1. Introduction
Antimicrobial peptides (AMPs) are a widely distributed group of short peptides that play a crucial function in the innate immune systems of various animals. The inhibitory effects of the substance are wide-ranging, encompassing bacteria, fungi, parasites, and viruses [1].
The urgency of the situation is emphasized in the 2019 Antibiotic Resistance Report published by the Centers for Disease Control and Prevention (CDC) in the United States. The report provides detailed information on the occurrence of approximately 2.8 million occurrences of illnesses that are resistant to antibiotics, leading to a total of 35,000 deaths. The occurrence of bacterial diseases that are resistant to drugs not only poses a threat to the health of both humans and animals, but also imposes a substantial economic burden [2].
It is worth mentioning that the presence of multi drug-resistant (MDR) bacteria, including Methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, Multi-drug resistant Pseudomonas aeruginosa, and Multi-drug resistant Stenotrophomonas maltophilia, intensifies the level of urgency surrounding this issue.
They are readily accessible and consistently utilized within the medical field. Positron emission tomography (PET) probes that utilize peptide targeting typically include of tiny peptides that exhibit a notable affinity and selectivity towards various cellular and tissue targets. The primary benefits associated with these probes encompass their cost-effectiveness compared to conventional antibody-based PET tracers, as well as their efficient chemical modification procedure that allows for radio-labeling with a wide range of radionuclides. These characteristics render them particularly appealing for clinical applications. Request for reconsideration. Presently, in line with the development of drug design, the utilization of computational procedures in the sector is progressively expanding, albeit not yet considered a conventional tool in radio-pharmaceutical design [3].
This revolutionary paradigm introduces a new direction to the study of therapeutic peptides, which includes a range of immunomodulatory functions, non-membrane mechanisms of action, peptide analogues, and formulation approaches to overcome protease degradation [4]. This review aims to provide a comprehensive understanding of the mechanisms underlying antimicrobial peptides, shedding light on this emerging paradigm. The technique presents intrinsic hurdles that are formidable, but it also holds the potential to develop more effective and cost-efficient broad-spectrum peptides, which could significantly impact the future of antimicrobial medicines.
2. Mechanism of antimicrobial peptides
2.1. Mechanism of peptide bond formation
The fundamental concept underlying peptide synthesis is the chemical condensation reaction between the carboxyl and amino groups of amino acids, resulting in the formation of peptide bonds. This chemical process is commonly denoted as a peptide bond-forming reaction or a peptide bond synthesis reaction. The process of peptide bond formation involves the creation of an amide bond between a carboxyl group and an amino group. Peptide bonds has the capability to undergo disassembly and reassembly via uncomplicated hydrolysis and transfer processes [5].
2.2. Mechanisms of antibiotic resistance
2.2.1. Causes of Antibiotic resistance
Microbial resistance to antibiotics is manifested by changes in antibiotic permeability, changes in target molecules, production of inactivating enzymes, and efflux of antibiotics from the cytoplasm. Bacteria and other microorganisms use all of these mechanisms to evade the toxic effects of antibiotics [6].
Antibacterial drug penetration barrier
In order to exert their antibacterial effect, antibiotics must penetrate the interior of bacteria and successfully reach the target spot. Alterations in the permeability of the bacterial cell wall and/or outer membrane can establish a protective barrier that hinders the penetration of antibacterial agents into the bacterial core, hence impeding their interaction with the intended target site. This phenomenon significantly compromises the efficacy of antibacterial treatments.
The outer membrane of Gram-negative bacteria is a multifaceted organelle that facilitates the uptake of nutrients while simultaneously serving as a safeguard and barrier. According to new findings from Enterobacteriaceae, it has been observed that the permeability of the outer membrane undergoes dynamic changes during bacterial development, which subsequently impacts the degree to which medications can enter the membrane [7].
Alteration of drug targets
Natural or acquired changes in antimicrobial drug targets that prevent drug binding are a common resistance mechanism. When the DNA or protein target of the drug is mutated or modified, the affinity between the drug and the target is reduced, and the inhibitory activity of antibacterial drugs is significantly weakened, leading to drug resistance. Alterations at target sites are often caused by spontaneous mutations in bacterial genes on chromosomes. Since the interaction of an antibiotic with a target molecule is usually very specific, small changes in the target molecule may have important effects on the binding of the antibiotic [8].
Production of inactivating enzymes
Many drug-resistant bacteria can produce inactivating or inactivating enzymes, hydrolyzing inactivating enzymes. Antibiotics can be modified by inactivating or modifying enzymes. These enzymes are located in transferable elements such as plasmids, transposons, and bacterial chromosomes, and spread between different bacteria, which are destructive to antimicrobial drugs and lead to drug inactivation. Bacteria often (develop resistance to antibiotics after exposure to them. Natural resistance is usually chromosomally mediated, whereas acquired resistance may result from chromosomal mutations or acquisition of resistance-encoding genes from external sources such as plasmids and transposons etc. Required and natural resistance are clinically important and can lead to treatment failure. However, acquired resistance is a major way of bacteria to be inherited [9].
Drug efflux
Drug efflux is a significant mechanism contributing to drug resistance in Gram-negative bacteria. These cellular mechanisms actively transport solutes out of the cell. Microbes employ efflux pumps as a means to manage their internal milieu by eliminating various detrimental chemicals, such as antimicrobials, metabolites, and quorum-sensing signaling molecules [10].
3. Classification of AMPs
The diversity of natural AMPs brings a lot of difficulties in their classification. AMPs are classified according to Source, Amino acid-rich species and Structure. (Figure 1). In the part of source, we will start form these aspects; Insect, mammalian, Aquatic and Microorganisms. In the part of Aminoacid-rich species, we will start from these aspects; Glycine, Proline and Histidine. Finally, in the part of structure, we will start from these aspects; Linear extension, α-helix and β-helix.
