrence rate of BV, it should still be given timely attention and treated as soon as possible. Clindamycin and metronidazole all act as bacterial inhibitors to prevent protein synthesis. They have been discovered very early and have been used for a long time, and people are still quite experienced in using them to treat BV. Because of being compared to metronidazole, clindamycin can be used in cases of recurrent infections, it may have a better efficacy. So far, many clinical trials have been conducted to study the safety and economic benefits of these two antibiotics. The effectiveness and safety of clindamycin vaginal cream are comparable to those of the established oral metronidazole therapy to treat bacterial vaginosis. Clindamycin administration has equivalent efficacy to metronidazole and stronger tolerability. When it comes to treating BV, clindamycin compared to metronidazole, clindamycin would be the better choice. It's worth mentioning that combination treatment of clindamycin and metronidazole is also common in treating bacterial vaginosis.
References
[1]. Bautista, C. T., Wurapa, E., Sateren, W. B., Morris, S., Hollingsworth, B., & Sanchez, J. L. (2016). Bacterial vaginosis: a synthesis of the literature on etiology, prevalence, risk factors, and relationship with chlamydia and gonorrhea infections. Military Medical Research, 3, 4. https://doi.org/10.1186/s40779-016-0074-5
[2]. Morar, M., Bhullar, K., Hughes, D. W., Junop, M., & Wright, G. D. (2009). Structure and mechanism of the lincosamide antibiotic adenylyltransferase LinB. Structure (London, England: 1993), 17(12), 1649–1659. https://doi.org/10.1016/j.str.2009.10.013
[3]. Johnson, M. D., & Decker, C. F. (2008). Antimicrobial agents in treatment of MRSA infections. Disease-a-month : DM, 54(12), 793–800. https://doi.org/10.1016/j.disamonth.2008.09.002
[4]. Fitzhugh A. L. (1998). Antibiotic inhibitors of the peptidyl transferase center. 1. Clindamycin as a composite analogue of the transfer RNA fragments L-Pro-Met and the D-ribosyl ring of adenosine. Bioorganic & medicinal chemistry letters, 8(1), 87–92. https://doi.org/10.1016/s0960-894x(97)10196-2
[5]. DrugBank. (n.d.). Clindamycin - DrugBank. Retrieved from https://www.drugbank.ca/drugs/DB01190
[6]. Murphy, P. B., Bistas, K. G., & Le, J. K. (2023, May 23). Clindamycin. In StatPearls. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK519574/
[7]. Medicines.org.uk. (n.d.). Clindamycin 150Mg Capsules-Summary Of Product Characteristics (Smpc) - (Emc). Retrieved from https://www.medicines.org.uk/emc/medicine/21628
[8]. De Cruz, R., Ferguson, J., Wee, J. S., & Akhras, V. (2015). Acute localised exanthematous pustulosis (ALEP) induced by clindamycin in pregnancy. The Australasian journal of dermatology, 56(3), e55–e58. https://doi.org/10.1111/ajd.12135
[9]. Drugs.com. (n.d.). Clindamycin Prices, Coupons & Patient Assistance Programs - Drugs.Com. Retrieved from https://www.drugs.com/price-guide/clindamycin
[10]. DrugBank. (n.d.). Metronidazole - DrugBank. Retrieved from https://go.drugbank.com/drugs/DB00916
[11]. Weir, C. B., & Le, J. K. (2023, February 12). Metronidazole. In StatPearls. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK539728/
[12]. Ahmed, F. (2022, April). Mechanism of action figure 2 above illustrates how metronidazole undergo therapeutic response ResearchGate. https://www.researchgate.net/figure/Mechanism-of-Action-Figure-2-above-illustrates-how-metronidazole-undergo-therapeutic_fig1_351867679
[13]. Vardanyan, R. S., & Hruby, V. J. (2006). Synthesis of Essential Drugs. In Synthesis of Essential Drugs (pp. 559-582). Department of Chemistry 1306 E. University. Retrieved May 9, 2007, from https://doi.org/10.1016/B978-044452166-8/50037-6
[14]. Lamp, K. C., Freeman, C. D., Klutman, N. E., & Lacy, M. K. (1999). Pharmacokinetics and pharmacodynamics of the nitroimidazole antimicrobials. Clinical pharmacokinetics, 36(5), 353–373. https://doi.org/10.2165/00003088-199936050-00004
[15]. Senthilkumaran, S., Shah, S., Balamurugan, N., & Thirumalaikolundusubramanian, P. (2015). Metronidazole encephalopathy: Uncommon reaction to a common drug. International journal of critical illness and injury science, 5(2), 123–124. https://doi.org/10.4103/2229-5151.158422
[16]. Hernández Ceruelos, A., Romero-Quezada, L. C., Ruvalcaba Ledezma, J. C., & López Contreras, L. (2019). Therapeutic uses of metronidazole and its side effects: an update. European review for medical and pharmacological sciences, 23(1), 397–401. https://doi.org/10.26355/eurrev_201901_16788
[17]. Andres, F. J., Parker, R., Hosein, I., & Benrubi, G. I. (1992). Clindamycin vaginal cream versus oral metronidazole in the treatment of bacterial vaginosis: a prospective double-blind clinical trial. Southern medical journal, 85(11), 1077–1080. https://doi.org/10.1097/00007611-199211000-00006
[18]. Jung, B., & Andrews, J. D. (1990). Effectiveness of an antibiotic cost containment measure. The Canadian journal of hospital pharmacy, 43(3), 116–122.
