1. Introduction
Cancer treatment remains a significant challenge, with conventional methods such as chemotherapy and radiation therapy offering only limited success. These traditional approaches, while effective at targeting rapidly dividing cells, often lack specificity, leading to damage to healthy tissues and causing severe side effects. Additionally, their effectiveness is often compromised by the development of drug resistance and the inability to fully eradicate tumors, especially in advanced stages. In response to these limitations, ADCs have emerged as a promising targeted therapy. ADCs combine the specificity of monoclonal antibodies with the potent cell-killing capability of cytotoxic drugs, allowing for targeted delivery directly to cancer cells while minimizing off-target effects on healthy tissue [1]. This novel approach holds significant potential to improve the precision and efficacy of cancer treatment, making it a focal point of ongoing research and clinical development. The main objective of this paper is to provide a comprehensive review of the mechanisms, clinical applications, and prospects of antibody-drug conjugates. By analyzing current research progress and clinical trial outcomes, this paper aims to highlight the potential of ADCs as an innovative targeted therapy in cancer treatment. In-depth research and optimization of ADCs are expected to further advance the development of cancer therapies.
2. Fundamentals and components of ADCs
2.1. Definition and working principle of ADCs
ADCs are a novel class of cancer therapeutics that chemically link a monoclonal antibody to a cytotoxic drug, enabling highly targeted drug delivery within the body. This technology combines the high specificity and selectivity of antibodies with the cytotoxic effects of the drug, aiming to enhance therapeutic efficacy while minimizing damage to normal tissues [2].
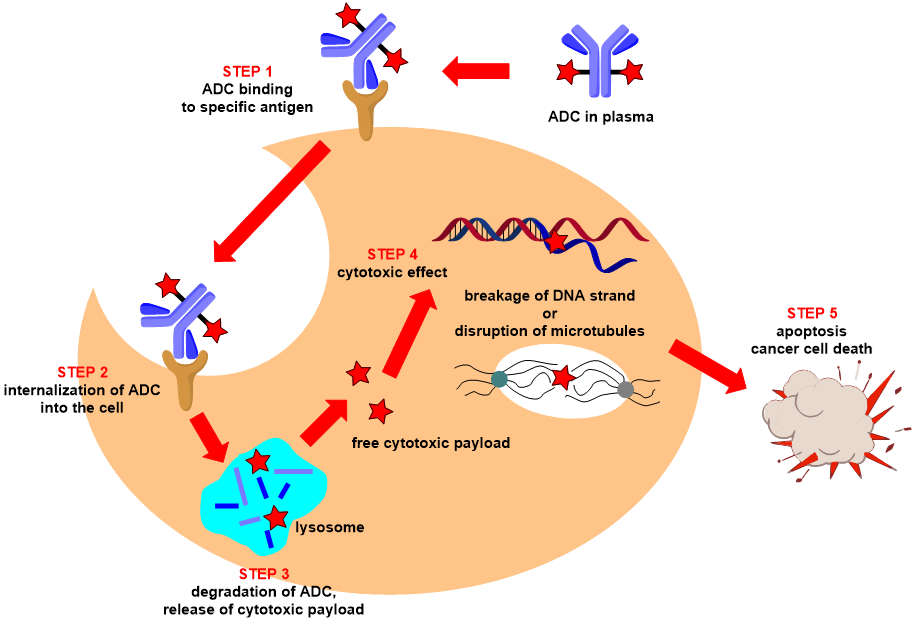
Figure 1. ADC mechanism of action (source: https://www.santiago-lab.com/)
As shown in Figure 1. The mechanism of action of antibody-drug conjugates involves several key steps. First, the antibody component of the ADC specifically recognizes and binds to a particular antigen on the surface of cancer cells, ensuring that the ADC selectively targets cancer cells without affecting normal cells. Next, the cancer cell internalizes the ADC complex through endocytosis, forming an endosome. The endosome then fuses with a lysosome, where, in the acidic environment and in the presence of proteases, the linker is cleaved, releasing the cytotoxic drug carried by the ADC. Once activated, these drugs enter the cytoplasm and exert their toxic effects by disrupting microtubules or causing DNA damage, interfering with the cell's survival processes, ultimately leading to cancer cell death. This entire process demonstrates the high specificity and precision of ADCs in targeting and killing cancer cells, effectively eliminating them while minimizing damage to normal cells [3].