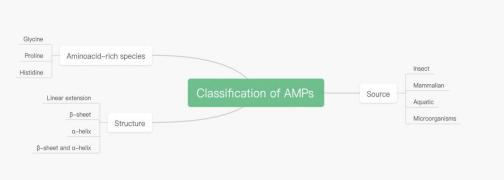
Figure 1. Classification of AMPs
3.1. Categorization of Antimicrobial Peptides (AMPs) According to their Origins
According to the statistical data provided by APD3, the sources of antimicrobial peptides (AMPs) can be categorized into three main groups: insect, mammalian, and microbes. Additionally, AMPs have the ability to attract marine organisms that inhabit the ocean. Through extensive research, it has been discovered that certain antibacterial peptides possess potent antimicrobial properties against fungi, protozoa, viruses, and cancer cells. Consequently, numerous researchers have been inclined to classify these bioactive peptides as “peptide antibiotics.”
3.2. Antimicrobial Peptides Derived from Insects
In instances where insects are exposed to pathogens or foreign chemicals, the hemolymph of these insects will generate a substantial quantity of antimicrobial proteins or antimicrobial peptides. The synthesis of these peptides mostly occurs within the fat bodies and subsequent secretion into the hemolymph. This process serves as a very efficient defense mechanism, capable of rapidly neutralizing or eradicating invasive germs. One example is the family of antimicrobial peptides known as Cecropin, which is widely recognized in insects. These peptides have been identified in several insect species, including guppies, silkworms, bees, and fruit flies. Cecropin A has demonstrated efficacy in combating several inflammatory conditions and malignancies [11]. It is important to note that there is significant variation in the quantity of antimicrobial peptides (AMPs) among different species. For instance, invasive Harlequin ladybirds (Harmonia axyridis) and Black flies (Hermetia illucens) possess as many as 50 AMPs, whereas the pea aphid (Acyrthosiphon pisum) does not possess any AMPs [12]. Jellyin, a peptide derived from royal jelly, exhibits robust biological activity, and demonstrates favorable effects against many bacteria and fungi. Additionally, its conjugated derivative, moonacid, has been found to possess inhibitory properties against Leishmania [13].
3.3. Antimicrobial Peptides in Mammals
Mammalian antimicrobial peptides have been identified in various species, including humans, sheep, cattle, and other vertebrates. The initial discovery of the mammalian antimicrobial peptide Cecropin P1 occurred in 1989, when it was isolated from the small intestine of pigs. The defensins present in the human body are a diverse group of antimicrobial peptides. They can be classified into three categories based on variations in their amino acid spatial structure and secretion site. These categories include human α-defensin (humandα-defensin), human β-defensin (human β-defensin), and human β-defensin (human β-defensin). Human θ-defensin (HβD) has been discovered.
There exist over 35 distinct types of human defensin, among which 10 defensin variants hold significant importance. Antimicrobial peptides can be categorized into many categories based on their structural characteristics, with Cathelicidin and Defensin being the predominant classifications. These entities can be categorized into five distinct classes: 1. Single chain α-helices lacking cysteine residues, or peptides consisting of two α-helices connected by a random coil region. 2. Antimicrobial peptides that exhibit a high abundance of specific amino acid residues, excluding cysteine residues. 3. Polypeptides with antibacterial properties characterized by the presence of a single disulfide bond. 4. Antimicrobial peptides possessing two or more disulfide bonds and adopting a β-folded conformation. 5. Peptides displaying antimicrobial activity derived from larger peptides with established functional roles. The earliest isolated Cecropins and Magainins derived from Xenopus are classified as the initial group of antimicrobial peptides, commonly known as Cecropin antimicrobial peptides. Currently, there is a greater level of depth in the study conducted on antimicrobial peptides. Furthermore, adenosine monophosphate (AMP) present in human breast milk serves a crucial function in the process of breastfeeding by mitigating the incidence of illness and death among infants who are nursed [14]. It is worth noting that Casein201, a peptide derived from beta-casein 201-220 amino acids, is present in varying quantities in colostrum from preterm and full-term humans [15]. Dairy products provide as a significant dietary source of AMP, which is generated through the process of enzymatic hydrolysis of milk. Multiple antimicrobial peptides (AMPs) have been discovered in various proteins such as alpha lactalbumin, beta-lactoglobulin, lactoferrin, and casein. Among these, lactoferrin B (LfcinB) is the most prominent peptide, as documented in previous research [16]. Furthermore, it is worth investigating the potential application of antimicrobial peptides derived from dairy products for the preservation of dairy products.
3.4. Microorganisms-Derived Antimicrobial Peptides
Bacterial antimicrobial peptides, commonly referred to as bacteriocins, encompass cationic peptides and neutral peptides, which are capable of being excreted by both gram-positive and gram-negative bacteria. Antimicrobial peptides can be derived from microorganisms such as bacteria and fungus. Notable examples of these peptides are nisin and gramicidin, which are obtained from Lactococcus lactis, Bacillus subtilis, and Bacillus brevis [17]. Bacteria have been revealed to possess four distinct classifications of antimicrobial peptides, including Bacitracin, Gramicidin S, Polymyxin E, and Nisin. Currently, the APD database comprises a total of 119 bacterial proteins. Among these proteins, nisin, a short peptide consisting of 3 to 4 amino acid residues, is produced by Lactococcus. Notably, nisin exhibits acid resistance and remains stable even in low pH environments, such as the stomach. Furthermore, it possesses the ability to inhibit gram-positive bacteria, including Clostridium and Listeria. Bacitracin and mersacidin, which are generated by certain strains of Bacillus spp., exhibit a significant inhibitory impact on methicillin-resistant Staphylococcus aureus (MRSA), a highly drug-resistant bacterium. Intraperitoneal dosing has been observed to effectively eliminate the presence of MRSA germs in various organs of mice, including the blood, lung, liver, kidney, and spleen. Furthermore, this method of delivery does not appear to induce any discernible damage to the organs of the animals.
