1. Introduction
Alzheimer’s disease (AD) is one of the most devastating diseases globally. According to statistics from the World Health Organization, AD is currently affecting 55 million people, and it is expected to have an increase of 10 million patients annually [1]. It is characterized by a gradual decline of cognitive functions, memory impairment, and changes in behavior. Research shows that there will be different degrees of shrinkage in the cortex and hippocampus; deposits of amyloid plaque and interneuron tau fibrillary tangles are present in the brains of patients [2]. While the precise pathogenesis of Alzheimer's disease is not fully understood, studies have shown that it is aroused by various interplays between genetics, ageing, environmental, and lifestyle factors. In this literature review, research on the current state of knowledge on the relationship between AD and genetics will be discussed. The biotechnology strategies to identify AD, cure AD, and help AD patients in rehabilitation will also be identified. The paper also aims at researching improvements in looking for a way to completely cure AD. In the long term, it would potentially be a guide for the caregivers to ease their pressure. By understanding the influence of the disease, it brings great social significance and arouses the attention of public health.
2. Genetics of late-onset Alzheimer's disease
Late-onset Alzheimer's disease (LOAD) is the most common form of AD; around 90–95% of AD patients are affected by LOAD, and it usually develops after the age of 65 and affects individuals over the age of 60 [3]. It is not caused by specific genetic mutations but rather by the interplay between genetic, environmental, and lifestyle factors [4]. However, it is believed that genetic factors play the most important role in LOAD.
The most significant genetic factor of LOAD is the apolipoprotein E ε4 allele (APOE ε4), which usually affects approximately 25–30% of AD patients. [3] The APOE ε4 allele is found to be associated with an increased risk of AD. Research found that the APOE ε4 allele causes a decline in cognitive ability, which is the main reason for the increased risk of dementia in older people. The significance of the APOE ε4 allele in the genetic influence of AD is remarkable; research suggests that the APOE ε4 allele accelerates the accumulation and deposit of Amyloid-beta (Aβ) in the brain, directly resulting in AD [5].
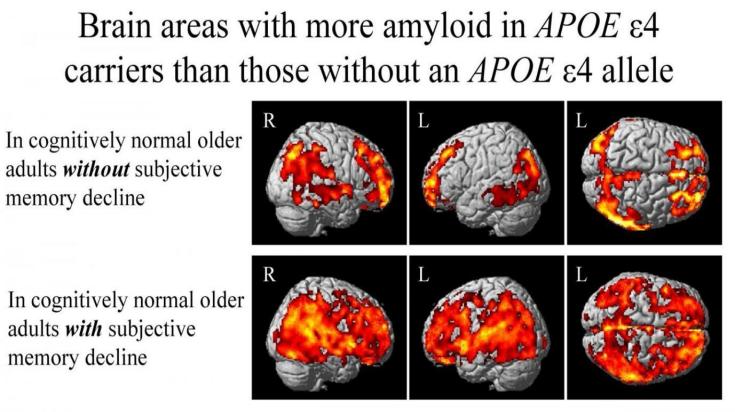
Figure 1. Brain Areas with more amyloid APOE ε4 carriers than those without an APOE ε4 allele [6]
A study led by investigators from Indiana University suggests that the genetic variant APOE ε4 as shown in figure 1, which is considered a major component of causing Alzheimer's disease, may promote the development of amyloid plaques in the brain before any measurable symptoms of the disease can be detected. The research focused on individuals who exhibited significant memory loss with normal cognitive abilities during testing. The scientists discovered evidence of Alzheimer's disease-related pathologies in individuals carrying the APOE ε4 gene variant. These also indicated that elevated levels of amyloid plaque and tau protein and reduced levels of the protein precursor to the plaques are linked to Alzheimer's disease. The study provides a foundation for further research among patients at risk of Alzheimer's long before the disease is diagnosed [6].
2.1. Mechanisms of the Development of Alzheimer’s Disease
The mechanisms by which genetic risk factors may contribute to the development of AD are not fully understood. However, two mechanisms have been discovered and deeply researched: the accumulation of amyloid beta and the accumulation of tau protein.
2.1.1. Accumulation of Amyloid Beta Polypeptide
The mechanism of accumulation of amyloid beta is mainly due to aging, the ability of the human body to degrade Aβ has decreased, causing the accumulation of amyloid beta in the brain. The mechanism of accumulation of amyloid beta is mainly due to aging; the ability of the human body to degrade Aβ has decreased, causing the accumulation of amyloid beta in the brain. In addition, research also supports the idea that the APOE ε4 protein aggregates with Aβ when associated with lipid [7]. Through this finding, it is believed that the cause of Late-Onset Alzheimer’s Disease (LOAD) is partially related to amyloid beta accumulation.