2.2. Antibody and antigen in ADCs
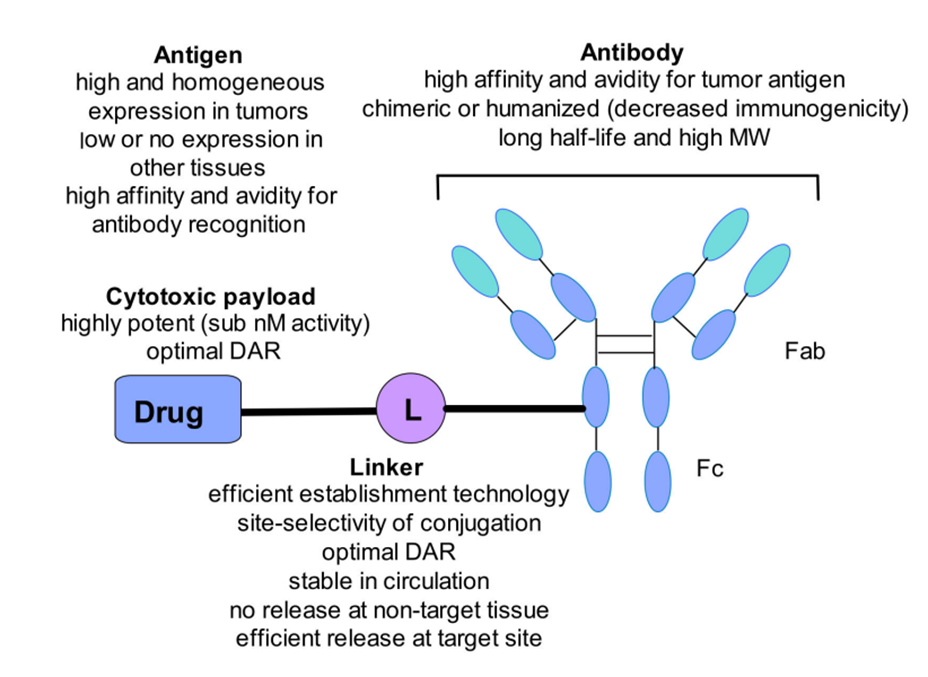
The antibody is the primary component of an Antibody-Drug Conjugate (ADC) responsible for recognizing and binding to tumor cells. It must possess high affinity for the target antigen and low immunogenicity, meaning the antibody can bind tightly and specifically to the antigen, even at low concentrations, effectively attaching to cancer cells [4]. Low immunogenicity also reduces the likelihood of the host immune system attacking the antibody, allowing the ADC to maintain prolonged activity in the body. Additionally, the antibody should have a long plasma half-life and the ability to facilitate rapid internalization, ensuring that the ADC circulates in the body for a longer period and increasing its chances of reaching the tumor site. There is a high degree of correlation between the antibody and antigen, and the antibody should not interfere with antigen internalization, ensuring the drug is effectively delivered into the cancer cells [5]. Moreover, selecting an appropriate target antigen is crucial; the antigen should be highly expressed on tumor cells while being minimally expressed on healthy cells to minimize adverse effects on normal tissues. The antigen should be present on the exterior surface of cancer cells, enabling the antibody to accurately and quickly recognize and bind to it, enhancing specificity. At the same time, the antigen should have a strong capacity for internalization, allowing the ADC molecule to enter the cell and release its payload at the intracellular target [6].
2.3. Cytotoxic Payloads in ADCs
The cytotoxic payload is the active component of an ADC, consisting of highly toxic compounds that exert therapeutic effects on target cells [7]. These compounds bind to intracellular targets, disrupt cellular processes, and promote cell death. Ideally, the payload should efficiently reach the target site without releasing any off-target effects and should be able to kill cancer cells without harming normal healthy cells. Notably, each payload must have very high toxicity, as each ADC molecule can only attach a limited number of payloads (typically 3-4 per antibody molecule). This high toxicity ensures that even with the limited amount of payload released after the ADC enters the tumor cell, it can effectively kill cancer cells at low concentrations. These payloads are generally categorized into two types: microtubule disruptors and DNA-damaging agents [8,9].
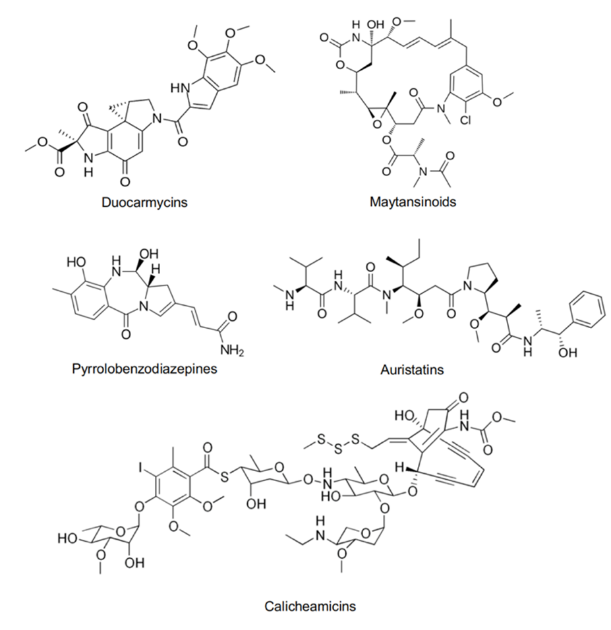
Microtubule disruptors prevent cell division by disturbing the dynamic stability of microtubules. Microtubules are crucial components of the cytoskeleton, composed of α and β-tubulin dimers, and play a key role during cell division process. The processes of microtubule polymerization and depolymerization are essential for forming the mitotic spindle, separating chromosomes, and completing cell division. The main function of microtubule disruptors is to inhibit microtubule assembly or disassembly, thereby blocking cell cycle progression and ultimately leading to apoptosis. As shown in the Figure 2a, common microtubule disruptors include Auristatins and Maytansinoids. DNA-damaging agents induce cell death by directly damaging the DNA structure or interfering with DNA repair mechanisms. These drugs primarily cause DNA double-strand breaks or interstrand cross-links, which obstruct DNA replication and transcription, ultimately resulting in apoptosis. Common DNA-damaging agents include Calicheamicins, Pyrrolobenzodiazepines, and Duocarmycins [10,11].