3.5. Classification of AMPs Based on Amino acid-rich species
3.5.1. Proline
Proline is a representative example of a non-polar amino acid. PrAMPs exhibit distinct behavior compared to other AMPs. Specifically, they gain entry into the bacterial cytoplasm by utilizing the inner membrane transporter SbmA, as opposed to exerting antibacterial effects by membrane disruption [18]. The molecular formula of the compound is C5H9NO2.
It solid formula is (Figure 2).
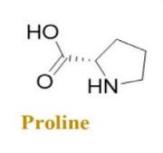
Figure 2. Proline
3.5.2. Histidine
Histidine is a prevalent amino acid with basic properties, and antimicrobial peptides (AMPs) that are rich in histidine demonstrate favorable activity in terms of membrane permeability. Moreover, it has been observed that HV2 exerts an inhibitory effect on bacterial motility, which is contingent upon its concentration. Additionally, HV2 demonstrates a robust anti-inflammatory action by suppressing the synthesis of tumor necrosis factor α (TNF-α) [19]. The molecular formula of the compound is C6H9N3O2. Its solid formula is (Figure 3).
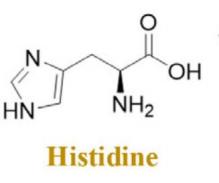
Figure 3. Histidine
3.5.3. Glycine
In the field of biology, the R group of glycine is commonly categorized as a non-polar amino acid. Glycine-rich antimicrobial peptides (AMPs), such as attacins and diptericins, are found abundantly in various natural environments [20]. Its chemical formula is C2H5NO2. Its solid formula is (Figure 4).
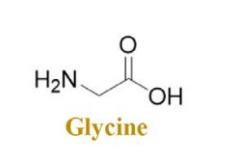
Figure 4. Glycine
3.6. Categorization of Antimicrobial Peptides (AMPs) According to Structural Characteristics
Antimicrobial peptides can be classified into four distinct categories according to their structural characteristics, namely linear α-helical peptides, β-sheet peptides, linear extension structure, and peptides exhibiting both α-helix and β-sheet conformations (Figure 5) [21]. Each individual possesses their own unique function, and hence, both individuals fulfill crucial roles.
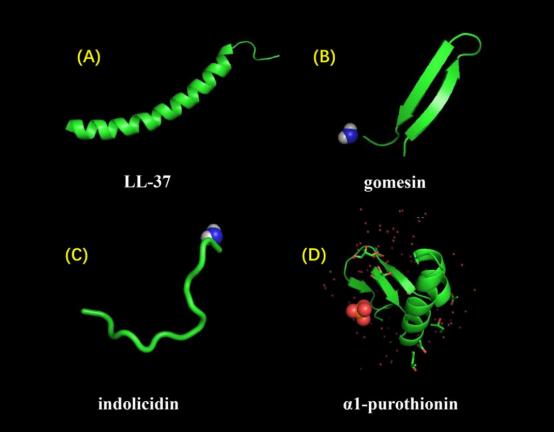
Figure 5. Displays several architectures of antimicrobial peptides (AMPs). LL-37 exhibits a characteristic α-helical structure, as reported in the literature (DOI: 10.2210/pdb2K6O/pdb). Gomesin is a peptide characterized by a β-sheet conformation and its stability is attributed to the presence of disulfide bonds (DOI: 10.2210/pdb1KFP/pdb). Indolicidin is an antimicrobial peptide (AMP) that possesses a linear extended structure rather than a well-defined three-dimensional conformation (DOI: 10.2210/pdb1G89/pdb). The protein α1-purothionin has a conformation that includes both alpha-helix and beta-sheet structures. The extension direction is shown by arrows (DOI: 10.2210/pdb2plh/pdb).
4. Designation
There are plenty of different approaches of designation, which is used to design antimicrobial peptides. Moreover, there are multiple types of Antimicrobial peptides (AMPs). AMPs have following questions with its property so it has a large number of methods on designation. Firstly, different function is an important aspect that should be taken into consideration of designing an antimicrobial peptides. In addition, costs of production and limited techniques narrow the possibility of manufacture. Another vital reason is that they have different stability in different conditions like temperature.
4.1. The Methodology of Template-Based Design
By comparing structurally comparable portions of natural antimicrobial peptides (AMPs) and identifying conservative patterns based on various types of residues (e.g., charged, polar, hydrophobic, etc) [22]. The propensity for helix formation, cationic properties, amphiphilicity, and overall hydrophobicity can be systematically altered by means of modification and other parameters. For example, cecropin, magainin, protegrin, and lactoferrin have been employed as templates for antimicrobial peptides (AMPs) [23]. The majority of antimicrobial peptides (AMPs) exhibit a cationic nature, resulting in reduced bactericidal efficacy in high salt conditions. This is attributed to the effective binding of cationic ions with the bacterial membrane [24]. The antimicrobial activity of a chimeric peptide H4, which is a combination of hBD3 and hBD4, was found to be more potent against bacteria like Enterococcus faecalis and S. aureus. Furthermore, this antibacterial activity was observed even in high salt circumstances [25]. Furthermore, the antibacterial activity of hBD3 was enhanced against various bacterial species, including E. coli and Enterococcus faecium, when the N-terminal region was truncated by three amino acids. This improvement was particularly notable under high salt conditions [26]. The antibacterial action of natural θ-defensins in rhesus macaques has been observed to be effective against both bacteria and fungi, even at low concentrations. As an illustration, it was observed that θ-defensin-1 (RTD-1) exhibited a three-fold increase in its bactericidal activity in comparison to the open-chain analog. Notably, this enhanced activity was not influenced by salt concentration [27]. In order to augment the antibacterial efficacy of antimicrobial peptides (AMPs) and mitigate their toxicity, the implementation of dimeric structures can be considered. However, the destabilizing effects on the membrane are diminished following the production of dimers [28].