There are different theories explaining the mechanism of the accumulation of amyloid beta. One theory is that amyloid beta deposits form plaques in the brain. Since amyloid beta has a glutinous characteristic in nature, when the APOE ε4 protein is cut, amyloid beta accumulates and forms plaques, which disrupt normal cellular communication and cause inflammation by activating the cellular immune response [8].
This leads to the death of brain cells, causing memory loss and other symptoms of Alzheimer's disease. Another theory is that amyloid beta influences the metabolism of brain cells, leading to oxidative stress and energy failure. This can cause apoptosis of brain cells, leading to memory loss and other Alzheimer's disease symptoms.
2.1.2. Accumulation of Tau Protein
The accumulation of tau protein in the brain area is also significant in the development of AD. Through the observation of tau protein accumulation in the brain, it is confirmed that AD strongly associates with the formation of neurofibrillary tangles.
Tau protein is a structural protein found in the brain that helps to maintain the shape and stability of nerve cell processes. In the brain of AD patients, tau protein forms neurofibrillary tangle clumps. These tangles interfere with the normal functioning of nerve cells, being detrimental to the nerve cells and causing brain shrinkage [9,10].
Research supports the idea that the abnormal tau protein disrupts the normal transportation of nutrients and other essential molecules within nerve cells. As shown in figures 2 and 3, this results in the death of nerve cells and contributes to the neurodegeneration seen in Alzheimer's disease.
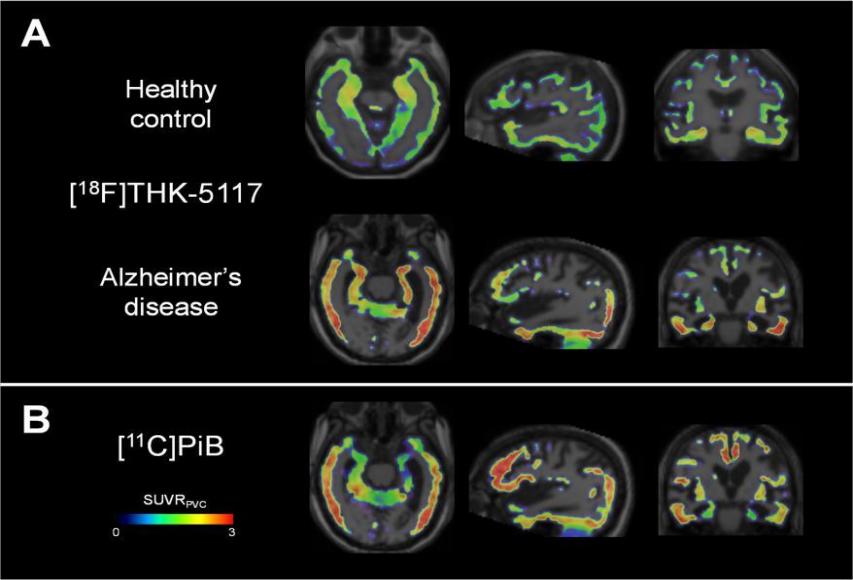
Figure 2. Comparison of Tau Deposition in Brain[9][10]
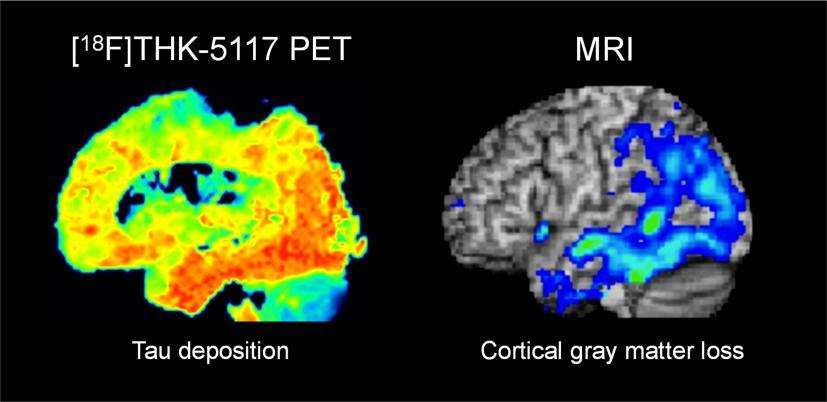
Figure 3. Accumulation of Tau Deposition causing the loss of cortical gray matter [9,10]
2.2. Interaction between Genetics and Environmental Factors
The cause of AD is not merely a genetic disorder; it consists of a complex interplay between genetic factors and environmental factors [11] [12]. Several studies show that there are several environmental factors associated with an increased risk of Alzheimer's disease, such as exposure to toxins, the intake of nicotine, and the misuse of caffeine [12] [13]. In addition, lifestyle factors, such as the habit of doing sports and the style of diet, may also influence the genetic risk factors for AD [13]. For example, working out regularly is often associated with a reduced risk of AD, even in individuals who carry the APOE ε4 allele. In terms of diet, a Mediterranean-style diet, which is rich in dietary fibre and protein, is considered to be associated with a reduced risk of AD.