2.4. The linkers in ADCs
In ADCs, linkers play a crucial role by attaching cytotoxic drugs to antibodies and ensuring that the drug is effectively released within specific tumor cells. Linkers need to be sufficiently stable to prevent degradation or premature release of the drug before it reaches its target, thereby avoiding off-target effects and unnecessary toxicity [12]. Additionally, linkers must be able to be activated after entering the tumor cells to release the drug in the intracellular environment. Based on their characteristics, linkers are categorized into two main types: non-cleavable linkers and cleavable linkers. Cleavable linkers are designed to break under specific conditions within tumor cells, ensuring precise drug release with high specificity and potent drug activity, but they may be unstable in systemic circulation, leading to off-target effects. Non-cleavable linkers, on the other hand, are more stable and do not break prematurely in the body, reducing non-specific toxicity [13,14]. However, their drug release efficiency is lower because release depends on the complete degradation of the ADC within the tumor cell.
(a)
(b)
Figure 2. (a) Molecular structure of several cytotoxic payloads including microtubule disruptors and DNA-damaging agents. (b) General structure of ADC with its key components and the pivotal considerations when combining the different components [3].
3. Development and clinical use of ADCs
3.1. Treatment for hematologic malignancies
ADCs have shown varying efficacy in treating hematologic malignancies, which encompass a variety of diseases, including acute myeloid leukemia (AML), Hodgkin lymphoma (HL), anaplastic large cell lymphoma (ALCL), and acute lymphoblastic leukemia (ALL), among others. Gemtuzumab ozogamicin is a representative ADC used in the treatment of hematologic malignancies. It consists of a monoclonal antibody targeting the CD33 antigen conjugated to the potent cytotoxic agent calicheamicin via an acid-sensitive linker. The CD33 antigen is highly expressed on most AML cells, and calicheamicin, a potent DNA-damaging agent, induces DNA double-strand breaks, leading to apoptosis. Therefore, Gemtuzumab ozogamicin might be expected to have significant effects in treating AML. However, clinical trials have shown that this ADC can cause serious side effects, such as delayed hematopoietic recovery and hepatic veno-occlusive disease (VOD), indicating that further research and optimization of this ADC are needed [15, 16].
Inotuzumab ozogamicin is another potent drug for treating hematologic malignancies. It consists of a monoclonal antibody targeting the B-cell antigen CD22 conjugated to calicheamicin via an acid-sensitive linker. The CD22 antigen is highly expressed on the surface of B cells, making this ADC particularly effective in treating ALL. Like Gemtuzumab ozogamicin, the cytotoxic drug calicheamicin induces DNA double-strand breaks, leading to cell death. Clinical trial data have shown that Inotuzumab ozogamicin achieves better remission rates and patient survival outcomes for ALL compared to other treatment options, indicating its significant efficacy in treating ALL [17].
Lastly, Brentuximab vedotin is composed of a monoclonal antibody targeting the CD30 antigen conjugated to the cytotoxic agent MMAE (Monomethyl auristatin E) via a cleavable valine-citrulline dipeptide linker [18]. CD30 is highly expressed on malignant cells in Hodgkin lymphoma (HL) and anaplastic large cell lymphoma (ALCL) [19], suggesting potential therapeutic effects of this ADC for these diseases. MMAE is a potent microtubule disruptor that destroys microtubule structures, preventing cell division and leading to tumor cell death. Clinical trials have shown that Brentuximab vedotin has higher remission and cure rates for HL and ALCL compared to other therapies[20]. However, it may cause some neurotoxicity, necessitating further evaluation of its safety and efficacy.
3.2. Treatment for solid tumors
Ado-trastuzumab emtansine is an ADC specifically designed for the treatment of HER2-positive breast cancer. It consists of the anti-HER2 monoclonal antibody trastuzumab conjugated to the cytotoxic drug DM1 (maytansinoid microtubule disruptor) via a non-cleavable linker, SMCC [21]. Ado-trastuzumab emtansine combines the targeting ability of trastuzumab with the cytotoxicity of DM1, enabling it to specifically target HER2-positive cancer cells and release DM1 inside the cells, thereby disrupting microtubule structures, inhibiting cell division, and inducing apoptosis [22,23]. In clinical trials, it has demonstrated significant antitumor activity, with improved response rates and patient survival.
Enfortumab vedotin is an ADC used for the treatment of urothelial carcinoma. It consists of a monoclonal antibody targeting the Nectin-4 antigen conjugated to the cytotoxic drug MMAE via a valine-citrulline dipeptide linker [24]. Enfortumab vedotin combines the targeting ability of the anti-Nectin-4 antibody with the cytotoxicity of MMAE, enabling it to specifically recognize and bind to the Nectin-4 antigen, which is highly expressed on the surface of urothelial carcinoma cells [25]. Once inside the cell, the linker is cleaved, releasing MMAE, which disrupts microtubule structures, inhibits cell division, and induces tumor cell death. In clinical trials, Enfortumab vedotin has demonstrated significant antitumor activity, especially in patients who have previously received platinum-based chemotherapy and PD-1/PD-L1 inhibitors, showing a high response rate [26].