4.2. Computer-assisted peptide
Computer-assisted peptide design (CAPD) pertains to the utilization of computer technology in the field of protein engineering. It involves the processing, prediction, and evaluation of various protein transformation schemes based on known protein sequence, molecular conformation, structure, and relationship data. CAPD aims to make optimal choices in protein design, with a particular focus on the development of software tools for protein engineering research. Computer design encompasses various methodologies, such as statistical modeling, quantitative structure-activity relationships research [29], neural networks [30], deep learning [31], word embedding [32], and machine learning. The backdrop of the research study
The field of quantitative structure-activity relationships (QSAR) has given rise to two distinct methodologies: a prediction method that relies on the therapeutic properties of AMPs.
In this study, we propose the utilization of an index for the identification of novel potential antimicrobial peptides (AMPs) from the expressed sequence tag (EST) database. Our approach is based on the concepts of highly conserved signal peptide subclasses that are associated with AMPs, as previously described [33]. The utilization of two active compounds to create chimeras is a well accepted practice in the field of computer-assisted drug discovery. This approach is employed by the software programs TOPAS [34] and BREED [35]. Nevertheless, this methodology may not always be suitable for peptides. The addition of acyl moieties is a significant approach for augmenting AMP activity, as these moieties can furnish the requisite hydrophobic regions for the formation of short peptides [36, 37, 38].
In relation to the present body of study on peptide molecules, it is evident that they universally possess a shared characteristic, namely, the ability to modify and enhance naturally occurring molecules. Nevertheless, over the course of conducting research, certain variables may arise that are outside the researcher’s control, such as spatial mutations. Computer-aided design (CAD) obviates the need for exhaustive evaluation of each option, resulting in significant time, cost, and labor savings. One of the primary constraints in current computer-directed approaches to forecasting amp function is the requirement for standardized and dependable biological data as an input to facilitate an efficient design process. The field of computational methods has undergone significant advancements, leading to the emergence of training computers and improved analysis techniques. Additionally, several types of Synthetic Aperture Radar (SAR) strategies have been established. These strategies have the potential to be integrated with diverse structures that are rooted in natural sequences. The production of peptides can be achieved with a high degree of precision and can be enhanced through the process of evolving templates in order to generate active peptides [39].
4.3. The general process of rational drug design
Rational drug design or structure-based drug design is based on the understanding of the molecular pathophysiology of the disease process, according to the molecular structure of the target, and reference to the chemical structure of the effector to design drug molecules for the disease, so as to guide the design to rationalization. The drugs designed by this method are often highly active, specific and have low side effects, so it is called rational drug design.Rational drug design is inseparable from computer, so it can also be called computer-aided drug design.In theory, computer-aided drug design avoids a certain degree of blindness in previous research, greatly speeds up the development of new drugs, and saves the human, material and financial resources for the development of new drugs. Janssen, a Belgian firm, for example, has increased the success rate of computer-aided drug design on a large scale from one in 10,000 to one in 3,000.
4.4. Computer aided drug design methods
4.4.1. Indirect drug design
3D structure search based on pharmacophore.
Pharmacophore model method.
It was found early that 2-deoxy-2, 3-dideoxyd-n-acetylneuraminic acid had an inhibitory effect on sialase bacteria, but the effect was not good in animal models.According to the crystal structure of sialase and its interaction with the enzyme inhibitor Neu5Ac2en, it was found that there were negative electric groups near the action site of the enzyme structure. If the inhibitor 4-carboxyl group was replaced by an amino group, the binding effect would be enhanced, because the amino group formed a salt bridge with the carboxyl side chain of the enzyme Glu119. If the 4-carboxyl group is replaced by guanidine, it can interact with Glu119 and Glu227, showing a stronger affinity.
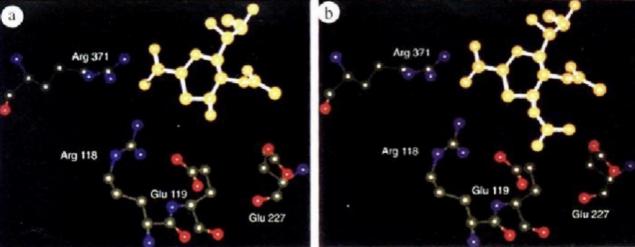
Figure 6. (a)2-deoxy-2, 3-dideoxyd-n-acetylneuraminic acid; (b)4-carboxyl group
4.4.2. Designing antimicrobial peptides
As antibiotic-resistant antibiotics gradually lose their effect, it is urgent to find new antimicrobial methods. Antimicrobial peptides are natural antimicrobials that protect the host organism from invasion, but their direct antimicrobial activity is moderate.[40]
4.5. Computer-assisted design of cyclic peptides and peptidomimetics
4.5.1. Cyclic peptidomimetics
The process of designing and synthesizing tiny molecules with conformational restrictions. The development and production of compact cyclic peptides with limited conformational flexibility is a compelling strategy to capture the essential molecular recognition components found in linear or bigger peptides and proteins. This approach aims to transfer these components to molecules that exhibit enhanced affinity, selectivity, stability, and bioavailability.