3. Biotechnology
The formation of AD is complex and effective treatments are still elusive. However, the invention of new techniques in the biotechnology field has the potential to revolutionize our understanding of the disease and inspire effective treatments.
3.1. Genetic Research
The improvement of biotechnology has accelerated the development of new treatments for AD. The use of genetic research has the potential to improve the accuracy of early stages AD diagnostics. This early diagnosis will allow for earlier initiation of treatment, which may slow or prevent the progression of the disease. Research done in 2011 has revealed that Late-Onset AD is related to the various genetic variations, using sophisticated biotechnology techniques [13]. With the collaboration of the Alzheimer Disease Genetics Consortium (ADGC), the research group has successfully identified one of the pathogeneses of AD. Based on these research results, and a combination of techniques of genetic research, it is also possible to manufacture personalized medicine for therapeutic use and health care.
3.2. Biomarker
Biotechnology has also enabled the development of biomarkers for AD. Biomarkers are biological measures that can be used to diagnose or predict the risk of developing a disease. In the case of AD, biomarkers may include measures of amyloid beta and tau protein levels in the brain, as well as measures of inflammation and oxidative stress.
Take tau protein as an example; research performed in 2016 has experimented with the feasibility of using plasma tau protein as a biomarker of AD, as a result, part of the plasma tau protein reflects the pathogenesis of AD [14]. However, the similarity between normal aged tau protein and AD-affected protein is very high. Under this circumstance, the biomarker is not clear and the directivity is low, so it cannot be used as a biomarker [14]. In contrast, several anti-amyloid monoclonal antibodies were developed and approved by the US food and Drug Association for the effective treatment of AD, such as Lecanemab and donanemab [15]. Studies showed that after getting monoclonal antibody treatment, many early-stage AD patients responded with a decrease in the speed of cognitive decline.
Additionally, a recent study in 2024 done by HKUST showed great improvement of biomarker development in detecting early-stage AD. Through this study, a new blood-based biomarker was developed with 21 proteins related to different pathological pathways. Corresponding machine-learning systems were developed to accurately evaluate the condition of AD and mild cognitive impairment patients [15]. This development of the system improved the effectiveness of the diagnosis of AD and, at the same time, ensured the safety of the patients. By using this examination system, more early-stage patients will be screened, and early treatment and prevention can be done to slow down the cognitive decline.
3.3. Therapeutic Approaches
Biotechnology has also facilitated the advancement of curative strategies for AD. Traditional treatments will be the monoclonal antibody treatment targeting amyloid beta, which has shown a reduction in the accumulation of this toxic peptide in the brain [16]. Novel therapeutic approaches also emerge, such as the use of gene therapy and stem cell therapy to repair or replace damaged cells in the brain to rehabilitate cognitive function.
While the development of new therapeutic approaches holds promise for advancing AD treatment and providing hope to those living with the condition, it is important to acknowledge that these approaches remain novel and their clinical potential is still under study. Inventions and investigations into therapeutic treatments demonstrate promising signs; however, many clinical trials are still needed before their risks and benefits are fully characterized. Continued research and clinical trials will be a crucial guide for the refinement of novel therapies, especially examining the safety and effectiveness of the therapy.
By 2021, stem cell therapy for Alzheimer's disease is still in the experimental phase; its positive and negative effects have not been fully determined. However, studies have shown that stem cell therapy has the potential to rehabilitate cognitive function and reduce symptoms in AD patients. A study conducted in 2016 examined the potential of human umbilical cord mesenchymal stem cells (hUC-MSCs) as a therapeutic approach for Alzheimer's disease [16]. Researchers transplanted hUC-MSCs onto mice with AD and observed some positive results. Specifically, the stem cells appeared effective at preserving cognitive function in the mice over time.
Upon further analysis, the research team found the implementation of hUC-MSCs helped reduce oxidative stress levels in the hippocampus region of the brain, which is particularly vulnerable to damage in AD. Signs of this included decreased malondialdehyde and increased nitric oxide, superoxide dismutase and neuronal nitric oxide synthase activity. What's more, the stem cells showed potential for promoting neurogenesis, that is the growth of new neurons [16].