Sacituzumab govitecan is used for the treatment of metastatic triple-negative breast cancer (TNBC). It consists of a monoclonal antibody targeting the Trop-2 antigen conjugated to the cytotoxic drug SN-38 via an acid-sensitive, cleavable linker (CL2A) [27]. Trop-2 is an antigen highly expressed in various epithelial cancers, and Sacituzumab govitecan combines the targeting ability of the anti-Trop-2 antibody with the cytotoxicity of SN-38. It specifically recognizes and binds to the Trop-2 antigen, which is highly expressed on the surface of TNBC cells. Once inside the cell, the linker is cleaved, releasing SN-38, a potent DNA inhibitor that suppresses DNA topoisomerase I activity, thereby preventing cell proliferation and inducing tumor cell death. In clinical trials, Sacituzumab govitecan has demonstrated a high response rate and prolonged progression-free survival in patients with metastatic TNBC who were previously unresponsive to other treatments [28,29].
Table 1. ADC types and their key features and corresponding cancers treated
ADC name | Target antigen | Cytotoxic payload | Linker | Tumor types |
Gemtuzumab ozogamicin | CD33 | Calicheamicin | Acid-labile hydrazone linker | Acute myeloid leukemia |
Inotuzumab ozogamicin | CD22 | Calicheamicin | Acid-labile hydrazone linker | Acute lymphoblastic leukemia |
Brentuximab vedotin | CD30 | MMAE | Valine-citrulline dipeptide linker | Anaplastic large cell lymphoma |
Ado-trastuzumab emtansine | HER2 | DM1 | SMCC | HER2-positive breast cancer |
Enfortumab vedotin | Nectin-4 | MMAE | Valine-citrulline dipeptide linker | Urothelial carcinoma |
Sacituzumab govitecan | Trop-2 | SN-38 | CL2A | Metastatic triple-negative breast cancer |
3.3. Challenges and advancements of ADCs
The preceding text detailed several ADCs used in the treatment of hematologic malignancies and solid tumors, demonstrating the significant potential of this technology in cancer therapy. However, despite the attention attracted by successful ADCs, the technique still faces several challenges in clinical application. ADCs suffer from a relatively poor balance between therapeutic efficacy and toxicity, as their potent payloads can lead to severe toxic reactions at slightly higher doses, limiting the range of usable doses. Additionally, tumor cells may develop resistance to ADCs over time by downregulating target antigens, increasing drug efflux capabilities, or altering intracellular drug processing mechanisms, thereby reducing efficacy [30]. Furthermore, the development of ADCs involves complex integration of antibodies, linkers, and payload drugs, with significant manufacturing challenges and high costs, making it difficult to ensure consistency and quality control across batches [31]. Future technological advancements should focus on enhancing antibody selectivity for tumors, developing more stable linker technologies capable of precise drug release in the tumor microenvironment, exploring new efficient payloads to enhance anticancer effects, and minimizing impact on healthy tissues. Moreover, further research into biomarkers to predict patient response to ADCs for personalized treatment could improve efficacy and safety. These improvements will help expand the application of ADCs and enhance their clinical effectiveness.
4. Future improvement of ADCs
4.1. Enhance selectivity and reducing toxicity
Optimization of antibody design can enhance the binding affinity of antibodies to tumor-specific antigens on the surface of cancer cells, thereby reducing attacks on normal cells. This can be achieved through structural biology techniques combined with antibody engineering to design antibodies with higher affinity, improving recognition of cancer-specific antigens. Simultaneously, liquid biopsy techniques can be integrated to detect circulating tumor DNA or exosomes containing tumor biomarkers, allowing real-time monitoring of the patient's response to ADC therapy, and enabling treatment adjustments [32,33]. Additionally, to minimize off-target effects, future research can explore the use of humanized or chimeric antibodies to reduce the immunogenicity of ADCs against healthy cells. Modifying the Fc region of the antibody can further reduce interactions with non-target cells, thereby lowering systemic toxicity. Nanotechnology also shows promise in reducing ADC toxicity, as combining antibodies with drug-loaded nanoparticles can enhance the precision of drug release, minimizing release in non-target areas [34].
4.2. Design more stable and controllable linker
First, it is essential to ensure that the linker remains highly stable during systemic circulation to prevent the premature release of cytotoxic drugs, thereby reducing damage to healthy tissues. Non-cleavable linkers, such as the maleimidocaproyl linker used for MMAF, can significantly enhance systemic stability. Additionally, introducing hydrophilic spacers like PEG can reduce the risk of ADC aggregation and extend circulation time. Moreover, effective release in the tumor environment is crucial. Cleavable linkers can be designed to take advantage of tumor-specific characteristics, such as the cleavage of specific peptide sequences by cathepsin B to release the drug, or acid-sensitive linkers that trigger drug release in the acidic environment of the tumor lysosome [12]. Furthermore, by incorporating site-specific conjugation techniques, such as engineered cysteines or non-natural amino acids, the linker can be precisely conjugated to the antibody, reducing heterogeneity and improving drug distribution [35]. By combining these techniques, more stable linkers with efficient release characteristics in the tumor microenvironment can be designed.