5. Conclusion
Antimicrobial peptides have emerged as a prominent area of research worldwide, with several crucial challenges in their design and application that require immediate attention. There are multiple restrictions that provide obstacles to the development and implementation of amplifier applications. The development of prospective antimicrobial peptides (AMPs) can be enhanced by multidisciplinary interactions including several fields such as biology, materials science, chemistry, bioinformatics, molecular informatics, and pharmacy. In order to enhance comprehension of the correlation between amp and different goals, it is advisable to move beyond unilateral conductive experimental research. This approach will enhance the experimental design, resulting in a more robust and rigorous demonstration of systematic and scientific principles. The process of applying antimicrobial peptides (AMPs) in clinical settings, starting from laboratory research through eventual implementation in medical practice, is expected to be a protracted and challenging endeavor. This research posits that as our understanding of the structural and functional interplay of polypeptide antimicrobials becomes more refined and their stability continues to increase, there will be additional advancements in their clinical use and the emergence of novel breakthroughs in the healthcare domain.
References
[1]. Huan Y, Kong Q, Mou H and Yi H (2020) Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 11:582779. doi: 10.3389/fmicb.2020.582779
[2]. Patamia V, Zagni C, Brullo I, Saccullo E, Coco A, Floresta G, Rescifina A. Computer-Assisted Design of Peptide-Based Radiotracers. International Journal ofMolecular Sciences. 2023; 24(7):6856. https://doi.org/10.3390/ijms24076856
[3]. Fjell, C., Hiss, J., Hancock, R. etal. Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov 11, 37–51 (2012). https://doi.org/10.1038/nrd3591.
[4]. Fjell CD, Hiss JA, Hancock REW, Schneider G. Designing antimicrobial peptides: Form follows function. Nature News. December 16, 2011. Accessed August 20, 2023. https://www.nature.com/articles/nrd3591.
[5]. Author links open overlay panelHenry Borsook, Publisher SummaryThis chapter discusses peptide bond formation. Peptide bonds may be broken and reconstituted by simple hydrolysis and its reversal by transferring reactions in which one of the participants need not be a peptide.Studies on the enzymatic . Peptide bond formation. Advances in Protein Chemistry. April 10, 2008. Accessed August 20, 2023. https://www.sciencedirect.com/science/article/abs/pii/ S0065323308600923.
[6]. Author links open overlay panelGerard D Wright, AbstractMicrobial resistance to antibiotics is manifested by changes in antibiotic permeability, Garau J, et al. Mechanisms of resistance to antibiotics. Current Opinion in Chemical Biology. August 29, 2003. Accessed August 20, 2023. https://www.sciencedirect.com/science/article/abs/pii/S1367593103001066#preview- section-references.
[7]. Darby, E.M., Trampari, E., Siasat, P. et al. Molecular mechanisms of antibiotic resistance revisited. Nat Rev Microbiol 21, 280–295 (2023). https://doi.org/ 10. 1038/s41579-022-00820-y
[8]. Kapoor G, Saigal S, Elongavan A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J Anaesthesiol Clin Pharmacol. 2017;33(3):300-305. doi:10.4103/joacp.JOACP_349_ 15
[9]. Antibiotics and the mechanisms of resistance to antibiotics - journalagent. Accessed August 20, 2023. https://jag.journalagent.com/ias/pdfs/IAS_21_4_ 138_ 142.pdf.
[10]. Soto SM. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence. 2013;4(3):223-229. doi:10.4161/viru.23724
[11]. Dutta, P., Sahu, R. K., Dey, T., Lahkar, M. D., Manna, P., and Kalita, J. (2019). Beneficial role of insect-derived bioactive components against inflammation and its associated complications (colitis and arthritis) and cancer. Chem. Biol.Interact. 313:108824. doi: 10.1016/j.cbi.2019.108824
[12]. Shelomi, M., Jacobs, C., Vilcinskas, A., and Vogel, H. (2020). The unique antimicrobial peptide repertoire of stick insects. Dev. Compar. Immunol. 103:103471. doi: 10.1016/j.dci.2019.103471
[13]. Zahedifard, F., Lee, H., No, J. H., Salimi, M., Seyed, N., Asoodeh, A., et al. (2020). Comparative study of different forms of jellein antimicrobial peptide on leishmania parasite. Exp. Parasitol. 209:107823. doi: 10.1016 j.exppara.2019.107823
[14]. ield, C. J. (2005). The immunological components of human milk and their effect on immune development in infants. J. Nutr. 135, 1–4. doi: 10.1093/jn/135.1.1
[15]. He, S.-W., Zhang, J., Li, N.-Q., Zhou, S., Yue, B., and Zhang, M. (2017). A TFPI-1peptide that induces degradation of bacterial nucleic acids, and inhibits bacterial and viral infection in half-smooth tongue sole, Cynoglossus semilaevis. Fish Shellf. Immunol. 60, 466–473. doi: 10.1016 j.fsi.2016.11.029
[16]. ibel Akalın, A. (2014). Dairy-derived antimicrobial peptides: action mechanisms, pharmaceutical uses and production proposals. Trends Food Sci. Technol. 36, 79–95. doi: 10.1016/j.tifs.2014.01.002
[17]. Cao, J., de la Fuente-Nunez, C., Ou, R. W., Torres, M. D. T., Pande, S. G., Sinskey, A. J., et al. (2018). Yeast-based synthetic biology platform for antimicrobial peptide production. ACS Synthet. Biol. 7, 896–902. doi: 10.1021/acssynbio.7b00396
[18]. Mattiuzzo, M., Bandiera, A., Gennaro, R., Benincasa, M., Pacor, S., Antcheva, N., et al. (2007). Role of the Escherichia coli SbmA in the antimicrobial activity of proline-rich peptides. Mol. Microbiol. 66, 151–163. doi: 10.1111/j.1365-2958.2007.05903.x
[19]. Cao, L., Jiang, W., Cao, S., Zhao, P., Liu, J., Dong, H., et al. (2019). In vitro leishmanicidal activity of antimicrobial peptide KDEL against Leishmania tarentolae. Acta Biochim. Biophys. Sin. 51, 1286–1292. doi: 10.1093/abbs/gmz128
[20]. Lee, J. H., Cho, K. S., Lee, J., Yoo, J., Lee, J., and Chung, J. (2001). Diptericinlike protein: an immune response gene regulated by the anti-bacterial gene induction pathway in drosophila. Gene 271, 233–238. doi: 10.1016/S0378-1119(01)00515-7
[21]. Lei, J., Sun, L., Huang, S., Zhu, C., Li, P., He, J., et al. (2019). The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 11, 3919–3931.