If the test on mice also validates during the clinical trials, the stem cells could provide an authentic treatment by addressing the molecular changes in the underlying genetic translation process. Of course, much remains to be investigated before determining safety and efficacy for AD patients. However, the study offers encouraging early signals of hUC-MSCs' therapeutic promise. More research in this area will be important to fully characterise their potential for helping Alzheimer's patients.
4. Conclusion
In conclusion, the relationship between AD and genetics is complex. Several genetic risk factors have been identified for AD, including the most significant APOE ε4 allele and other genes. However, the presence of hereditary risk factors does not guarantee the development of AD. The impact of genetic risk factors for AD may also be influenced by environmental factors such as eating habits and lifestyle choices. More studies are needed to better understand the interaction between genetics and the environment. Advances in biotechnology have enhanced the understanding of the genetic factor behind AD, and it is encouraging to observe that the development of treatments for AD has been emerging. Although there are still many technological difficulties in treatment development that remain unsolved, the understanding of the underlying mechanism and pathogenesis has greatly encouraged the enhancement of techniques, and many possibilities have been found. The more AD is studied, the more biological antibody treatments are able to be discovered and different biomarkers are able to be developed. In addition, the potential of stem cell therapy on AD treatment has been continuously researched, and it would be more optimistic that more experiments could be done before the clinical trial in order to understand the mechanism and the potential negative impact it might bring. These advances have the potential to revolutionise our understanding of AD and lead to the development of new and effective treatments.
References
[1]. World Health Organization. (n.d.). Dementia. World Health Organization.
[2]. Sepulcre, J., Schultz, A. P., Sabuncu, M., Gomez-Isla, T., Chhatwal, J., Becker, A., ... & Johnson, K. A. (2016). In vivo tau, amyloid, and gray matter profiles in the aging brain. Journal of Neuroscience, 36(28), 7364-7374.
[3]. Isik, A. T. (2010). Late onset Alzheimer’s disease in older people. Clinical interventions in aging, 307-311.
[4]. Kivipelto, M., & Solomon, A. (2008). Alzheimer’s disease—the ways of prevention. The Journal of Nutrition Health and Aging, 12, S89-S94.
[5]. Gharbi-Meliani, A., Dugravot, A., Sabia, S., Regy, M., Fayosse, A., Schnitzler, A., ... & Dumurgier, J. (2021). The association of APOE ε4 with cognitive function over the adult life course and incidence of dementia: 20 years follow-up of the Whitehall II study. Alzheimer's Research & Therapy, 13, 1-11.
[6]. Liu, C. C., Kanekiyo, T., Xu, H., & Bu, G. (2013). Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nature Reviews Neurology, 9(2), 106-118.
[7]. Hampel, H., Hardy, J., Blennow, K., Chen, C., Perry, G., Kim, S. H., ... & Vergallo, A. (2021). The amyloid-β pathway in Alzheimer’s disease. Molecular psychiatry, 26(10), 5481-5503.
[8]. Kametani, F., & Hasegawa, M. (2018). Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer's disease. Frontiers in neuroscience, 12, 25.
[9]. Okamura, N., Harada, R., Furukawa, K., Furumoto, S., Tago, T., Yanai, K., ... & Kudo, Y. (2016). Advances in the development of tau PET radiotracers and their clinical applications. Aging research reviews, 30, 107-113.
[10]. NBCUniversal News Group. (2016, July 27). Four things to know about tau therapy for Alzheimer’s disease.NBCNews.com. https://www.nbcnews.com/better/wellness/four-things-know-about-tau-therapy-alzheimer-s-disease-n618091
[11]. Lashley, T., Gami, P., Valizadeh, N., Li, A., Revesz, T., & Balazs, R. (2015). Alterations in global DNA methylation and hydroxymethylation are not detected in Alzheimer's disease. Neuropathology and applied neurobiology, 41(4), 497-506.
[12]. Moceri, V., Kukull, W., Emanual, I., Belle, G., Starr, J., Schellenberg, G., … & Larson, E. (2001). Using Census Data and Birth Certificates To Reconstruct The Early-life Socioeconomic Environment And The Relation To The Development Of Alzheimer’s Disease. Epidemiology, 4(12), 383-389.
[13]. Naj, A. C., Jun, G., Beecham, G. W., Wang, L. S., Vardarajan, B. N., Buros, J., ... & Foroud, T. M. (2011). Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nature genetics, 43(5), 436-441.
[14]. Mattsson, N., Zetterberg, H., Janelidze, S., Insel, P. S., Andreasson, U., Stomrud, E., ... & Blennow, K. (2016). Plasma tau in Alzheimer disease. Neurology, 87(17), 1827-1835.