4.3. Overcome drug resistance mechanism
Over time, cancer cells can develop resistance to ADCs through several pathways, including the downregulation of the target antigens that ADCs recognize or by increasing the activity of efflux pumps, which actively expel the cytotoxic drug from the cancer cells. To address these challenges, research should focus on thoroughly understanding the underlying mechanisms of resistance. One potential strategy is to employ combination therapies that target multiple pathways simultaneously, reducing the likelihood of resistance development [36]. For example, combining ADCs with small-molecule inhibitors, immune checkpoint inhibitors, or other chemotherapeutic agents could enhance the overall therapeutic effect and prevent or delay the emergence of resistance. Additionally, modifying ADC design, such as using novel payloads or more potent linkers, can help to bypass or mitigate these resistance mechanisms, ensuring sustained efficacy in the long term [37].
4.4. Other aspects
In the future, the success of Antibody-Drug Conjugates (ADCs) in cancer treatment will increasingly depend on the principles of personalized medicine. Identifying biomarkers that can predict a patient's response to specific ADC therapies is a crucial step in improving efficacy and safety. By studying biomarkers such as tumor antigen expression levels, drug resistance mechanisms, or the genetic characteristics of tumors, companion diagnostic tools can be developed to help clinicians tailor the most suitable ADC treatment plan for individual patients. Furthermore, the complex production process of ADCs, which involves the conjugation of antibodies, cytotoxic drugs, and linkers, presents challenges in manufacturing and quality control, affecting the accessibility and cost of these therapies. Therefore, future research should focus on optimizing production processes, improving batch consistency, and reducing overall costs, making ADCs available to a broader range of patients. Although ADCs are primarily used in cancer treatment, their unique mechanism of precisely targeting specific cells holds promise for the treatment of other diseases. Future exploration of ADCs in autoimmune and infectious diseases could help reduce systemic side effects, further expanding the scope of this technology's applications.
5. Conclusion
ADC as an innovative cancer therapy, demonstrate the unique advantage of combining the targeting specificity of monoclonal antibodies with the potent cytotoxic effects of chemotherapy drugs. By selectively targeting cancer cells and releasing the drug within the cells, ADCs have made significant progress in treating various cancers, including solid tumors and hematologic malignancies. However, despite the notable therapeutic potential of ADCs, their clinical application still faces several challenges, such as toxicity management, the development of drug resistance, and the complexity and cost of manufacturing. In the future, the key lies in further optimizing the selectivity of antibodies, developing more stable and controllable linker technologies, and finding new, highly effective cytotoxic payloads. Additionally, the development of personalized treatment strategies and biomarkers for different patients will help enhance the efficacy and safety of ADCs. Overall, with continuous technological advancements and in-depth research, ADCs are expected to become an important tool in the field of cancer treatment, significantly improving patient outcomes and quality of life.
References
[1]. Pysz, I., Jackson, P. J., & Thurston, D. E. (2019). Introduction to antibody–drug conjugates (ADCs).
[2]. Pettinato, M. C. (2021). Introduction to antibody-drug conjugates. Antibodies, 10(4), 42.
[3]. Kostova, V., Désos, P., Starck, J. B., & Kotschy, A. (2021). The chemistry behind ADCs. Pharmaceuticals, 14(5), 442.
[4]. Tang, H., Liu, Y., Yu, Z., Sun, M., Lin, L., Liu, W., ... & Jin, Y. (2019). The analysis of key factors related to ADCs structural design. Frontiers in Pharmacology, 10, 373.
[5]. Hoffmann, R. M., Coumbe, B. G., Josephs, D. H., Mele, S., Ilieva, K. M., Cheung, A., ... & Karagiannis, S. N. (2018). Antibody structure and engineering considerations for the design and function of Antibody Drug Conjugates (ADCs). Oncoimmunology, 7(3), e1395127.
[6]. Esapa, B., Jiang, J., Cheung, A., Chenoweth, A., Thurston, D. E., & Karagiannis, S. N. (2023). Target antigen attributes and their contributions to clinically approved antibody-drug conjugates (ADCs) in haematopoietic and solid cancers. Cancers, 15(6), 1845.
[7]. Thurston, D. E., & Jackson, P. J. (Eds.). (2019). Cytotoxic payloads for antibody–drug conjugates (Vol. 71). Royal Society of Chemistry.
[8]. Yaghoubi, S., Karimi, M. H., Lotfinia, M., Gharibi, T., Mahi‐Birjand, M., Kavi, E., ... & Abdollahpour‐Alitappeh, M. (2020). Potential drugs used in the antibody–drug conjugate (ADC) architecture for cancer therapy. Journal of cellular physiology, 235(1), 31-64.
[9]. Best, R. L., LaPointe, N. E., Azarenko, O., Miller, H., Genualdi, C., Chih, S., ... & Stagg, N. J. (2021). Microtubule and tubulin binding and regulation of microtubule dynamics by the antibody drug conjugate (ADC) payload, monomethyl auristatin E (MMAE): Mechanistic insights into MMAE ADC peripheral neuropathy. Toxicology and Applied Pharmacology, 421, 115534.
[10]. Lutz, R. J. (2019). The Future of Antibody–Drug Conjugate (ADC) Payloads.
[11]. Yao, H. P., Zhao, H., Hudson, R., Tong, X. M., & Wang, M. H. (2021). Duocarmycin-based antibody–drug conjugates as an emerging biotherapeutic entity for targeted cancer therapy: pharmaceutical strategy and clinical progress. Drug Discovery Today, 26(8), 1857-1874.
[12]. Jain, N., Smith, S. W., Ghone, S., & Tomczuk, B. (2015). Current ADC linker chemistry. Pharmaceutical research, 32(11), 3526-3540.