[22]. Zelezetsky, I., and Tossi, A. (2006). Alpha-helical antimicrobial peptides—using a sequence template to guide structure-activity relationship studies. Biochim. Biophys. Acta Biomembr. 1758, 1436– 1449. doi: 10. 1016/j.bbamem.2006.03.021
[23]. Fjell, C. D., Hiss, J. A., Hancock, R. E. W., and Schneider, G. (2012). Designing antimicrobial peptides: form follows function. Nat. Rev. Drug Discov. 11, 37–51. doi: 10. 1038/nrd3591
[24]. Yang, M.; Zhang, C.; Zhang, M.Z.; Zhang, S. Beta-defensin derived cationic antimicrobial peptides with potent killing activity against gram negative and gram positive bacteria. BMC Microbiol. 2018, 18, 54.
[25]. Yu, W.; Ning, N.; Xue, Y.; Huang, Y.; Guo, F.; Li, T.; Yang, B.; Luo, D.; Sun, Y.; Li, Z.; et al. A Chimeric Cationic Peptide Composed of Human β-Defensin 3 and Human β-Defensin 4 Exhibits Improved Antibacterial Activity and Salt Resistance. Front. Microbiol. 2021, 12, 663151.
[26]. Li, T.; Guo, F.; Wang, Q.; Fang, H.; Li, Z.; Wang, D.; Wang, H. N-terminus three residues deletion mutant of human beta-defensin 3 with remarkably enhanced salt-resistance. PLoS ONE 2015, 10, e0117913.
[27]. Tang, Y.Q.; Yuan, J.; Osapay, G.; Osapay, K.; Tran, D.; Miller, C.J.; Ouellette, A.J.; Selsted, M.E. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science 1999, 286, 498–502.
[28]. Malekkhaiat Häffffner, S., and Malmsten, M. (2018). Inflfluence of self-assembly on the performance of antimicrobial peptides. Curr. Opin. Colloids Interf. Sci. 38, 56–79. doi: 10. 1016/j.cocis.2018.09.002
[29]. Abdel Monaim, S. A. H., Jad, Y. E., El-Faham, A., de la Torre, B. G., and Albericio, F. (2018). Teixobactin as a scaffffold for unlimited new antimicrobial peptides: SAR study. Bioorgan. Med. Chem. 26, 2788–2796. doi: 10. 1016/j.bmc.2017.09.040
[30]. Müller, A. T., Hiss, J. A., and Schneider, G. (2018). Recurrent neural network model for constructive peptide design. J. Chem. Inform. Model. 58, 472–479. doi:10. 1021/acs.jcim.7b00414
[31]. Veltri, D., Kamath, U., and Shehu, A. (2018). Deep learning improves antimicrobial peptide recognition. Bioinformatics 34, 2740–2747. doi: 10. 1093/ bioinformatics/bty179
[32]. Hamid, M.-N., and Friedberg, I. (2018). Identifying antimicrobial peptides using word embedding with deep recurrent neural networks. Bioinformatics 35, 2009–2016. doi: 10. 1093/bioinformatics/bty937
[33]. Jureti´c, D., Vuki ˇcevi´c,D., Petrov, D., Novkovi´c,M., Bojovi´c, V., Luˇci´c, B., et al. ´ (2011). Knowledge-based computational methods for identifying or designing novel, non-homologous antimicrobial peptides. Eur. Biophys. J. 40, 371–385. doi: 10. 1007/s00249-011-0674-7
[34]. Schneider, G., Lee, M. L., Stahl, M. & Schneider, P. De novo design of molecular architectures by evolutionary assembly of drug-derived building blocks. J. Comput. Aided Mol. Des. 14, 487–494 (2000).
[35]. Pierce, A. C., Rao, G. & Bemis, G. W. BREED: generating novel inhibitors through hybridization of known ligands. Application to CDK2, p38, and HIV protease. J. Med. Chem. 47, 2768–2775 (2004).
[36]. Radzishevsky, I. S. et al. Effects of acyl versus aminoacyl conjugation on the properties of antimicrobial peptides. Antimicrob. Agents Chemother. 49, 2412–2420 (2005).
[37]. Serrano, G. N., Zhanel, G. G. & Schweizer, F. Antibacterial activity of ultrashort cationic lipo-β-peptides. Antimicrob. Agents Chemother. 53, 2215–2217 (2009).
[38]. Avrahami, D. & Shai, Y. A new group of antifungal and antibacterial lipopeptides derived from nonmembrane active peptides conjugated to palmitic acid. J. Biol. Chem. 279, 12277– 12285 (2004).