[15]. Jiang Y, Uhm H, Ip FC, et al. A blood-based multi-pathway biomarker assay for early detection and staging of Alzheimer's disease across ethnic groups. Alzheimer's Dement. 2024; 1-16.
[16]. Cui, Y., Ma, S., Zhang, C., Cao, W., Liu, M., Li, D., ... & Guan, F. (2017). Human umbilical cord mesenchymal stem cells transplantation improves cognitive function in Alzheimer’s disease mice by decreasing oxidative stress and promoting hippocampal neurogenesis. Behavioural Brain Research, 320, 291-301.
Cite this article
Liang,Y.H.C. (2024). The role of genetics and biotechnology in Alzheimer’s Disease. Theoretical and Natural Science,59,201-206.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. World Health Organization. (n.d.). Dementia. World Health Organization.
[2]. Sepulcre, J., Schultz, A. P., Sabuncu, M., Gomez-Isla, T., Chhatwal, J., Becker, A., ... & Johnson, K. A. (2016). In vivo tau, amyloid, and gray matter profiles in the aging brain. Journal of Neuroscience, 36(28), 7364-7374.
[3]. Isik, A. T. (2010). Late onset Alzheimer’s disease in older people. Clinical interventions in aging, 307-311.
[4]. Kivipelto, M., & Solomon, A. (2008). Alzheimer’s disease—the ways of prevention. The Journal of Nutrition Health and Aging, 12, S89-S94.
[5]. Gharbi-Meliani, A., Dugravot, A., Sabia, S., Regy, M., Fayosse, A., Schnitzler, A., ... & Dumurgier, J. (2021). The association of APOE ε4 with cognitive function over the adult life course and incidence of dementia: 20 years follow-up of the Whitehall II study. Alzheimer's Research & Therapy, 13, 1-11.
[6]. Liu, C. C., Kanekiyo, T., Xu, H., & Bu, G. (2013). Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nature Reviews Neurology, 9(2), 106-118.
[7]. Hampel, H., Hardy, J., Blennow, K., Chen, C., Perry, G., Kim, S. H., ... & Vergallo, A. (2021). The amyloid-β pathway in Alzheimer’s disease. Molecular psychiatry, 26(10), 5481-5503.
[8]. Kametani, F., & Hasegawa, M. (2018). Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer's disease. Frontiers in neuroscience, 12, 25.
[9]. Okamura, N., Harada, R., Furukawa, K., Furumoto, S., Tago, T., Yanai, K., ... & Kudo, Y. (2016). Advances in the development of tau PET radiotracers and their clinical applications. Aging research reviews, 30, 107-113.
[10]. NBCUniversal News Group. (2016, July 27). Four things to know about tau therapy for Alzheimer’s disease.NBCNews.com. https://www.nbcnews.com/better/wellness/four-things-know-about-tau-therapy-alzheimer-s-disease-n618091
[11]. Lashley, T., Gami, P., Valizadeh, N., Li, A., Revesz, T., & Balazs, R. (2015). Alterations in global DNA methylation and hydroxymethylation are not detected in Alzheimer's disease. Neuropathology and applied neurobiology, 41(4), 497-506.
[12]. Moceri, V., Kukull, W., Emanual, I., Belle, G., Starr, J., Schellenberg, G., … & Larson, E. (2001). Using Census Data and Birth Certificates To Reconstruct The Early-life Socioeconomic Environment And The Relation To The Development Of Alzheimer’s Disease. Epidemiology, 4(12), 383-389.
[13]. Naj, A. C., Jun, G., Beecham, G. W., Wang, L. S., Vardarajan, B. N., Buros, J., ... & Foroud, T. M. (2011). Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nature genetics, 43(5), 436-441.
[14]. Mattsson, N., Zetterberg, H., Janelidze, S., Insel, P. S., Andreasson, U., Stomrud, E., ... & Blennow, K. (2016). Plasma tau in Alzheimer disease. Neurology, 87(17), 1827-1835.
[15]. Jiang Y, Uhm H, Ip FC, et al. A blood-based multi-pathway biomarker assay for early detection and staging of Alzheimer's disease across ethnic groups. Alzheimer's Dement. 2024; 1-16.
[16]. Cui, Y., Ma, S., Zhang, C., Cao, W., Liu, M., Li, D., ... & Guan, F. (2017). Human umbilical cord mesenchymal stem cells transplantation improves cognitive function in Alzheimer’s disease mice by decreasing oxidative stress and promoting hippocampal neurogenesis. Behavioural Brain Research, 320, 291-301.