[13]. Su, Z., Xiao, D., Xie, F., Liu, L., Wang, Y., Fan, S., ... & Li, S. (2021). Antibody–drug conjugates: recent advances in linker chemistry. Acta Pharmaceutica Sinica B, 11(12), 3889-3907.
[14]. Nolting, B. (2013). Linker technologies for antibody–drug conjugates. Antibody-drug conjugates, 71-100.
[15]. Sievers, E. L., Larson, R. A., Stadtmauer, E. A., Estey, E., Löwenberg, B., Dombret, H., ... & Mylotarg Study Group. (2001). Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. Journal of Clinical Oncology, 19(13), 3244-3254.
[16]. Bross, P. F., Beitz, J., Chen, G., Chen, X. H., Duffy, E., Kieffer, L., ... & Pazdur, R. (2001). Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clinical cancer research, 7(6), 1490-1496.
[17]. DiJoseph, J. F., Armellino, D. C., Boghaert, E. R., Khandke, K., Dougher, M. M., Sridharan, L., ... & Damle, N. K. (2004). Antibody-targeted chemotherapy with CMC-544: a CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies. Blood, 103(5), 1807-1814.
[18]. Senter, P. D., & Sievers, E. L. (2012). The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nature biotechnology, 30(7), 631-637.
[19]. Younes, A., Gopal, A. K., Smith, S. E., Ansell, S. M., Rosenblatt, J. D., Savage, K. J., ... & Chen, R. (2012). Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. Journal of clinical oncology, 30(18), 2183-2189.
[20]. Pro, B., Advani, R., Brice, P., Bartlett, N. L., Rosenblatt, J. D., Illidge, T., ... & Shustov, A. (2012). Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. Journal of clinical oncology, 30(18), 2190-2196.
[21]. Lambert, J. M., & Chari, R. V. (2014). Ado-trastuzumab emtansine (T-DM1): an antibody–drug conjugate (ADC) for HER2-positive breast cancer.
[22]. Lewis Phillips, G. D., Li, G., Dugger, D. L., Crocker, L. M., Parsons, K. L., Mai, E., ... & Sliwkowski, M. X. (2008). Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody–cytotoxic drug conjugate. Cancer research, 68(22), 9280-9290.
[23]. Erickson, H. K., Lewis Phillips, G. D., Leipold, D. D., Provenzano, C. A., Mai, E., Johnson, H. A., ... & Tibbitts, J. (2012). The effect of different linkers on target cell catabolism and pharmacokinetics/pharmacodynamics of trastuzumab maytansinoid conjugates. Molecular cancer therapeutics, 11(5), 1133-1142.
[24]. Challita-Eid, P. M., Satpayev, D., Yang, P., An, Z., Morrison, K., Shostak, Y., ... & Stover, D. R. (2016). Enfortumab vedotin antibody–drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer research, 76(10), 3003-3013.
[25]. Hashimoto, H., Tanaka, Y., Murata, M., & Ito, T. (2022). Nectin-4: A novel therapeutic target for skin cancers. Current Treatment Options in Oncology, 23(4), 578-593.
[26]. Evan, Y. Y., Petrylak, D. P., O'Donnell, P. H., Lee, J. L., van der Heijden, M. S., Loriot, Y., ... & Balar, A. V. (2021). Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV201): a multicentre, single-arm, phase 2 trial. The Lancet Oncology, 22(6), 872-882.
[27]. Goldenberg, D. M., & Sharkey, R. M. (2019, August). Antibody-drug conjugates targeting TROP-2 and incorporating SN-38: A case study of anti-TROP-2 sacituzumab govitecan. In MAbs (Vol. 11, No. 6, pp. 987-995). Taylor & Francis.
[28]. Matusz-Fisher, A., & Tan, A. R. (2022). Sacituzumab govitecan and other antibody-drug conjugates targeting trophoblast cell-surface antigen 2 (Trop-2) in breast cancer. Annals of Translational Medicine, 10(18).
[29]. Bardia, A., Vahdat, L. T., Diamond, J. R., Starodub, A., Moroose, R. L., Isakoff, S. J., ... & Mayer, I. A. (2015). Therapy of refractory/relapsed metastatic triple-negative breast cancer (TNBC) with an anti-Trop-2-SN-38 antibody-drug conjugate (ADC), sacituzumab govitecan (IMMU-132): Phase I/II clinical experience.
[30]. Abdollahpour‐Alitappeh, M., Lotfinia, M., Gharibi, T., Mardaneh, J., Farhadihosseinabadi, B., Larki, P., ... & Bagheri, N. (2019). Antibody–drug conjugates (ADCs) for cancer therapy: Strategies, challenges, and successes. Journal of cellular physiology, 234(5), 5628-5642.
[31]. Dean, A. Q., Luo, S., Twomey, J. D., & Zhang, B. (2021, January). Targeting cancer with antibody-drug conjugates: Promises and challenges. In MAbs (Vol. 13, No. 1, p. 1951427). Taylor & Francis
[32]. Chari, R. V., Miller, M. L., & Widdison, W. C. (2014). Antibody–drug conjugates: an emerging concept in cancer therapy. Angewandte Chemie International Edition, 53(15), 3796-3827.