[39]. Marcelo D.T.Torres,Jicong Cao,Octavio L.Franco,Timothy K.Lu,and Cesar de la Fuente-Nunez
[40]. Fjell, Christopher D.; Hiss, Jan A.; Hancock, Robert E. W.; Schneider, Gisbert (2011). Designing antimicrobial peptides: form follows function. Nature Reviews Drug Discovery, doi:10. 1038/nrd3591
Cite this article
He,X.;Zhang,Y.;Yuan,S.;Mao,M. (2024). A comprehensive review of antimicrobial peptides: Mechanisms, classifications and designations. Theoretical and Natural Science,46,131-141.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Huan Y, Kong Q, Mou H and Yi H (2020) Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 11:582779. doi: 10.3389/fmicb.2020.582779
[2]. Patamia V, Zagni C, Brullo I, Saccullo E, Coco A, Floresta G, Rescifina A. Computer-Assisted Design of Peptide-Based Radiotracers. International Journal ofMolecular Sciences. 2023; 24(7):6856. https://doi.org/10.3390/ijms24076856
[3]. Fjell, C., Hiss, J., Hancock, R. etal. Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov 11, 37–51 (2012). https://doi.org/10.1038/nrd3591.
[4]. Fjell CD, Hiss JA, Hancock REW, Schneider G. Designing antimicrobial peptides: Form follows function. Nature News. December 16, 2011. Accessed August 20, 2023. https://www.nature.com/articles/nrd3591.
[5]. Author links open overlay panelHenry Borsook, Publisher SummaryThis chapter discusses peptide bond formation. Peptide bonds may be broken and reconstituted by simple hydrolysis and its reversal by transferring reactions in which one of the participants need not be a peptide.Studies on the enzymatic . Peptide bond formation. Advances in Protein Chemistry. April 10, 2008. Accessed August 20, 2023. https://www.sciencedirect.com/science/article/abs/pii/ S0065323308600923.
[6]. Author links open overlay panelGerard D Wright, AbstractMicrobial resistance to antibiotics is manifested by changes in antibiotic permeability, Garau J, et al. Mechanisms of resistance to antibiotics. Current Opinion in Chemical Biology. August 29, 2003. Accessed August 20, 2023. https://www.sciencedirect.com/science/article/abs/pii/S1367593103001066#preview- section-references.
[7]. Darby, E.M., Trampari, E., Siasat, P. et al. Molecular mechanisms of antibiotic resistance revisited. Nat Rev Microbiol 21, 280–295 (2023). https://doi.org/ 10. 1038/s41579-022-00820-y
[8]. Kapoor G, Saigal S, Elongavan A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J Anaesthesiol Clin Pharmacol. 2017;33(3):300-305. doi:10.4103/joacp.JOACP_349_ 15
[9]. Antibiotics and the mechanisms of resistance to antibiotics - journalagent. Accessed August 20, 2023. https://jag.journalagent.com/ias/pdfs/IAS_21_4_ 138_ 142.pdf.
[10]. Soto SM. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence. 2013;4(3):223-229. doi:10.4161/viru.23724
[11]. Dutta, P., Sahu, R. K., Dey, T., Lahkar, M. D., Manna, P., and Kalita, J. (2019). Beneficial role of insect-derived bioactive components against inflammation and its associated complications (colitis and arthritis) and cancer. Chem. Biol.Interact. 313:108824. doi: 10.1016/j.cbi.2019.108824
[12]. Shelomi, M., Jacobs, C., Vilcinskas, A., and Vogel, H. (2020). The unique antimicrobial peptide repertoire of stick insects. Dev. Compar. Immunol. 103:103471. doi: 10.1016/j.dci.2019.103471
[13]. Zahedifard, F., Lee, H., No, J. H., Salimi, M., Seyed, N., Asoodeh, A., et al. (2020). Comparative study of different forms of jellein antimicrobial peptide on leishmania parasite. Exp. Parasitol. 209:107823. doi: 10.1016 j.exppara.2019.107823
[14]. ield, C. J. (2005). The immunological components of human milk and their effect on immune development in infants. J. Nutr. 135, 1–4. doi: 10.1093/jn/135.1.1
[15]. He, S.-W., Zhang, J., Li, N.-Q., Zhou, S., Yue, B., and Zhang, M. (2017). A TFPI-1peptide that induces degradation of bacterial nucleic acids, and inhibits bacterial and viral infection in half-smooth tongue sole, Cynoglossus semilaevis. Fish Shellf. Immunol. 60, 466–473. doi: 10.1016 j.fsi.2016.11.029
[16]. ibel Akalın, A. (2014). Dairy-derived antimicrobial peptides: action mechanisms, pharmaceutical uses and production proposals. Trends Food Sci. Technol. 36, 79–95. doi: 10.1016/j.tifs.2014.01.002
[17]. Cao, J., de la Fuente-Nunez, C., Ou, R. W., Torres, M. D. T., Pande, S. G., Sinskey, A. J., et al. (2018). Yeast-based synthetic biology platform for antimicrobial peptide production. ACS Synthet. Biol. 7, 896–902. doi: 10.1021/acssynbio.7b00396
[18]. Mattiuzzo, M., Bandiera, A., Gennaro, R., Benincasa, M., Pacor, S., Antcheva, N., et al. (2007). Role of the Escherichia coli SbmA in the antimicrobial activity of proline-rich peptides. Mol. Microbiol. 66, 151–163. doi: 10.1111/j.1365-2958.2007.05903.x
[19]. Cao, L., Jiang, W., Cao, S., Zhao, P., Liu, J., Dong, H., et al. (2019). In vitro leishmanicidal activity of antimicrobial peptide KDEL against Leishmania tarentolae. Acta Biochim. Biophys. Sin. 51, 1286–1292. doi: 10.1093/abbs/gmz128
[20]. Lee, J. H., Cho, K. S., Lee, J., Yoo, J., Lee, J., and Chung, J. (2001). Diptericinlike protein: an immune response gene regulated by the anti-bacterial gene induction pathway in drosophila. Gene 271, 233–238. doi: 10.1016/S0378-1119(01)00515-7
[21]. Lei, J., Sun, L., Huang, S., Zhu, C., Li, P., He, J., et al. (2019). The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 11, 3919–3931.