[19]. Jung, B., & Andrews, J. D. (1990). Effectiveness of an antibiotic cost containment measure. The Canadian journal of hospital pharmacy, 43(3), 116-122.
Cite this article
Ding,K. (2024). Comparison of clindamycin and metronidazole in treating vaginitis. Theoretical and Natural Science,45,239-247.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Bautista, C. T., Wurapa, E., Sateren, W. B., Morris, S., Hollingsworth, B., & Sanchez, J. L. (2016). Bacterial vaginosis: a synthesis of the literature on etiology, prevalence, risk factors, and relationship with chlamydia and gonorrhea infections. Military Medical Research, 3, 4. https://doi.org/10.1186/s40779-016-0074-5
[2]. Morar, M., Bhullar, K., Hughes, D. W., Junop, M., & Wright, G. D. (2009). Structure and mechanism of the lincosamide antibiotic adenylyltransferase LinB. Structure (London, England: 1993), 17(12), 1649–1659. https://doi.org/10.1016/j.str.2009.10.013
[3]. Johnson, M. D., & Decker, C. F. (2008). Antimicrobial agents in treatment of MRSA infections. Disease-a-month : DM, 54(12), 793–800. https://doi.org/10.1016/j.disamonth.2008.09.002
[4]. Fitzhugh A. L. (1998). Antibiotic inhibitors of the peptidyl transferase center. 1. Clindamycin as a composite analogue of the transfer RNA fragments L-Pro-Met and the D-ribosyl ring of adenosine. Bioorganic & medicinal chemistry letters, 8(1), 87–92. https://doi.org/10.1016/s0960-894x(97)10196-2
[5]. DrugBank. (n.d.). Clindamycin - DrugBank. Retrieved from https://www.drugbank.ca/drugs/DB01190
[6]. Murphy, P. B., Bistas, K. G., & Le, J. K. (2023, May 23). Clindamycin. In StatPearls. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK519574/
[7]. Medicines.org.uk. (n.d.). Clindamycin 150Mg Capsules-Summary Of Product Characteristics (Smpc) - (Emc). Retrieved from https://www.medicines.org.uk/emc/medicine/21628
[8]. De Cruz, R., Ferguson, J., Wee, J. S., & Akhras, V. (2015). Acute localised exanthematous pustulosis (ALEP) induced by clindamycin in pregnancy. The Australasian journal of dermatology, 56(3), e55–e58. https://doi.org/10.1111/ajd.12135
[9]. Drugs.com. (n.d.). Clindamycin Prices, Coupons & Patient Assistance Programs - Drugs.Com. Retrieved from https://www.drugs.com/price-guide/clindamycin
[10]. DrugBank. (n.d.). Metronidazole - DrugBank. Retrieved from https://go.drugbank.com/drugs/DB00916
[11]. Weir, C. B., & Le, J. K. (2023, February 12). Metronidazole. In StatPearls. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK539728/
[12]. Ahmed, F. (2022, April). Mechanism of action figure 2 above illustrates how metronidazole undergo therapeutic response ResearchGate. https://www.researchgate.net/figure/Mechanism-of-Action-Figure-2-above-illustrates-how-metronidazole-undergo-therapeutic_fig1_351867679
[13]. Vardanyan, R. S., & Hruby, V. J. (2006). Synthesis of Essential Drugs. In Synthesis of Essential Drugs (pp. 559-582). Department of Chemistry 1306 E. University. Retrieved May 9, 2007, from https://doi.org/10.1016/B978-044452166-8/50037-6
[14]. Lamp, K. C., Freeman, C. D., Klutman, N. E., & Lacy, M. K. (1999). Pharmacokinetics and pharmacodynamics of the nitroimidazole antimicrobials. Clinical pharmacokinetics, 36(5), 353–373. https://doi.org/10.2165/00003088-199936050-00004
[15]. Senthilkumaran, S., Shah, S., Balamurugan, N., & Thirumalaikolundusubramanian, P. (2015). Metronidazole encephalopathy: Uncommon reaction to a common drug. International journal of critical illness and injury science, 5(2), 123–124. https://doi.org/10.4103/2229-5151.158422
[16]. Hernández Ceruelos, A., Romero-Quezada, L. C., Ruvalcaba Ledezma, J. C., & López Contreras, L. (2019). Therapeutic uses of metronidazole and its side effects: an update. European review for medical and pharmacological sciences, 23(1), 397–401. https://doi.org/10.26355/eurrev_201901_16788
[17]. Andres, F. J., Parker, R., Hosein, I., & Benrubi, G. I. (1992). Clindamycin vaginal cream versus oral metronidazole in the treatment of bacterial vaginosis: a prospective double-blind clinical trial. Southern medical journal, 85(11), 1077–1080. https://doi.org/10.1097/00007611-199211000-00006
[18]. Jung, B., & Andrews, J. D. (1990). Effectiveness of an antibiotic cost containment measure. The Canadian journal of hospital pharmacy, 43(3), 116–122.
[19]. Jung, B., & Andrews, J. D. (1990). Effectiveness of an antibiotic cost containment measure. The Canadian journal of hospital pharmacy, 43(3), 116-122.