[33]. Beck, A., Goetsch, L., Dumontet, C., & Corvaïa, N. (2017). Strategies and challenges for the next generation of antibody–drug conjugates. Nature reviews Drug discovery, 16(5), 315-337.
[34]. Aggarwal, D., Yang, J., Salam, M. A., Sengupta, S., Al-Amin, M. Y., Mustafa, S., ... & Pawar, J. S. (2023). Antibody-drug conjugates: the paradigm shifts in the targeted cancer therapy. Frontiers in immunology, 14, 1203073.
[35]. Agarwal, P., & Bertozzi, C. R. (2015). Site-specific antibody–drug conjugates: the nexus of bioorthogonal chemistry, protein engineering, and drug development. Bioconjugate chemistry, 26(2), 176-192.
[36]. Teicher, B. A., & Chari, R. V. (2011). Antibody conjugate therapeutics: challenges and potential. Clinical cancer research, 17(20), 6389-6397.
[37]. Perez, H. L., Cardarelli, P. M., Deshpande, S., Gangwar, S., Schroeder, G. M., Vite, G. D., & Borzilleri, R. M. (2014). Antibody–drug conjugates: current status and future directions. Drug discovery today, 19(7), 869-881
Cite this article
Sun,S. (2024). Fundamental research on Antibody-Drug Conjugates and their applications in solid tumors and hematologic malignancies. Theoretical and Natural Science,73,8-16.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Pysz, I., Jackson, P. J., & Thurston, D. E. (2019). Introduction to antibody–drug conjugates (ADCs).
[2]. Pettinato, M. C. (2021). Introduction to antibody-drug conjugates. Antibodies, 10(4), 42.
[3]. Kostova, V., Désos, P., Starck, J. B., & Kotschy, A. (2021). The chemistry behind ADCs. Pharmaceuticals, 14(5), 442.
[4]. Tang, H., Liu, Y., Yu, Z., Sun, M., Lin, L., Liu, W., ... & Jin, Y. (2019). The analysis of key factors related to ADCs structural design. Frontiers in Pharmacology, 10, 373.
[5]. Hoffmann, R. M., Coumbe, B. G., Josephs, D. H., Mele, S., Ilieva, K. M., Cheung, A., ... & Karagiannis, S. N. (2018). Antibody structure and engineering considerations for the design and function of Antibody Drug Conjugates (ADCs). Oncoimmunology, 7(3), e1395127.
[6]. Esapa, B., Jiang, J., Cheung, A., Chenoweth, A., Thurston, D. E., & Karagiannis, S. N. (2023). Target antigen attributes and their contributions to clinically approved antibody-drug conjugates (ADCs) in haematopoietic and solid cancers. Cancers, 15(6), 1845.
[7]. Thurston, D. E., & Jackson, P. J. (Eds.). (2019). Cytotoxic payloads for antibody–drug conjugates (Vol. 71). Royal Society of Chemistry.
[8]. Yaghoubi, S., Karimi, M. H., Lotfinia, M., Gharibi, T., Mahi‐Birjand, M., Kavi, E., ... & Abdollahpour‐Alitappeh, M. (2020). Potential drugs used in the antibody–drug conjugate (ADC) architecture for cancer therapy. Journal of cellular physiology, 235(1), 31-64.
[9]. Best, R. L., LaPointe, N. E., Azarenko, O., Miller, H., Genualdi, C., Chih, S., ... & Stagg, N. J. (2021). Microtubule and tubulin binding and regulation of microtubule dynamics by the antibody drug conjugate (ADC) payload, monomethyl auristatin E (MMAE): Mechanistic insights into MMAE ADC peripheral neuropathy. Toxicology and Applied Pharmacology, 421, 115534.
[10]. Lutz, R. J. (2019). The Future of Antibody–Drug Conjugate (ADC) Payloads.
[11]. Yao, H. P., Zhao, H., Hudson, R., Tong, X. M., & Wang, M. H. (2021). Duocarmycin-based antibody–drug conjugates as an emerging biotherapeutic entity for targeted cancer therapy: pharmaceutical strategy and clinical progress. Drug Discovery Today, 26(8), 1857-1874.
[12]. Jain, N., Smith, S. W., Ghone, S., & Tomczuk, B. (2015). Current ADC linker chemistry. Pharmaceutical research, 32(11), 3526-3540.
[13]. Su, Z., Xiao, D., Xie, F., Liu, L., Wang, Y., Fan, S., ... & Li, S. (2021). Antibody–drug conjugates: recent advances in linker chemistry. Acta Pharmaceutica Sinica B, 11(12), 3889-3907.
[14]. Nolting, B. (2013). Linker technologies for antibody–drug conjugates. Antibody-drug conjugates, 71-100.
[15]. Sievers, E. L., Larson, R. A., Stadtmauer, E. A., Estey, E., Löwenberg, B., Dombret, H., ... & Mylotarg Study Group. (2001). Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. Journal of Clinical Oncology, 19(13), 3244-3254.
[16]. Bross, P. F., Beitz, J., Chen, G., Chen, X. H., Duffy, E., Kieffer, L., ... & Pazdur, R. (2001). Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clinical cancer research, 7(6), 1490-1496.
[17]. DiJoseph, J. F., Armellino, D. C., Boghaert, E. R., Khandke, K., Dougher, M. M., Sridharan, L., ... & Damle, N. K. (2004). Antibody-targeted chemotherapy with CMC-544: a CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies. Blood, 103(5), 1807-1814.