[22]. Zelezetsky, I., and Tossi, A. (2006). Alpha-helical antimicrobial peptides—using a sequence template to guide structure-activity relationship studies. Biochim. Biophys. Acta Biomembr. 1758, 1436– 1449. doi: 10. 1016/j.bbamem.2006.03.021
[23]. Fjell, C. D., Hiss, J. A., Hancock, R. E. W., and Schneider, G. (2012). Designing antimicrobial peptides: form follows function. Nat. Rev. Drug Discov. 11, 37–51. doi: 10. 1038/nrd3591
[24]. Yang, M.; Zhang, C.; Zhang, M.Z.; Zhang, S. Beta-defensin derived cationic antimicrobial peptides with potent killing activity against gram negative and gram positive bacteria. BMC Microbiol. 2018, 18, 54.
[25]. Yu, W.; Ning, N.; Xue, Y.; Huang, Y.; Guo, F.; Li, T.; Yang, B.; Luo, D.; Sun, Y.; Li, Z.; et al. A Chimeric Cationic Peptide Composed of Human β-Defensin 3 and Human β-Defensin 4 Exhibits Improved Antibacterial Activity and Salt Resistance. Front. Microbiol. 2021, 12, 663151.
[26]. Li, T.; Guo, F.; Wang, Q.; Fang, H.; Li, Z.; Wang, D.; Wang, H. N-terminus three residues deletion mutant of human beta-defensin 3 with remarkably enhanced salt-resistance. PLoS ONE 2015, 10, e0117913.
[27]. Tang, Y.Q.; Yuan, J.; Osapay, G.; Osapay, K.; Tran, D.; Miller, C.J.; Ouellette, A.J.; Selsted, M.E. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science 1999, 286, 498–502.
[28]. Malekkhaiat Häffffner, S., and Malmsten, M. (2018). Inflfluence of self-assembly on the performance of antimicrobial peptides. Curr. Opin. Colloids Interf. Sci. 38, 56–79. doi: 10. 1016/j.cocis.2018.09.002
[29]. Abdel Monaim, S. A. H., Jad, Y. E., El-Faham, A., de la Torre, B. G., and Albericio, F. (2018). Teixobactin as a scaffffold for unlimited new antimicrobial peptides: SAR study. Bioorgan. Med. Chem. 26, 2788–2796. doi: 10. 1016/j.bmc.2017.09.040
[30]. Müller, A. T., Hiss, J. A., and Schneider, G. (2018). Recurrent neural network model for constructive peptide design. J. Chem. Inform. Model. 58, 472–479. doi:10. 1021/acs.jcim.7b00414
[31]. Veltri, D., Kamath, U., and Shehu, A. (2018). Deep learning improves antimicrobial peptide recognition. Bioinformatics 34, 2740–2747. doi: 10. 1093/ bioinformatics/bty179
[32]. Hamid, M.-N., and Friedberg, I. (2018). Identifying antimicrobial peptides using word embedding with deep recurrent neural networks. Bioinformatics 35, 2009–2016. doi: 10. 1093/bioinformatics/bty937
[33]. Jureti´c, D., Vuki ˇcevi´c,D., Petrov, D., Novkovi´c,M., Bojovi´c, V., Luˇci´c, B., et al. ´ (2011). Knowledge-based computational methods for identifying or designing novel, non-homologous antimicrobial peptides. Eur. Biophys. J. 40, 371–385. doi: 10. 1007/s00249-011-0674-7
[34]. Schneider, G., Lee, M. L., Stahl, M. & Schneider, P. De novo design of molecular architectures by evolutionary assembly of drug-derived building blocks. J. Comput. Aided Mol. Des. 14, 487–494 (2000).
[35]. Pierce, A. C., Rao, G. & Bemis, G. W. BREED: generating novel inhibitors through hybridization of known ligands. Application to CDK2, p38, and HIV protease. J. Med. Chem. 47, 2768–2775 (2004).
[36]. Radzishevsky, I. S. et al. Effects of acyl versus aminoacyl conjugation on the properties of antimicrobial peptides. Antimicrob. Agents Chemother. 49, 2412–2420 (2005).
[37]. Serrano, G. N., Zhanel, G. G. & Schweizer, F. Antibacterial activity of ultrashort cationic lipo-β-peptides. Antimicrob. Agents Chemother. 53, 2215–2217 (2009).
[38]. Avrahami, D. & Shai, Y. A new group of antifungal and antibacterial lipopeptides derived from nonmembrane active peptides conjugated to palmitic acid. J. Biol. Chem. 279, 12277– 12285 (2004).
[39]. Marcelo D.T.Torres,Jicong Cao,Octavio L.Franco,Timothy K.Lu,and Cesar de la Fuente-Nunez
[40]. Fjell, Christopher D.; Hiss, Jan A.; Hancock, Robert E. W.; Schneider, Gisbert (2011). Designing antimicrobial peptides: form follows function. Nature Reviews Drug Discovery, doi:10. 1038/nrd3591