[18]. Senter, P. D., & Sievers, E. L. (2012). The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nature biotechnology, 30(7), 631-637.
[19]. Younes, A., Gopal, A. K., Smith, S. E., Ansell, S. M., Rosenblatt, J. D., Savage, K. J., ... & Chen, R. (2012). Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. Journal of clinical oncology, 30(18), 2183-2189.
[20]. Pro, B., Advani, R., Brice, P., Bartlett, N. L., Rosenblatt, J. D., Illidge, T., ... & Shustov, A. (2012). Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. Journal of clinical oncology, 30(18), 2190-2196.
[21]. Lambert, J. M., & Chari, R. V. (2014). Ado-trastuzumab emtansine (T-DM1): an antibody–drug conjugate (ADC) for HER2-positive breast cancer.
[22]. Lewis Phillips, G. D., Li, G., Dugger, D. L., Crocker, L. M., Parsons, K. L., Mai, E., ... & Sliwkowski, M. X. (2008). Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody–cytotoxic drug conjugate. Cancer research, 68(22), 9280-9290.
[23]. Erickson, H. K., Lewis Phillips, G. D., Leipold, D. D., Provenzano, C. A., Mai, E., Johnson, H. A., ... & Tibbitts, J. (2012). The effect of different linkers on target cell catabolism and pharmacokinetics/pharmacodynamics of trastuzumab maytansinoid conjugates. Molecular cancer therapeutics, 11(5), 1133-1142.
[24]. Challita-Eid, P. M., Satpayev, D., Yang, P., An, Z., Morrison, K., Shostak, Y., ... & Stover, D. R. (2016). Enfortumab vedotin antibody–drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer research, 76(10), 3003-3013.
[25]. Hashimoto, H., Tanaka, Y., Murata, M., & Ito, T. (2022). Nectin-4: A novel therapeutic target for skin cancers. Current Treatment Options in Oncology, 23(4), 578-593.
[26]. Evan, Y. Y., Petrylak, D. P., O'Donnell, P. H., Lee, J. L., van der Heijden, M. S., Loriot, Y., ... & Balar, A. V. (2021). Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV201): a multicentre, single-arm, phase 2 trial. The Lancet Oncology, 22(6), 872-882.
[27]. Goldenberg, D. M., & Sharkey, R. M. (2019, August). Antibody-drug conjugates targeting TROP-2 and incorporating SN-38: A case study of anti-TROP-2 sacituzumab govitecan. In MAbs (Vol. 11, No. 6, pp. 987-995). Taylor & Francis.
[28]. Matusz-Fisher, A., & Tan, A. R. (2022). Sacituzumab govitecan and other antibody-drug conjugates targeting trophoblast cell-surface antigen 2 (Trop-2) in breast cancer. Annals of Translational Medicine, 10(18).
[29]. Bardia, A., Vahdat, L. T., Diamond, J. R., Starodub, A., Moroose, R. L., Isakoff, S. J., ... & Mayer, I. A. (2015). Therapy of refractory/relapsed metastatic triple-negative breast cancer (TNBC) with an anti-Trop-2-SN-38 antibody-drug conjugate (ADC), sacituzumab govitecan (IMMU-132): Phase I/II clinical experience.
[30]. Abdollahpour‐Alitappeh, M., Lotfinia, M., Gharibi, T., Mardaneh, J., Farhadihosseinabadi, B., Larki, P., ... & Bagheri, N. (2019). Antibody–drug conjugates (ADCs) for cancer therapy: Strategies, challenges, and successes. Journal of cellular physiology, 234(5), 5628-5642.
[31]. Dean, A. Q., Luo, S., Twomey, J. D., & Zhang, B. (2021, January). Targeting cancer with antibody-drug conjugates: Promises and challenges. In MAbs (Vol. 13, No. 1, p. 1951427). Taylor & Francis
[32]. Chari, R. V., Miller, M. L., & Widdison, W. C. (2014). Antibody–drug conjugates: an emerging concept in cancer therapy. Angewandte Chemie International Edition, 53(15), 3796-3827.
[33]. Beck, A., Goetsch, L., Dumontet, C., & Corvaïa, N. (2017). Strategies and challenges for the next generation of antibody–drug conjugates. Nature reviews Drug discovery, 16(5), 315-337.
[34]. Aggarwal, D., Yang, J., Salam, M. A., Sengupta, S., Al-Amin, M. Y., Mustafa, S., ... & Pawar, J. S. (2023). Antibody-drug conjugates: the paradigm shifts in the targeted cancer therapy. Frontiers in immunology, 14, 1203073.
[35]. Agarwal, P., & Bertozzi, C. R. (2015). Site-specific antibody–drug conjugates: the nexus of bioorthogonal chemistry, protein engineering, and drug development. Bioconjugate chemistry, 26(2), 176-192.
[36]. Teicher, B. A., & Chari, R. V. (2011). Antibody conjugate therapeutics: challenges and potential. Clinical cancer research, 17(20), 6389-6397.
[37]. Perez, H. L., Cardarelli, P. M., Deshpande, S., Gangwar, S., Schroeder, G. M., Vite, G. D., & Borzilleri, R. M. (2014). Antibody–drug conjugates: current status and future directions. Drug discovery today, 19(7), 869-881









